Abstract
Olefin/paraffin separation is an important but challenging and energy-intensive process in petrochemical industry. The realization of carbons with size-exclusion capability is highly desirable but rarely reported. Herein, we report polydopamine-derived carbons (PDA-Cx, where x refers to the pyrolysis temperature) with tailorable sub-5 Å micropore orifices together with larger microvoids by one-step pyrolysis. The sub-5 Å micropore orifices centered at 4.1–4.3 Å in PDA-C800 and 3.7–4.0 Å in PDA-C900 allow the entry of olefins while entirely excluding their paraffin counterparts, performing a precise cut-off to discriminate olefin/paraffin with sub-angstrom discrepancy. The larger voids enable high C2H4 and C3H6 capacities of 2.25 and 1.98 mmol g−1 under ambient conditions, respectively. Breakthrough experiments confirm that a one-step adsorption-desorption process can obtain high-purity olefins. Inelastic neutron scattering further reveals the host–guest interaction of adsorbed C2H4 and C3H6 molecules in PDA-Cx. This study opens an avenue to exploit the sub-5 Å micropores in carbon and their desirable size-exclusion effect.
Similar content being viewed by others
Introduction
Light olefins, especially ethylene (C2H4) and propylene (C3H6), are crucial chemical feedstocks in petrochemical industries for the broad production of polyethylene, polypropylene, and highly value-added products1,2,3,4. The world demand for C2H4 and C3H6 in 2022 exceeds 210 and 140 million metric tons, respectively. Steam cracking is the major industrial approach for olefin production, in which the paraffins are inevitably entrained as the byproducts5,6. The separation of olefins from their paraffin counterparts is thus an essential process to achieve high-purity olefins7,8,9, and has been listed as one of the seven major chemical separations to change the world10. Currently, industrial separation of olefin/paraffin mainly relies on energy-intensive cryogenic distillation (number of trays >100, pressure >20 bar, temperature <258 K), which accounts for approximately 0.3% of the total global energy consumption11. Driven by the world’s energy footprint, adsorptive separation has become an attractive and reliable alternative due to its high efficiency, energy-saving, and flexible operation12,13,14.
The keystone for adsorptive separation is the development of advanced physisorbents with excellent separation performance and scalability15,16. Porous carbon materials have demonstrated great prospects and broad application in gas separation due to their rich porosity, excellent stability, and low cost17,18,19. However, regarding the olefin/paraffin separation, the conventional equilibrium separation based on favorable enthalpic interaction toward olefins or paraffins cannot confer carbon materials with desirable selectivity due to their similar physicochemical properties20. In principle, to maximize the separation factor, the ideal physisorbents should have narrower sub-nanometer micropores to match the guest molecule size, thus taking up smaller olefins while completely excluding larger paraffin counterparts by precise regulation of pore sizes or geometries15,21. However, the major barrier is the broadly distributed pore size of carbonaceous materials, ranging from sub-nanometer to micrometer scale, due to the random arrangement of carbonaceous nanodomains with uncontrollable defects22. Such broad pore size distributions (PSD) inevitably cause the co-adsorption of both olefins and paraffins, thus poor selectivity at a low uptake ratio23,24.
In recent years, research endeavors have been devoted to designing carbon materials with tailorable porosity, especially with favorable ultrahigh surface area and pore volume for gas adsorption and storage25,26, and only a few works reported carbons with suitable small micropores matching olefins over the slightly larger paraffin counterparts for selective separation. The carbon of C-CDMOF-2-700 derived from a metal-organic framework (MOF) was recently reported to enable the separation of C3H6 and C3H8 via a size-sieving effect27. Still, to our best knowledge, it is rather challenging to tailor the micropores (or micropore orifices) in carbon to a lower size range to distinguish C2H4 and C2H6 at sub-angstrom precision. Meanwhile, such small micropores in carbon can be readily tuned to recognize C3H6 over the C3H8 counterpart. Furthermore, the conventional single gas probe technique such as N2 at 77 K and Ar at 87 K, mainly detects the larger micropores beyond 5 Å28, thereby the contribution from small micropore below 5 Å in carbons can often be veiled and underestimated. As a complement, multiple gas probe molecules could provide a more comprehensive assessment29, with cautiously chosen groups of probe molecules. In addition, unlike crystalline materials with precise crystallographic data as an alternative, carbon materials with amorphous structures cannot generalize such an approach. The resolution of small micropores below 5 Å and their manipulation for important industrial separations is worthy of investigation and may open an avenue in the highly selective separation of olefin/paraffin at similar sizes on carbons.
