Abstract
In this work, the enantioselective total synthesis of (–)-quinine has been accomplished in a pot-economical manner using five reaction vessels. In the first pot, reactions involve the diphenylprolinol silyl ether-mediated Michael reaction, aza-Henry reaction, hemiaminalization, and elimination of HNO2 (five reactions), affording a chiral tetrahydropyridine with excellent enantioselectivity. In the second pot, five reactions proceed with excellent diastereoselectivity to afford a trisubstituted piperidine with the desired stereochemistry. A further five reactions are carried out in the last one-pot sequence.
Similar content being viewed by others
Introduction
Quinine is one of the cinchona alkaloids; it is extracted from the bark of cinchona trees1. It is an important antimalarial medication2. Its derivatives are used not only as ligands of asymmetric metal catalysts, but also as effective asymmetric organocatalysts3,4,5,6,7,8,9,10. Quinine has fascinated synthetic organic chemists for several decades11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28. It contains four chiral centers, and stereoselective synthesis is a challenge.
In 1944, Woodward and Doering synthesized homomeroquinene—the first formal synthesis of a racemic quinine4,5. Stork then reported the first synthesis of chiral (–)-quinine from a chiral starting material using highly diastereoselective reactions17. Jacobsen18 and Kobayashi20 independently synthesized (–)-quinine by constructing a quinuclidine skeleton, by a C8–N bond formation, using an epoxide as an electrophile. Jacobsen used an asymmetric catalytic reaction for the enantioselective synthesis. Aggarwal used a chiral Corey–Chaykovsky reaction as a key step22,23, while Maulide used a C–H activation reaction and an aldol reaction25. Chen synthesized quinine and quinidine using a local desymmetrization-based strategy26.
Recently, organocatalysts have been used for the synthesis of quinine. Hatakeyama used a proline-mediated intramolecular aldol reaction for the formation of a piperidine skeleton24, while the Michael reaction of dimethyl malonate and α,β-unsaturated aldehyde catalyzed by diphenylprolinol silyl ether is a key step in Córdova’s synthesis27. Ishikawa has reported a synthesis of (–)-quinine involving a one-pot synthesis of a chiral piperidine skeleton by a diphenylprolinol silyl ether-mediated formal aza-[3 + 3] cycloaddition/Strecker-type cyanation reaction28. Although there are several asymmetric total syntheses of (–)-quinine, it is still desirable to develop an efficient and practical method for its synthesis. Moreover, a method that facilitates derivatization is desirable, particularly because of its importance as a medication and as an organocatalyst.
One-pot operations are effective methods for making several bonds and generating a complexity of molecules in a single-pot sequence29,30,31. Moreover, one-pot operations circumvent several purification steps via in situ quenching events, thereby minimizing chemical waste generation and saving time. Based on this, we proposed the concept of “pot economy.” We subsequently accomplished several total syntheses based on this concept32,33,34,35,36,37,38.
In this work, we describe the “pot-economical” enantioselective total synthesis of (–)-quinine using organocatalyst-mediated reaction.
Results
The retrosynthetic analysis of (–)-quinine
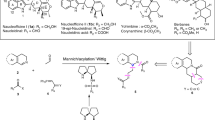
The retrosynthetic analysis of (–)-quinine is shown in Fig. 1. It would be synthesized by making a C6–N bond from a piperidine precursor 2 via an intramolecular SN2 reaction. 2 would be prepared by the addition reaction of a quinoline unit to piperdinecarbaldehyde 3. 3 would be synthesized from a substituted piperidine derivative 4 by functional group transformation. 4 would be prepared by a three-component coupling reaction of aldehyde 5, nitroalkene 6, and imine 7, as developed by our group39.
Earlier, we reported a one-pot synthesis of a piperidine skeleton via a sequential reaction of the diphenylprolinol silyl ether-mediated Michael reaction40, aza-Henry reaction, and hemiaminalization reaction (Fig. 2). As simple starting materials were used in the earlier study, we considered it now a challenge to determine whether the key reaction proceeds using densely functionalized substrates and whether high stereoselectivity is realized.
