Abstract
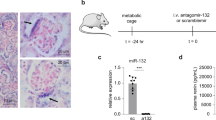
BPH/2J mice are a genetic model of hypertension with overactivity of the sympathetic nervous system (SNS) and renin–angiotensin system (RAS). BPH/2J display higher renal renin mRNA and low levels of its negative regulator microRNA-181a (miR-181a). We hypothesise that high renal SNS activity may reduce miR-181a expression, which contributes to elevated RAS activity and hypertension in BPH/2J. Our aim was to determine whether in vivo administration of a renal-specific miR-181a mimic or whether renal denervation could increase renal miR-181a abundance to reduce renal renin mRNA, RAS activity and hypertension in BPH/2J mice. Blood pressure (BP) in BPH/2J and normotensive BPN/3J mice was measured via radiotelemetry probes. Mice were administered miR-181a mimic or a negative control (1–25 nmol, i.v., n = 6–10) with BP measured for 48 h after each dose or they underwent renal denervation or sham surgery (n = 7–9). Injection of 5–25 nmol miR-181a mimic reduced BP in BPH/2J mice after 36–48 h (−5.3 ± 1.8, −6.1 ± 1.9 mmHg, respectively, P < 0.016). Treatment resulted in lower renal renin and inflammatory marker (TLR4) mRNA levels in BPH/2J. The mimic abolished the hypotensive effect of blocking the RAS with enalaprilat (P < 0.01). No differences between mimic or vehicle were observed in BPN/3J mice except for a higher level of renal angiotensinogen in the mimic-treated mice. Renal miR-181a levels that were lower in sham BPH/2J mice were greater following renal denervation and were thus similar to those of BPN/3J. Our findings suggest that the reduced renal miR-181a may partially contribute to the elevated BP in BPH/2J mice, through an interaction between the renal sympathetic nerves and miR-181a regulation of the RAS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jackson KL, Palma-Rigo K, Nguyen-Huu T-P, Davern PJ, Head GA. Major contribution of the medial amygdala to hypertension in BPH/2J genetically hypertensive mice. Hypertension. 2014;63:811–8.
Jackson KL, Marques FZ, Watson AMD, Palma Rigo K, Nguyen-Huu T-P, Morris BJ, et al. A novel interaction between sympathetic overactivity and aberrant regulation of renin by mir-181a in BPH/2J genetically hypertensive mice. Hypertension. 2013;62:775–81.
Davern PJ, Nguyen-Huu T, La Greca L, Head GA. Role of the sympathetic nervous system in Schlager genetically hypertensive mice. Hypertension. 2009;54:852–9.
Gueguen C, Jackson KL, Marques FZ, Eikelis N, Phillips S, Stevenson ER, et al. Renal nerves contribute to hypertension in Schlager BPH/2J mice. Hypertens Res. 2019;42:306–18.
Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YH, Charchar FJ, et al. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension. 2011;58:1093–8.
Hulsmans M, Sinnaeve P, Van der Schueren B, Mathieu C, Janssens S, Holvoet P. Decreased miR-181a expression in monocytes of obese patients is associated with the occurrence of metabolic syndrome and coronary artery disease. J Clin Endocrinol Metab. 2012;97:E1213–8.
Marques FZ, Romaine SP, Denniff M, Eales J, Dormer J, Garrelds IM, et al. Signatures of miR-181a on renal transcriptome and blood pressure. Mol Med. 2015;21:739–48.
Adams DJ, Head GA, Markus MA, Lovicu FJ, van der Weyden L, Köntgen F, et al. Renin enhancer is critical for regulation of renin gene expression and control of cardiovascular function. J Biol Chem. 2006;281:31753–61.
Jackson K, Dampney B, Moretti J-L, Stevenson E, Davern P, Carrive P, et al. The contribution of orexin to the neurogenic hypertension In BPH/2J mice. Hypertension. 2016;67:959–69.
Huang Y, Ratovitski EA. Phospho-DeltaNp63alpha/Rpn13-dependent regulation of LKB1 degradation modulates autophagy in cancer cells. Aging. 2010;2:959–68.
Ji J, Yamashita T, Wang XW. Wnt/beta-catenin signaling activates microRNA-181 expression in hepatocellular carcinoma. Cell Biosci. 2011;1:4.
Arthurs AL, Lumbers ER, Pringle KG. MicroRNA mimics that target the placental renin-angiotensin system inhibit trophoblast proliferation. Mol Hum Reprod. 2019;25:218–27.
Ramkumar N, Ying J, Stuart D, Kohan DE. Overexpression of renin in the collecting duct causes elevated blood pressure. Am J Hypertens. 2013;26:965–72.
Ramkumar N, Stuart D, Rees S, Hoek AV, Sigmund CD, Kohan DE. Collecting duct-specific knockout of renin attenuates angiotensin II-induced hypertension. Am J Physiol Ren Physiol. 2014;307:F931–8.
Marques FZ, Vizi D, Khammy O, Mariani JA, Kaye DM. The transcardiac gradient of cardio-microRNAs in the failing heart. Eur J Heart Fail. 2016;18:1000–8.