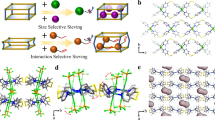
Herein, we move toward an insight into specific pore-engineering on small micropore orifices below 5 Å in carbons, which realizes precise molecular recognition of C2H4/C2H6 and C3H6/C3H8 based on a size-exclusion effect. The PDA-Cx carbons were synthesized by the self-assembling of dopamine (DA) followed by pyrolysis of assembled polydopamine (PDA) with π-electron-rich backbones stacked along the z axis30,31 (Fig. 1a). Being recognized as a non-porous solid by N2 adsorption at 77 K, PDA-Cx possesses a high surface area up to 400 m2 g−1 by CO2 adsorption at 195 K. Small sub-5 Å micropore orifices were resolved comprehensively by a series of gas probe molecules ranging from 3.3 to 5.0 Å at sub-angstrom size discrepancies. The PDA-C800 and PDA-C900 are sizing narrowly in the range of 4.1–4.3 Å and 3.7–4.0 Å, respectively. Such refined micropore orifices matched well with the pore size required for size-sieving separation of C2- and C3- olefin/paraffin pairs (Fig. 1b). Hence, PDA-Cx not only takes up high amounts of C2H4 (2.25 mmol g−1) or C3H6 (1.98 mmol g−1) at 298 K and 1.0 bar but also excludes C2H6 and C3H8 counterparts. Inelastic neutron scattering (INS) further examines host-guest interaction in carbons.
a Schematic illustration of the synthetic procedure. Dopamine undergoes self-assembly to prepare polydopamine firstly and subsequent pyrolysis to synthesize PDA-Cx with narrowly distributed sub-5 Å micropore orifices; b Schematic illustrations of a side view of the pore system. The small micropore orifices in PDA-C800 (4.1–4.3 Å) and PDA-C900 (3.7–4.0 Å) were probed by a series of angstrom-sized gas probes ranging from 3.3 to 5.0 Å; PDA-C800 admits C3H6 (4.0 Å) while rejects C3H8 (4.3 Å) completely and PDA-C900 admits C2H4 (3.7 Å) while rejects C2H6 (4.1 Å) completely; such refined small micropores enable the production of high-purity olefins (>97%) through a one-step adsorption-desorption process.
Results
Thermal-regulated structural evolution
The thermal stability of the polymer determines its successful transition to the carbonaceous structure during pyrolysis. TG analysis showed that even heated up to 900 °C for PDA polymer, 53.9% of the residues remained (Supplementary Fig. 1), much higher than many other polymer precursors and classical biomaterials7,32. Such excellent thermal stability contributes to the good preservation of sphere-shaped morphology (Supplementary Fig. 2). The homogeneous elemental distribution of C, O, and N were found throughout the PDA in the EDS mapping, indicating a remarkable heteroatom-doping structure (Supplementary Fig. 3). To track the structural change of heteroatom bonding nature during pyrolysis, X-ray photoelectron spectra (XPS) was utilized, which indicates the transformation from pyrrolic-N to pyridinic-N and thermally stable graphitic-N, existing at edges and defects sites (Supplementary Figs. 4–7 and Table 1). 13C NMR spectra validate the loss of original aliphatic CHx groups (x = 1–3) and functional groups (O–H/N–H) (Fig. 2a). A rather graphitic carbon phase formed, although the signal intensity of aromatic rings reduced due to the lack of hydrogen at the sites33, consistent with Fourier transform infrared (FTIR) spectroscopy (Supplementary Fig. 8). Raman spectroscopy showed higher intensity ratio of ID (A1g-symmetry, disorder-induced defects) to IG (E2g-symmetry, graphene layer edges) from PDA (0.87) to PDA-C700 (1.01), indicating the increased defects in carbon matrix (Supplementary Fig. 9)34. When the temperature was above 700°C, heat energy was provided to grow the graphene lattice, favoring a lower ID to IG ratio.