The first pot reaction
4-Arylthiobutanal 541 was selected as a starting aldehyde, taking into consideration of the required introduction of a C10–C11 double bond in a latter stage of the synthesis. The asymmetric Michael reaction of 5 and nitroalkene 6, catalyzed by diphenylprolinol silyl ether, proceeded to afford 8 in good yield and with excellent diastereo- and enantioselectivities (Fig. 3). However, the next aza-Henry reaction was found to be problematic. In our previous study, we used p-nitrophenylsulfonyl (Ns)42 imine generated from arylaldehyde (Fig. 2). The Ns imine of ethyl glyoxylate 7a is too unstable and difficult to handle (Fig. 4). Imine 7b derived from p-anisidine is too stable to react with 8. On the other hand, the corresponding N-Boc imine 7c possesses suitable stability and reactivity, but it is difficult to isolate it. Thus, it was generated in situ from the corresponding sulfonyl precursor 943 with DBU. A domino reaction of 8 and 9, involving the generation of the imine and the aza-Henry reaction, proceeded well to afford piperidine 4 and tetrahydropyridine 10 in 35% and 50% yield, respectively (Fig. 5). Although 4 was an expected product, 10 would be a more suitable intermediate for the total synthesis, because of the harsh reaction conditions that are usually necessary for the reductive removal of a NO2 group from 444,45,46. 4 can be converted to 10 by the treatment with DBU.
These reactions (5 + 6→10) can be conducted in a single pot (Fig. 6). The first pot reaction starts from the Michael reaction. The asymmetric Michael reaction of 5 and 6 proceeded to afford product 8. Upon the addition of imine precursor 9 and DBU to the same vessel, a domino reaction proceeded. That is, in situ imine generation occurred, and an aza-Henry reaction proceeded, sequentially, followed by hemiaminalization to afford 4. Elimination of HNO2 with DBU afforded 10 with a mixture of diastereomers at C2. Good overall yield, high diastereoselectivity (dr = 10:1 at C3), and excellent enantioselectivity (98% ee) were obtained. The reaction proceeded deca-gram scale efficiently. It should be noted that DBU plays three roles as a base in this transformation: (1) to generate imine (9→7c), (2) to promote the aza-Henry reaction, and (3) to eliminate HNO2.
Organocatalyst-mediated construction of the piperidine ring in one-pot. After two one-pot reaction and introduction of quinoline ring, sequential deprotection, mesylation and cyclization afforded (–)-quinine (1). Ac acetyl, DIBAL diisobutylaluminium hydride, LDBBA lithium diisobutyl-tert-butoxyaluminum hydride, Ms methansulfonyl, Pr propyl.
The second pot reaction
The second pot reaction starts with the reductive removal of a hydroxy group at C2 by the treatment of 10 with Et3SiH and CF3CO2H (TFA) at –20 °C39. Upon increasing the temperature of a reaction mixture to room temperature, the deprotection of a Boc group took place, to afford an enamine 12, which was tautomerized to afford an iminium ion 13. Upon the addition of NaBH(OAc)3 to the reaction vessel, stereoselective reduction occurred to afford 1417. Addition of CH3CHO led to decomposition of the remaining reducing reagent, and the excess CH3CHO and TFA were removed under reduced pressure. Thus, through this in situ quenching method30, a one-pot sequential reaction can be realized.
Further addition of Boc2O and Et3N to the reaction mixture afforded a highly substituted piperidine derivative 15 as a single isomer with the desired configuration. It should be noted that TFA plays three roles as an acid. It facilitates the formation of tetrahydropyridium from hemiaminal 10 at low temperature (–20 °C), to deprotect the Boc group at higher temperature, and to generate the iminium ion 13. It is synthetically efficient that the same reagent plays several roles in a one-pot reaction29. The reaction proceeded in a gram scale and the overall yield of this second pot (10→15) was 78%. In this transformation, procedures of deprotection and reprotection of Boc group were involved. The stereoselective reduction of Boc protected alkene 11 did not proceed well, while stereoselective reduction of the iminium ion 13 was successful. Thus, the deprotection step of Boc group is necessary.