Hori D, Dunkerly-Eyring B, Nomura Y, Biswas D, Steppan J, Henao-Mejia J, et al. miR-181b regulates vascular stiffness age dependently in part by regulating TGF-beta signaling. PLoS ONE. 2017;12:e0174108.
Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–9.
Zhang Z, Qin YW, Brewer G, Jing Q. MicroRNA degradation and turnover: regulating the regulators. Wiley Interdiscip Rev RNA. 2012;3:593–600.
Johns DW, Peach MJ, Gomez RA, Inagami T, Carey RM. Angiotensin II regulates renin gene expression. Am J Physiol. 1990;259:F882–7.
Wang Y, Lumbers ER, Arthurs AL, Corbisier de Meaultsart C, Mathe A, Avery-Kiejda KA, et al. Regulation of the human placental (pro)renin receptor-prorenin-angiotensin system by microRNAs. Mol Hum Reprod. 2018;24:453–64.
Tonevitsky AG. Dynamically regulated miRNA-mRNA networks revealed by exercise. BMC Physiol. 2013;13:9.
Nishi EE, Lopes NR, Gomes GN, Perry JC, Sato AYS, Naffah-Mazzacoratti MG, et al. Renal denervation reduces sympathetic overactivation, brain oxidative stress, and renal injury in rats with renovascular hypertension independent of its effects on reducing blood pressure. Hypertens Res. 2019;42:628–40.
Saba R, Storchel PH, Aksoy-Aksel A, Kepura F, Lippi G, Plant TD, et al. Dopamine-regulated microRNA MiR-181a controls GluA2 surface expression in hippocampal neurons. Mol Cell Biol. 2012;32:619–32.
Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, et al. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Investig. 2011;121:2845–54.
Boccardi V, Ambrosino I, Papa M, Fiore D, Rizzo MR, Paolisso G, et al. Potential role of TCF7L2 gene variants on cardiac sympathetic/parasympathetic activity. Eur J Hum Genet. 2010;18:1333–8.
Deng M, Tufan T, Raza MU, Jones TC, Zhu MY. MicroRNAs 29b and 181a down-regulate the expression of the norepinephrine transporter and glucocorticoid receptors in PC12 cells. J Neurochem. 2016;139:197–207.
Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension. 2004;43:169–75.
Zhou J. Norepinephrine transporter inhibitors and their therapeutic potential. Drugs Future. 2004;29:1235–44.
Marques FZ, Eikelis N, Bayles RG, Lambert EA, Straznicky NE, Hering D, et al. A polymorphism in the norepinephrine transporter gene is associated with affective and cardiovascular disease through a microRNA mechanism. Mol Psychiatry. 2017;22:134–41.
Head GA, Saigusa T, Mayorov DN. Angiotensin and baroreflex control of the circulation. Braz J Med Biol Res. 2002;35:1047–59.
Matsukawa S, Reid IA. Role of the area postrema in the modulation of the baroreflex control of heart rate by angiotensin-II. Circ Res. 1990;67:1462–73.
Li X, Wei Y, Wang Z. microRNA-21 and hypertension. Hypertens Res. 2018;41:649–61.
Acknowledgements
We acknowledge the technical assistance of Thu-Phuc Nguyen-Huu and John-Luis Moretti. We thank Dr Anna Watson for kindly providing the TLR4, ACE, AT2 and CD36 Taqman mixes and Dr Christos Tikellis for the ACE2 and AT1aR Taqman mixes.
Funding
This work was supported by a grant from the National Health & Medical Research Council of Australia (NHMRC, Project grant 1065714) and in part by the Victorian Government’s OIS Program. Investigators were supported by NHMRC/National Heart Foundation (NHF) Postdoctoral Fellowships (NHMRC APP1091688 to KLJ, NHMRC APP1052659 and NHF PF12M6785 and 101185 to FZM) and NHMRC Research Fellowships (APP1042492 to GWL and APP1002186 to GAH).
Author information
Authors and Affiliations
Contributions
KLJ, CG, KL, FZM, NE, ERS and MRP performed the study including experimental data collection, assays and analyses. CG, KLJ, KL, FZM, NE, ERS, FJC, GWL, SLB, MRP and GAH contributed to the analysis, writing and preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
GWL received research support from Medtronic and has acted as a consultant for Medtronic. GAH has received research support from Boehringer Ingelheim. Both are unrelated to the current study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Jackson, K.L., Gueguen, C., Lim, K. et al. Neural suppression of miRNA-181a in the kidney elevates renin expression and exacerbates hypertension in Schlager mice. Hypertens Res 43, 1152–1164 (2020). https://doi.org/10.1038/s41440-020-0453-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-0453-x
Keywords
This article is cited by
-
The pharmaco-epigenetics of hypertension: a focus on microRNA
Molecular and Cellular Biochemistry (2024)
-
Connecting sympathetic and renin–angiotensin system overdrive in neurogenic hypertension through miRNA-181a
Hypertension Research (2020)