Abstract
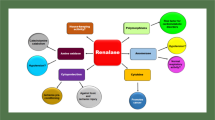
Cardiovascular and renal complications cover a wide array of diseases. The most commonly known overlapping complications include cardiac and renal fibrosis, cardiomyopathy, cardiac hypertrophy, hypertension, and cardiorenal failure. The known or reported causes for the abovementioned complications include injury, ischemia, infection, and metabolic stress. To date, various targets have been reported and investigated in detail that are considered to be the cause of these complications. In the past 5 years, the role of noncoding RNAs has emerged in the area of cardiovascular and renal research, especially in relation to metabolic stress. The long noncoding RNA MALAT1 (metastasis-associated lung adenocarcinoma transcript 1) has shown immense promise among the long noncoding RNA targets for treating cardiorenal complications. In this review, we shed light on the role of MALAT1 as a primary and novel target in treating cardiovascular and renal diseases as a whole.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Chatterjee K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol. 2005;95:8B–13B.
Skvortsova VI, Platonova IA, Tvorogova TV, Volkovenko OV, Demidova LI, Ostrovtsev IV. The effects of hormones of the hypothalamo-hypophyseal-adrenal, renin-angiotensin, and thyroid hormone systems on the formation of dyscirculatory encephalopathy. Neurosci Behav Physiol. 2004;34:939–47.
Hering D, Winklewski PJ. Autonomic nervous system in acute kidney injury. Clin Exp Pharm Physiol. 2017;44:162–71.
Fu Q, Cao L, Li H, Wang B, Li Z. Cardiorenal syndrome: pathophysiological mechanism, preclinical models, novel contributors and potential therapies. Chin Med J (Engl). 2014;127:3011–8.
Lekawanvijit S, Krum H. Cardiorenal syndrome: acute kidney injury secondary to cardiovascular disease and role of protein-bound uraemic toxins. J Physiol. 2014;592:3969–83.
Rajapakse NW, Nanayakkara S, Kaye DM. Pathogenesis and treatment of the cardiorenal syndrome: implications of L-arginine-nitric oxide pathway impairment. Pharm Ther. 2015;154:1–12.
Rubattu S, Mennuni S, Testa M, Mennuni M, Pierelli G, Pagliaro B, et al. Pathogenesis of chronic cardiorenal syndrome: is there a role for oxidative stress? Int J Mol Sci. 2013;14:23011–32.
Sumida M, Doi K, Ogasawara E, Yamashita T, Hamasaki Y, Kariya T, et al. Regulation of mitochondrial dynamics by dynamin-related protein-1 in acute cardiorenal syndrome. J Am Soc Nephrol. 2015;26:2378–87.
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39.
Fonarow GC, Corday E, Committee ASA. Overview of acutely decompensated congestive heart failure (ADHF): a report from the ADHERE registry. Heart Fail Rev. 2004;9:179–85.
Heywood JT. The cardiorenal syndrome: lessons from the ADHERE database and treatment options. Heart Fail Rev. 2004;9:195–201.
Yancy CW, Fonarow GC, ADHERE Scientific Advisory Committee. Quality of care and outcomes in acute decompensated heart failure: the ADHERE Registry. Curr Heart Fail Rep. 2004;1:121–8.
Chowdhury EK, Langham RG, Ademi Z, Owen A, Krum H, Wing LM, et al. Rate of change in renal function and mortality in elderly treated hypertensive patients. Clin J Am Soc Nephrol. 2015;10:1154–61.
Yancy CW. Treatment with B-type natriuretic peptide for chronic decompensated heart failure: insights learned from the follow-up serial infusion of nesiritide (FUSION) trial. Heart Fail Rev. 2004;9:209–16.
Yancy CW, Krum H, Massie BM, Silver MA, Stevenson LW, Cheng M, et al. Safety and efficacy of outpatient nesiritide in patients with advanced heart failure: results of the second follow-up serial infusions of nesiritide (FUSION II) trial. Circ Heart Fail. 2008;1:9–16.
Uneda K, Tamura K, Wakui H, Azushima K, Haku S, Kobayashi R, et al. Comparison of direct renin inhibitor and angiotensin II receptor blocker on clinic and ambulatory blood pressure profiles in hypertension with chronic kidney disease. Clin Exp Hypertens. 2016;38:738–43.
Kobayashi R, Tamura K, Wakui H, Ohsawa M, Azushima K, Haku S, et al. Effect of single-pill irbesartan/amlodipine combination-based therapy on clinic and home blood pressure profiles in hypertension with chronic kidney diseases. Clin Exp Hypertens. 2016;38:744–50.