We employed two reducing reagents such as Et3SiH and NaBH(OAc)3 for the reduction of hemiaminal (10→11) and iminium ion (13→14). It would be synthetically simple to use a single reducing reagent in a one-pot reaction. Although Et3SiH can act as a reducing reagent in both reactions, the second reaction was slow in the low yield with the partial deprotection of TBDPS group. In the case of NaBH(OAc)3 the reaction from 10 to 11 did not proceed well with the generation of several byproducts. On the other hand, the sequential use of two reducing reagents gave a good result in the second pot reaction.
The third pot reaction
The third pot involves the preparation of a C10–C11 alkene and half-reduction of an ester. When arylthiol ether 15 was treated with 16, according to Matsuo’s protocol, alkene 17 was generated47. In the same reaction vessel, the addition of DIBAL at low temperature (–95 °C) reduced an ester, and aldehyde 18 was obtained in good yield (77%) over two steps, in one pot. The conventional method of arylsulfanylethyl moiety into vinyl substituent is a two-step process: oxidation to sulfoxide and elimination of ArSOH. However, by applying Matsuo’s protocol, it becomes a one-step process. Moreover, the reaction conditions here are comparable to those of the one-pot sequential reaction of the next DIBAL reduction. This step is also scalable.
The fourth pot reaction
The next step is the introduction of a quinoline moiety to aldehyde 18—this was found to be very difficult. According to Ishikawa, several quinoline metals generated from 23 did not react with a similar aldehyde with formation of dimer of 23 because the C2 position of quinoline is electrophilic—thus, dihydroquinoline lithium reagent generated from 24 was developed as a nucleophile (Fig. 7)28. In order to synthesize C2’ derivatives of quinine and also to reduce the electrophilicity at the C2 of quinoline, we used 2,4-dibromo-6-methoxyquinoline (19) as a quinoline precursor. However, the selective halogen–metal exchange reaction of Br at C4 over Br at C2 is another concern. Pleasingly, the selective halogen–metal exchange of Br at C4 occurred selectively. But neither lithium nor magnesium species were added to aldehyde 18 (Table 1; entries 1, 2).
Then, the use of an additive was investigated. Whereas ZnCl248 did not afford the desired product (entry 3), a moderate yield was obtained in the presence of CeCl349,50 (entry 4). After numerous investigations, we achieved success with LaCl3·2LiCl51; the addition products were obtained in good yield (20: 41%, epi-20: 44%, entry 5). BuLi could be used for the first halogen–metal exchange reaction, but the yield was low (entry 7). The reaction also proceeded in a gram scale (entry 6). Although the undesired diastereomer epi-20 was obtained, epi-20 can be converted to the desired isomer 20 via oxidation with DMP (90%) and stereoselective reduction using LDBBA (lithium diisobutyl-tert-butoxyaluminum hydride)52,53, in which 20 was obtained in 85% with epi-20 (14%).
The fifth pot reaction
The last pot reaction begins with 20. Upon treatment of 20 with Zn and acetic acid, the C–Br bond was reduced to a C–H bond to afford 2123. Addition of AcCl and MeOH to the reaction mixture generated HCl, which then deprotected both the Boc group and the TBDPS group, to afford piperidine 22. Using MsCl and pyridine as a base, selective mesylation of a primary alcohol in the presence of a secondary alcohol and piperidine took place17, in which piperidine was protected as its HCl salt. Upon the addition of ammonia, the piperidine·HCl salt was converted to free piperidine 2, and excess ammonia was removed under reduced pressure. An intramolecular SN2 reaction proceeded in the presence of NaI and K2CO3 at 60 °C to afford (–)-quinine (1). The physical properties of the synthetic (–)-quinine (1) were identical in all respects to the reported data18.The yield of the last pot reaction (20→1) was 55%.
