Abstract
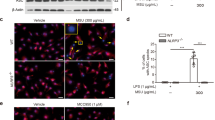
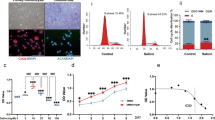
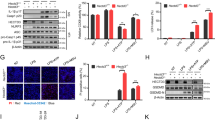
Monosodium urate (MSU) crystals activate inflammatory pathways that overlap with interleukin-1β (IL-1β) signaling. However, the post-translational mechanisms involved and the role of signaling proteins in this activation are unknown. In the present study, we investigated the intracellular signaling mechanisms involved in MSU-induced activation of THP-1 macrophages and human nondiseased synovial fibroblasts (NLSFs) and the in vivo efficacy of an inhibitor of tumor growth factor-β (TGF-β)-activated kinase 1 (TAK1), 5Z-7-oxozeaenol, in MSU-induced paw inflammation in C57BL/6 mice. THP-1 macrophage activation with MSU crystals (25–200 µg/ml) resulted in the rapid and sustained phosphorylation of interleukin-1 receptor-activated kinase 1 (IRAK1 Thr209) and TAK1 (Thr184/187) and their association with the E3 ubiquitin ligase TRAF6. At the cellular level, MSU inhibited the deubiquitinases A20 and UCHL2 and increased 20s proteasomal activity, leading to a global decrease in K63-linked ubiquitination and increase in K48-linked ubiquitination in THP-1 macrophages. While MSU did not stimulate cytokine production in NLSFs, it significantly amplified IL-1β-induced IL-6, IL-8, and ENA-78/CXCL5 production. Docking studies and MD simulations followed by TAK1 in vitro kinase assays revealed that uric acid molecules are capable of arresting TAK1 in an active-state conformation, resulting in sustained TAK1 kinase activation. Importantly, MSU-induced proinflammatory cytokine production was completely inhibited by 5Z-7-oxozeaenol but not IRAK1/4 or TRAF6 inhibitors. Administration of 5Z-7-oxozeaenol (5 or 15 mg/kg; orally) significantly inhibited MSU-induced paw inflammation in C57BL/6 mice. Our study identifies a novel post-translational mechanism of TAK1 activation by MSU and suggests the therapeutic potential of TAK1 in regulating MSU-induced inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rees, F., Hui, M. & Doherty, M. Optimizing current treatment of gout. Nat. Rev. Rheumatol. 10, 271–283 (2014).
So, A. & Busso, N. A magic bullet for gout? Ann. Rheum. Dis. 68, 1517–1519 (2009).
Chen, C. J. et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J. Clin. Invest. 116, 2262–2271 (2006).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Martinon, F. & Glimcher, L. H. Gout: new insights into an old disease. J. Clin. Invest. 116, 2073–2075 (2006).
Bardin, T. Acute inflammatory arthritis: interleukin-1 blockade: a magic wand for gout? Nat. Rev. Rheumatol. 5, 594–596 (2009).
Dumusc, A. & So, A. Interleukin-1 as a therapeutic target in gout. Curr. Opin. Rheumatol. 27, 156–163 (2015).
Jiang, X. & Chen, Z. J. The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 12, 35–48 (2011).
Kulathu, Y. & Komander, D. Atypical ubiquitylation—the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat. Rev. Cell. Mol. Biol. 13, 508–523 (2012).
Fechtner, S., Fox, D. A. & Ahmed, S. Transforming growth factor beta activated kinase 1: a potential therapeutic target for rheumatic diseases. Rheumatology 56, 1060–1068 (2017).
Jones, D. S. et al. Profiling drugs for rheumatoid arthritis that inhibit synovial fibroblast activation. Nat. Chem. Biol. 13, 38–45 (2017).
Singh, A. K., Umar, S., Riegsecker, S., Chourasia, M. & Ahmed, S. Regulation of transforming growth factor beta-activated kinase activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts: suppression of K(63)-linked autoubiquitination of tumor necrosis factor receptor-associated factor 6. Arthritis Rheum. 68, 347–358 (2016).