Asleh R, Snipelisky D, Hathcock M, Kremers W, Liu D, Batzler A, et al. Genomewide association study reveals novel genetic loci associated with change in renal function in heart transplant recipients. Clin Transplant. 2018;32:e13395.
Denby L, Baker AH. Targeting non-coding RNA for the therapy of renal disease. Curr Opin Pharm. 2016;27:70–7.
Lorenzen JM, Thum T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol. 2016;12:360–73.
Shang D, Zheng T, Zhang J, Tian Y, Liu Y. Profiling of mRNA and long non-coding RNA of urothelial cancer in recipients after renal transplantation. Tumour Biol. 2016;37:12673–84.
Virzi GM, Clementi A, Brocca A, de Cal M, Ronco C. Epigenetics: a potential key mechanism involved in the pathogenesis of cardiorenal syndromes. J Nephrol. 2018;31:333–41.
Li X, Wei Y, Wang Z. microRNA-21 and hypertension. Hypertens Res. 2018;41:649–61.
Chuppa S, Liang M, Liu P, Liu Y, Casati MC, Cowley AW, et al. MicroRNA-21 regulates peroxisome proliferator-activated receptor alpha, a molecular mechanism of cardiac pathology in cardiorenal syndrome type 4. Kidney Int. 2018;93:375–89.
Rana I, Velkoska E, Patel SK, Burrell LM, Charchar FJ. MicroRNAs mediate the cardioprotective effect of angiotensin-converting enzyme inhibition in acute kidney injury. Am J Physiol Ren Physiol. 2015;309:F943–54.
Alvarez ML, DiStefano JK. Functional characterization of the plasmacytoma variant translocation 1 gene (PVT1) in diabetic nephropathy. PLoS One. 2011;6:e18671.
Lorenzen JM, Schauerte C, Kielstein JT, Hubner A, Martino F, Fiedler J, et al. Circulating long noncoding RNATapSaki is a predictor of mortality in critically ill patients with acute kidney injury. Clin Chem. 2015;61:191–201.
Wang F, Li L, Xu H, Liu Y, Yang C, Cowley AW Jr., et al. Characteristics of long non-coding RNAs in the brown Norway rat and alterations in the Dahl salt-sensitive rat. Sci Rep. 2014;4:7146.
Chen W, Zhang L, Zhou ZQ, Ren YQ, Sun LN, Man YL, et al. Effects of long non-coding RNA LINC00963 on renal interstitial fibrosis and oxidative stress of rats with chronic renal failure via the foxo signaling pathway. Cell Physiol Biochem. 2018;46:815–28.
Gomez J, Lorca R, Reguero JR, Martin M, Moris C, Alonso B, et al. Genetic variation at the long noncoding RNA H19 gene is associated with the risk of hypertrophic cardiomyopathy. Epigenomics. 2018;10:865–73.
Wang YN, Shan K, Yao MD, Yao J, Wang JJ, Li X, et al. Long noncoding RNA-GAS5: a novel regulator of hypertension-induced vascular remodeling. Hypertension. 2016;68:736–48.
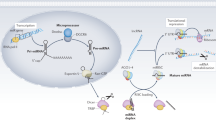
Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–38.
Zong X, Tripathi V, Prasanth KV. RNA splicing control: yet another gene regulatory role for long nuclear noncoding RNAs. RNA Biol. 2011;8:968–77.
Bernard D, Prasanth KV, Tripathi V, Colasse S, Nakamura T, Xuan Z, et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010;29:3082–93.
Rajaram V, Knezevich S, Bove KE, Perry A, Pfeifer JD. DNA sequence of the translocation breakpoints in undifferentiated embryonal sarcoma arising in mesenchymal hamartoma of the liver harboring the t(11;19)(q11;q13.4) translocation. Genes Chromosom Cancer. 2007;46:508–13.
Gutschner T, Hammerle M, Diederichs S. MALAT1—a paradigm for long noncoding RNA function in cancer. J Mol Med (Berl). 2013;91:791–801.
Gutschner T, Hammerle M, Eissmann M, Hsu J, Kim Y, Hung G, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–9.
Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–41.
Guffanti A, Iacono M, Pelucchi P, Kim N, Solda G, Croft LJ, et al. A transcriptional sketch of a primary human breast cancer by 454 deep sequencing. BMC Genom. 2009;10:163.
Xu C, Yang M, Tian J, Wang X, Li Z. MALAT-1: a long non-coding RNA and its important 3' end functional motif in colorectal cancer metastasis. Int J Oncol. 2011;39:169–75.