The order of the reaction steps and reaction conditions are essential for the success of the last one-pot operations. There are several other reaction conditions suitable for each reaction step; however, a combination of these steps in a single reaction vessel presents difficulties. For instance, Zn reduction of bromoquinoline did not proceed well after the formation of a quinuclidine skeleton. Reduction of bromoquinoline 20 proceeded well using NaBH4 and TMEDA in the presence of a catalytic amount of PdCl2(dppf)54, but subsequent deprotection of TBDPS and Boc did not proceed in a one-pot operation. The TBDPS group could be selectively deprotected without affecting the Boc group by the treatment of n-Bu4NF, but the next mesylation could not proceed in the presence of n-Bu4NF.
The derivatization of (–)-quinine
As Br(C2)-quinoline derivative 19 was employed as a nucleophile, derivatization of (–)-quinine at the C2′ position would be possible. Br(C2′)-(–)-quinine 2555 was synthesized from 20 by a method similar to that of our last pot reaction for the synthesis of (–)-quinine, except for the Zn reduction. Four reaction steps, i.e., the deprotection of both (1) TBDPS and (2) Boc groups, (3) mesylation of a primary alcohol, and (4) an intramolecular SN2 reaction, proceeded efficiently to afford 25 in good overall yield (77%) in a single vessel (Fig. 8). The introduction of alkyne, aryl, and heteroaryl moieties was successful, affording 26, 27, and 28, respectively in good yields, by the Sonogashira reaction and Suzuki reaction.
Discussion
In summary, an efficient, enantioselective, and pot-economical total synthesis of (–)-quinine has been accomplished. The present synthesis has several noteworthy features. (1) A highly functionalized chiral tetrahydropyridine can be synthesized in the first one-pot operation, which consists of a succession of five reaction steps, including a diphenylprolinol silyl ether-mediated asymmetric Michael reaction as developed in our group, a domino aza-Henry reaction/hemiacetal formation involving the in situ generation of imine, and elimination of HNO2. (2) Five reactions can be successfully conducted in the second one-pot operation: the reductive removal of a hydroxy group, deprotection of Boc, iminium ion generation, stereoselective reduction, and Boc protection, thus affording the trisubstituted piperidine as a single isomer with the correct configuration. (3) Five reactions can proceed efficiently by careful choice of the reaction order and the reaction conditions: the reductive removal of Br, deprotection of Boc and TBDPS groups, selective synthesis of a mono-Ms derivative, and an intramolecular SN2 reaction. (4) The use of 2,4-dibromo-6-methoxy quinoline for the introduction of a quinoline moiety makes feasible the derivatization at the C2′ position of (–)-quinine.
This synthesis requires a total of five separate one-pot operations and five purifications by column chromatography, plus two additional operations for the conversion of the undesired epi-20 to the desired 20. The total yield of (–)-quinine was 14%.
Methods
General information
All reactions were carried out under argon atmosphere and monitored by thin-layer chromatography using Merck 60 F254 precoated silica gel plates (0.25 mm thickness). Specific optical rotations were measured using a JASCO P-2200 polarimeter. FT-IR spectra were recorded on a JASCO FT/IR-4600HC1 spectrometer. 1H and 13C NMR spectra were recorded on an Agilent-400 MR (400 MHz for 1H NMR, 100 M Hz for 13C NMR) instrument. Data for 1H NMR are reported as chemical shift (δ ppm), integration multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, septet = sep, dd = doublet of doublets, ddd = doublet of doublet of doublets, ddt = doublet of doublet of triplets, dt = doublet of triplets, dq = doublet of quartets, m = multiplet, brs = broad singlet, brd = broad doublet, brt = broad triplet), coupling constant (Hz), Data for 13C NMR are reported as chemical shift. High resolution ESI-TOF mass spectra were measured by Themo Orbi-trap LTQ XL instrument. HPLC analysis was performed on a HITACHI Elite LaChrom Series HPLC, UV detection monitored at appropriate wavelength respectively, using CHIRALPACK® IB (0.46 cm × 25 cm). Melting point was measured using Yanaco MP-J3. Flash chromatography was performed using silica gel 60 N (spherical) or silica gel 60 N (spherical) NH2 of Kanto Chemical Co. Int., Tokyo, Japan. All reagents were purchased from commercial sources (Aldrich, FUJIFILM Wako chemicals, Kanto Chemical, TCI).