Bruegel, M., Teupser, D., Haffner, I., Mueller, M. & Thiery, J. Statins reduce macrophage inflammatory protein-1alpha expression in human activated monocytes. Clin. Exp. Pharm. Physiol. 33, 1144–1149 (2006).
Pazar, B. et al. Basic calcium phosphate crystals induce monocyte/macrophage IL-1beta secretion through the NLRP3 inflammasome in vitro. J. Immunol. 186, 2495–2502 (2011).
Ahmed, S., Pakozdi, A. & Koch, A. E. Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 54, 2393–2401 (2006).
Akhtar, N., Singh, A. K. & Ahmed, S. MicroRNA-17 suppresses TNF-alpha signaling by interfering with TRAF2 and cIAP2 association in rheumatoid arthritis synovial fibroblasts. J. Immunol. 197, 2219–2228 (2016).
Ahmed, S. et al. Epigallocatechin-3-gallate inhibits IL-6 synthesis and suppresses transsignaling by enhancing soluble gp130 production. Proc. Natl Acad. Sci. USA 105, 14692–14697 (2008).
Singh, A. K., Fechtner, S., Chourasia, M., Sicalo J. & Ahmed S. Critical role of IL-1alpha in IL-1beta-induced inflammatory responses: cooperation with NF-kappaBp65 in transcriptional regulation. FASEB J. https://doi.org/10.1096/fj201801513R (2018).
Fechtner, S., Singh, A., Chourasia, M. & Ahmed, S. Molecular insights into the differences in anti-inflammatory activities of green tea catechins on IL-1beta signaling in rheumatoid arthritis synovial fibroblasts. Toxicol. Appl Pharm. 329, 112–120 (2017).
Lacey, D. C. et al. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J. Immunol. 188, 5752–5765 (2012).
Reber, L. L. et al. Contribution of mast cell-derived interleukin-1beta to uric acid crystal-induced acute arthritis in mice. Arthritis Rheum. 66, 2881–2891 (2014).
Fechtner, S. et al. Cannabinoid receptor 2 agonist JWH-015 inhibits interleukin-1beta-induced inflammation in rheumatoid arthritis synovial fibroblasts and in adjuvant induced arthritis rat via glucocorticoid receptor. Front. Immunol. 10, 1027 (2019).
Wu, J. et al. Mechanism and in vitro pharmacology of TAK1 inhibition by (5Z)-7-oxozeaenol. ACS Chem. Biol. 8, 643–650 (2013).
Crisan, T. O. et al. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann. Rheum. Dis. 75, 755–762 (2016).
Xiao, J. et al. Soluble monosodium urate, but not its crystal, induces toll like receptor 4-dependent immune activation in renal mesangial cells. Mol. Immunol. 66, 310–318 (2015).
Ninomiya-Tsuji, J. et al. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398, 252–256 (1999).
Lamothe, B. et al. Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of I kappa B kinase activation. J. Biol. Chem. 282, 4102–4112 (2007).
Edwards, N. L. & So, A. Emerging therapies for gout. Rheum. Dis. Clin. N. Am. 40, 375–387 (2014).
Weiss, W. A., Taylor, S. S. & Shokat, K. M. Recognizing and exploiting differences between RNAi and small-molecule inhibitors. Nat. Chem. Biol. 3, 739–744 (2007).
Mevissen, T. E. T. & Komander, D. Mechanisms of deubiquitinase specificity and regulation. Annu. Rev. Biochem. 86, 159–192 (2017).
Chen, Z. J. Ubiquitination in signaling to and activation of IKK. Immunol. Rev. 246, 95–106 (2012).
Duong, B. H. et al. A20 restricts ubiquitination of pro-interleukin-1beta protein complexes and suppresses NLRP3 inflammasome activity. Immunity 42, 55–67 (2015).
Lork, M., Verhelst, K. & Beyaert, R. CYLD, A20 and OTULIN deubiquitinases in NF-kappaB signaling and cell death: so similar, yet so different. Cell Death Differ. 24, 1172–1183 (2017).
Shembade, N., Ma, A. & Harhaj, E. W. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327, 1135–1139 (2010).
Skaug, B. et al. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol. Cell 44, 559–571 (2011).