Gutschner T, Baas M, Diederichs S. Noncoding RNA gene silencing through genomic integration of RNA destabilizing elements using zinc finger nucleases. Genome Res. 2011;21:1944–54.
Lelli A, Nolan KA, Santambrogio S, Goncalves AF, Schonenberger MJ, Guinot A, et al. Induction of long noncoding RNA MALAT1 in hypoxic mice. Hypoxia (Auckl). 2015;3:45–52.
Michalik KM, You X, Manavski Y, Doddaballapur A, Zornig M, Braun T, et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014;114:1389–97.
Puthanveetil P, Chen S, Feng B, Gautam A, Chakrabarti S. Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J Cell Mol Med. 2015;19:1418–25.
Li X, Zeng L, Cao C, Lu C, Lian W, Han J, et al. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res. 2017;350:327–35.
Song Y, Yang L, Guo R, Lu N, Shi Y, Wang X. Long noncoding RNA MALAT1 promotes high glucose-induced human endothelial cells pyroptosis by affecting NLRP3 expression through competitively binding miR-22. Biochem Biophys Res Commun. 2019;509:359–66.
Li Y, Ren D, Xu G. Long noncoding RNA MALAT1 mediates high glucose-induced glomerular endothelial cell injury by epigenetically inhibiting klotho via methyltransferase G9a. IUBMB Life. 2019;71:873–81.
Eissmann M, Gutschner T, Hammerle M, Gunther S, Caudron-Herger M, Gross M, et al. Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. RNA Biol. 2012;9:1076–87.
Nakagawa S, Ip JY, Shioi G, Tripathi V, Zong X, Hirose T, et al. Malat1 is not an essential component of nuclear speckles in mice. RNA. 2012;18:1487–99.
Zhang X, Tang X, Liu K, Hamblin MH, Yin KJ. Long noncoding RNA Malat1 regulates cerebrovascular pathologies in ischemic stroke. J Neurosci. 2017;37:1797–806.
Cremer S, Michalik KM, Fischer A, Pfisterer L, Jae N, Winter C, et al. Hematopoietic deficiency of the long noncoding RNA MALAT1 promotes atherosclerosis and plaque inflammation. Circulation. 2019;139:1320–34.
Kolling M, Genschel C, Kaucsar T, Hubner A, Rong S, Schmitt R, et al. Hypoxia-induced long non-coding RNA Malat1 is dispensable for renal ischemia/reperfusion-injury. Sci Rep. 2018;8:3438.
Yu SY, Dong B, Tang L, Zhou SH. LncRNA MALAT1 sponges miR-133 to promote NLRP3 inflammasome expression in ischemia-reperfusion injured heart. Int J Cardiol. 2018;254:50.
Chen H, Wang X, Yan X, Cheng X, He X, Zheng W. LncRNA MALAT1 regulates sepsis-induced cardiac inflammation and dysfunction via interaction with miR-125b and p38 MAPK/NFkappaB. Int Immunopharmacol. 2018;55:69–76.
Huang S, Zhang L, Song J, Wang Z, Huang X, Guo Z, et al. Long noncoding RNA MALAT1 mediates cardiac fibrosis in experimental postinfarct myocardium mice model. J Cell Physiol. 2019;234:2997–3006.
Xiang Y, Zhang Y, Tang Y, Li Q. MALAT1 modulates TGF-beta1-induced endothelial-to-mesenchymal transition through downregulation of miR-145. Cell Physiol Biochem. 2017;42:357–72.
Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee SS. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids. 2017;8:132–43.
Sinclair A, Islam S, Jones S. Gene therapy: an overview of approved and pipeline technologies. In: CADTH issues in emerging health technologies. Ottawa, ON; 2016. p. 1–23. https://www.ncbi.nlm.nih.gov/books/NBK378971/.
Hoy SM. Patisiran: first global approval. Drugs. 2018;78:1625–31.
Weng Y, Xiao H, Zhang J, Liang XJ, Huang Y. RNAi therapeutic and its innovative biotechnological evolution. Biotechnol Adv. 2019;37:801–25.
Acknowledgements
PP is supported by Research Start Up Funds from the Department of Pharmacology, College of Graduate Studies, Midwestern University, Downers Grove, IL.
Author information
Authors and Affiliations
Contributions
PP conceived the idea, wrote, and edited the manuscript. The figures were also created by PP. TG and JL wrote and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Puthanveetil, P., Gutschner, T. & Lorenzen, J. MALAT1: a therapeutic candidate for a broad spectrum of vascular and cardiorenal complications. Hypertens Res 43, 372–379 (2020). https://doi.org/10.1038/s41440-019-0378-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0378-4