Data availability
Experimental procedures, characterizations, spectra are available in the Supplementary Information.
References
Delepine, M., Pelletier, J. & Caventou, J. J. Chem. Educ. 28, 454–461 (1951).
Achan, J. et al. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar. J. 10, 144–155 (2011).
Hentges, S. G. & Sharpless, K. B. Asymmetric induction in the reaction of osmium tetroxide with olefins. J. Am. Chem. Soc. 102, 4263–4265 (1980).
Jacobsen, E. N., Marko, I., Mungall, W. S., Schroeder, G. & Sharpless, K. B. Asymmetric dihydroxylation via ligand-accelerated catalysis. J. Am. Chem. Soc. 110, 1968–1970 (1988).
France, S., Guerin, D. J., Miller, S. J. & Lectka, T. Nucleophilic chiral amines as catalysts in asymmetric synthesis. Chem. Rev. 103, 2985–3012 (2003).
Tian, S.-K. et al. Asymmetric organic catalysis with modified cinchona alkaloid. Acc. Chem. Res. 37, 621–631 (2004).
Gaunt, M. J. & Johansson, C. C. C. Recent developments in the use of catalytic asymmetric ammonium enolates in chemical synthesis. Chem. Rev. 107, 5596–5605 (2007).
Ooi, T. & Maruoka, K. Recent advances in asymmetric phase-transfer catalysis. Angew. Chem., Int. Ed. 46, 4222–4266 (2007).
Song, C. E. Cinchona Alkaloids in Synthesis and Catalysis: Ligand, Immobilization and Organocatalysis (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2009).
Ishikawa, H. & Shiomi, S. The alkaloids: Chemistry and Biology (ed. Knölker, H.-J.) vol. 79, 1–70 (Academic Press, Cambridge, 2018).
Woodward, R. B. & Doering, W. E. The total synthesis of quinine. J. Am. Chem. Soc. 66, 849 (1944).
Woodward, R. B. & Doering, W. E. The total synthesis of quinine. J. Am. Chem. Soc. 67, 860–874 (1945).
Uskoković, M. R., Gutzwiller, J. & Henderson, T. Total synthesis of quinine and quinidine. I. J. Am. Chem. Soc. 92, 203–204 (1970).
Uskoković, M. R. & Gutzwiller, J. T. Total synthesis of quinine. II. J. Am. Chem. Soc. 92, 204–205 (1970).
Gates, M., Sugavanam, B. & Schreiber, W. L. Syntheses in the cinchona alkaloid series. J. Am. Chem. Soc. 92, 205–207 (1970).
Gutzwiller, J. T. & Uskoković, M. R. Total synthesis of cinchona alkaloids. 2. Stereoselective total syntheses of quinine and quinidine. J. Am. Chem. Soc. 100, 576–581 (1978).
Stork, G. et al. The first stereoselective total synthesis of quinine. J. Am. Chem. Soc. 123, 3239–3242 (2001).
Raheem, I. T., Goodman, S. N. & Jacobsen, E. N. Catalytic asymmetric total syntheses of quinine and quinidine. J. Am. Chem. Soc. 126, 706–707 (2004).
Igarashi, J., Katsukawa, M., Wang, Y.-G., Acharya, H. P. & Kobayashi, Y. Stereocontrolled synthesis of quinine and quinidine. Tetrahedron Lett. 45, 3783–3786 (2004).
Igarashi, J. & Kobayashi, Y. Improved synthesis of quinine alkaloids with the Teoc protective group. Tetrahedron Lett. 46, 6381–6384 (2005).
Webber, P. & Krische, M. J. Concise stereocontrolled formal synthesis of (±)-quinine and total synthesis of (±)−7- hydroxyquinine via merged Morita-Baylis-Hillman-Tsuji-Trost cyclization. J. Org. Chem. 73, 9379–9387 (2008).