Liu, R., Liote, F., Rose, D. M., Merz, D. & Terkeltaub, R. Proline-rich tyrosine kinase 2 and Src kinase signaling transduce monosodium urate crystal-induced nitric oxide production and matrix metalloproteinase 3 expression in chondrocytes. Arthritis Rheum. 50, 247–258 (2004).
Liu-Bryan, R., Pritzker, K., Firestein, G. S. & Terkeltaub, R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J. Immunol. 174, 5016–5023 (2005).
Zheng, S. C. et al. Role of the NLRP3 inflammasome in the transient release of IL-1beta induced by monosodium urate crystals in human fibroblast-like synoviocytes. J. Inflamm. 12, 30 (2015).
Baldwin, A. G., Brough, D. & Freeman, S. Inhibiting the inflammasome: a chemical perspective. J. Med. Chem. 59, 1691–1710 (2016).
Cavalli, G. & Dinarello, C. A. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology 54, 2134–2144 (2015).
Davies K. & Bukhari M. A. S. Recent pharmacological advances in the management of gout. Rheumatology https://doi.org/10.1093/rheumatology/kex343 (2017).
Sato, S. et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6, 1087–1095 (2005).
Cao, H. et al. TAK1 inhibition prevents the development of autoimmune diabetes in NOD mice. Sci. Rep. 5, 14593 (2015).
Xiao, Y. et al. TPL2 mediates autoimmune inflammation through activation of the TAK1 axis of IL-17 signaling. J. Exp. Med. 211, 1689–1702 (2014).
Brown, K. et al. Structural basis for the interaction of TAK1 kinase with its activating protein TAB1. J. Mol. Biol. 354, 1013–1020 (2005).
Singer, J. W. et al. Inhibition of interleukin-1 receptor-associated kinase 1 (IRAK1) as a therapeutic strategy. Oncotarget 9, 33416–33439 (2018).
Pauls, E. et al. Two phases of inflammatory mediator production defined by the study of IRAK2 and IRAK1 knock-in mice. J. Immunol. 191, 2717–2730 (2013).
Della Mina, E. et al. Inherited human IRAK-1 deficiency selectively impairs TLR signaling in fibroblasts. Proc. Natl Acad. Sci. USA 114, E514–E523 (2017).
Acknowledgements
We thank the National Disease Research Interchange, Philadelphia, PA and the Cooperative Human Tissue Network (CHTN), Columbus, OH for providing synovial tissues. We would also like to thank Sadiq Umar and Bhanupriya Madarampalli for providing technical support in animal experiments. We also thank Ruby Siegel for critical reading of the manuscript. This study was supported by start-up funds from Washington State University.
Author information
Authors and Affiliations
Contributions
A.K.S. and S.A. designed this study. A.K.S., K.O.S. and M.H. performed the experiments and participated in drafting the manuscript. S.A. participated in writing the manuscript and provided his support to the study. M.C. participated in writing the manuscript and conducted and analyzed docking and MD simulation experiments. M.O. performed histological analysis and interpreted the results. S.A. is the corresponding author of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Singh, A.K., Haque, M., O’Sullivan, K. et al. Suppression of monosodium urate crystal-induced inflammation by inhibiting TGF-β-activated kinase 1-dependent signaling: role of the ubiquitin proteasome system. Cell Mol Immunol 18, 162–170 (2021). https://doi.org/10.1038/s41423-019-0284-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0284-3
Keywords
This article is cited by
-
Paracrine activin B-NF-κB signaling shapes an inflammatory tumor microenvironment in gastric cancer via fibroblast reprogramming
Journal of Experimental & Clinical Cancer Research (2023)
-
Comparison of the different monosodium urate crystals in the preparation process and pro-inflammation
Advances in Rheumatology (2023)
-
TGF-β is elevated in hyperuricemic individuals and mediates urate-induced hyperinflammatory phenotype in human mononuclear cells
Arthritis Research & Therapy (2023)
-
Silencing TAK1 reduces MAPKs-MMP2/9 expression to reduce inflammation-driven neurohistological disruption post spinal cord injury
Cell Death Discovery (2021)
-
TAK1-AMPK Pathway in Macrophages Regulates Hypothyroid Atherosclerosis
Cardiovascular Drugs and Therapy (2021)