Illa, O., Arshad, M., Ros, A., MacGarrigle, E. M. & Aggarwal, V. K. Practical and highly selective sulfur ylide mediated asymmetric epoxidations and aziridinations using an inexpensive, readily available chiral sulfide. Applications to the synthesis of quinine and quinidine. J. Am. Chem. Soc. 132, 1828–1830 (2010).
Arshad, M., Fernández, M. A., MacGarrigle, E. M. & Aggarwal, V. K. Synthesis of quinine and quinidine using sulfur ylide-mediated asymmetric epoxidation as a key step. Tetrahedron: Asymmetry 21, 1771–1776 (2010).
Sarkar, S. M. et al. Organocatalytic asymmetric synthesis of quinine and quinidine. Tetrahedron Lett. 52, 923–927 (2011).
OʼDonovan, D. H. et al. C-H activation enables a concise total synthesis of quinine and analogues with enhanced antimalarial activity. Angew. Chem. Int. Ed. 57, 10737–10741 (2018).
Lee, J. & Chen, D. Y.-K. A Local-desymmetrization-based divergent synthesis of quinine and quinidine. Angew. Chem. Int. Ed. 58, 488–493 (2019).
Jiang, Y., Deiana, L., Zhang, K., Lin, S. & Córdova, A. Total asymmetric synthesis of quinine, quinidine, and analogues via catalytic enantioselective cascade transformations. Eur. J. Org. Chem. 2019, 6016–6023 (2019).
Shiomi, S., Misaka, R., Kaneko, M. & Ishikawa, H. Enantioselective total synthesis of the unnatural enantiomer of quinine. Chem. Sci. 10, 9433–9437 (2019).
Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 7, 866–880 (2016).
Hayashi, Y. Time economy in total synthesis. J. Org. Chem. 86, 1–23 (2021).
Hayashi, Y. Time and pot economy in total synthesis. Acc. Chem. Res. 54, 1385–1398 (2021).
Ishikawa, H., Suzuki, T. & Hayashi, Y. High-yielding synthesis of the anti-influenza neuramidase inhibitor (–)-Oseltamivir by three “One-Pot” operations. Angew. Chem. Int. Ed. 48, 1304–1307 (2009).
Ishikawa, H., Honma, M. & Hayashi, Y. One-pot high-yielding synthesis of the DPP4-selective inhibitor ABT-341 by a four-component coupling mediated by a diphenylprolinol silyl ether. Angew. Chem. Int. Ed. 50, 2824–2827 (2011).
Hayashi, Y. & Umemiya, S. Pot economy in the synthesis of prostaglandin A1 and E1 methyl esters. Angew. Chem. Int. Ed. 52, 3450–3452 (2013).
Hayashi, Y. & Ogasawara, S. Time economical total synthesis of (–)-Oseltamivir. Org. Lett. 18, 3426–3429 (2016).
Hayashi, Y., Sakamoto, D. & Okamura, D. One-pot synthesis of (S)-baclofen via aldol condensation of acetaldehyde with diphenylprolinol silyl ether mediated asymmetric michael reaction as a key step. Org. Lett. 18, 4–7 (2016).
Hayashi, Y., Koshino, S., Ojima, K. & Kwon, E. Pot Economy in the Total Synthesis of Estradiol Methyl Ether by Using an Organocatalyst. Angew. Chem. Int. Ed. 56, 11812–11815 (2017).
Umekubo, N., Suga, Y. & Hayashi, Y. Pot and time economies in the total synthesis of Corey lactone. Chem. Sci. 11, 1205–1209 (2020).
Urushima, T., Sakamoto, D., Ishikawa, H. & Hayashi, Y. Enantio- and diastereoselective synthesis of piperidines by coupling of four components in a “One-Pot” sequence involving diphenylprolinol silyl ether mediated Michael reaction. Org. Lett. 12, 4588–4591 (2010).
Hayashi, Y., Gotoh, H., Hayashi, T. & Shoji, M. Diphenylprolinol silyl ethers as efficient organocatalysts for the asymmetric Michael reaction of aldehydes and nitroalkenes. Angew. Chem. Int. Ed. 44, 4212–4215 (2005).
Rakumitsu, K., Sakamoto, J. & Ishikawa, H. Total syntheses of (–)-Secologanin, (–)−5-carboxystrictosidine, and (–)-rubenine. Chem. Eur. J. 25, 8996–9000 (2019).
Kan, T. & Fukuyama, T. Ns strategies: a highly versatile synthetic method for amines. Chem. Commun. 353–359 (2004).
Nagano, T. & Kinoshita, H. A new and convenient method for the synthesis of dehydroamino acids starting from ethyl N-Boc- and N-Z-α-Tosylglycinates and various nitro compounds. Bull. Chem. Soc. Jpn. 73, 1605–1613 (2000).
Barton, D. H. R. & McCombie, S. W. A new method for the deoxygenation of secondary alcohols. J. Chem. Soc. Perkin Trans. 1. 1574–1585 (1975).
Ono, N., Miyake, H., Tamura, R. & Kaji, A. A new synthetic method: direct replacement of the nitro group by hydrogen or deuterium. Tetrahedron Lett. 22, 1705–1708 (1981).
Tanner, D. D., Blackburn, E. V. & Diaz, G. E. A free-radical chain reaction involving electron transfer. The replacement of the nitro group by hydrogen using trialkyltin hydride, a variation of the Kornblum reaction. J. Am. Chem. Soc. 103, 1557–1559 (1981).
Matsuo, J., Kozai, T. & Ishibashi, H. Mild preparation of alkenes from phenyl sulfides: one-pot elimination of phenylthio group via sulfilimine at ambient temperature. Org. Lett. 8, 6095–6098 (2006).
Hatano, M., Suzuki, S. & Ishihara, K. Highly chemoselective stoichiometric alkylation of ketones with grignard reagent derived zinc(II) ate complexes. Synlett. 2010, 321–324 (2010).
Imamoto, T., Takiyama, N., Nakamura, K., Hatajima, T. & Kamiya, Y. Reactions of carbonyl compounds with grignard reagents in the presence of cerium chloride. J. Am. Chem. Soc. 111, 4392–4398 (1989).
Takeda, N. & Imamoto, T. Use of cerium(III) chloride in the reactions of carbonyl compounds with organolithiums or Grignard reagents for the suppression of abnormal reactions:1-butyl-1,2,3,4-tetrahydro-1-naphthol. Org. Synth. 76, 228–238 (1998).
Krasovskiy, A., Kopp, F. & Knochel, P. Soluble lanthanide salts (LnCl3·2LiCl) for the improved addition of organomagnesium reagents to carbonyl compounds. Angew. Chem. Int. Ed. 45, 497–500 (2006).
Kim, M. S., Choi, Y. M. & An, D. K. Lithium diisobutyl-t-butoxyaluminum hydride, a new and efficient reducing agent for the conversion of esters to aldehydes. Tetrahedron Lett. 48, 5061–5064 (2007).
Liu, W. et al. Bioinspired synthesis of (+)-cinchonidine using cascade reactions. Angew. Chem. Int. Ed. 57, 12299–12302 (2018).
Chelucci, G. & Figus, S. NaBH4-TMEDA and a palladium catalyst as efficient regio- and chemoselective system for the hydrodehalogenation of halogenated heterocycles. J. Mol. Catal. A: Chem. 393, 191–209 (2014).
Wang, D. et al. A highly practical and convenient halogenation of fused heterocyclic N-oxides. Tetrahedron 72, 5762–5768 (2016).
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP19H05630 (Y.H.).
Author information
Authors and Affiliations
Contributions
T.T. performed the experiments. Y.H. conceived the concept and prepared the manuscript with feedback from T.T.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yong Qin, Shu-Yu Zhang and the other, anonymous, reviewer for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Terunuma, T., Hayashi, Y. Organocatalyst-mediated five-pot synthesis of (–)-quinine. Nat Commun 13, 7503 (2022). https://doi.org/10.1038/s41467-022-34916-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-34916-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.