a 13C CP MAS NMR experiments recorded on PDA and PDA-Cx; b PXRD patterns of PDA and PDA-Cx. The dotted lines mark the (002) and (100) peaks; c The average pore size of PDA-Cx derived from the PALS. The dashed line is a linear data fit as a visual guide. The error bars represent the standard deviations based on the nonlinear least squares fitting process carried out using the LTv9 program.
To unveil the change of carbonaceous structure of PDA and PDA-Cx, Powder X-ray diffraction (PXRD) was performed (Fig. 2b). The broad peak of the (002) plane of the graphite phase right shifted from 21.0° of PDA to 22.6° of PDA-C900, revealing the decreased average inter-plane distance (d-spacing) from 4.2 to 3.9 Å based on Bragg’s equation35,36. The peak of the (100) line at 44.0° does not change position but becomes stronger, indicative of a modest increase in aromatic crystalline size. Positron annihilation lifetime spectroscopy (PALS) is a useful microprobe capable of direct determining the local void spaces and average pore size in a 3D network at the atomic scale37. The detailed positron lifetime components (τ1, τ2, τ3) and their corresponding intensities were listed in the supplementary Table 2. The second-component positron lifetime (τ2) was used to estimate the average pore size (Eq. 1), as nearly no o-Ps component (τ3) was detected in PALS due to the electronic conductivity of PDA-Cx38. PALS-detected average pore size increased from 4.68 Å of PDA-C300 to 5.25 Å of PDA-C900, revealing the formation of slightly larger angstrom-sized voids in carbon matrix induced by higher pyrolysis temperature (Fig. 2c).
Pore structure analysis via probe gases
The above PALS technique reflects the variation of average pore size of the accessible free-volume in porous frameworks39, whereas the probe gases can estimate the size of pore orifices accurately15. To further unveil the size distribution of pore orifices, N2 physisorption at 77 K was performed. Negligible N2 uptake (<0.1 mmol g−1) was detected in PDA-Cx except for PDA-C700 (Supplementary Fig. 10). However, by further applying probe gases of H2 and CO2, PDA-Cx exhibited remarkably higher H2 and CO2 uptakes at higher pyrolysis temperature, where PDA-C900 realized 5.28 mmol g−1 for H2 capture at 77 K and 1.0 bar, and 5.60 mmol g−1 for CO2 capture at 195 K and 1.0 bar (Supplementary Figs. 11–12). Above phenomena suggest that smaller sub-5 Å micropores are present in PDA-Cx as the orifice40,41, to limit N2 to diffuse at critical temperature to the larger voids (4.68-5.25 Å) detected from PALS spectra, but still accessible for the smaller H2 and CO2 probes, which is also supported by wide-angle X-ray scattering (Supplementary Fig. 13). Such a pore system confers PDA-C900 a high surface area up to 400 m2 g−1 analyzed by CO2 sorption at 195 K (Supplementary Fig. 14). Nonetheless, the existing theoretical models of PSD for CO2 are relatively insensitive to fit the complex domain structure of carbon42, as shown by the similar PSD results (Supplementary Fig. 12). Thus, the size distribution of small micropores below 5 Å requires further comprehensive evaluation. Additionally, it was noted that the CO2 adsorption of PDA-Cx at 273 K exhibited less capacity increase at low pressure compared with that at 195 K (Supplementary Figs. 12 and 15), revealing the weak host-guest interaction between CO2 molecules and non-polar carbon surfaces by Van de Waals forces. Inelastic neutron scattering (INS) in the later part can further confirm the weak physisorption. The adsorption is thus reduced to steric restrictions to probe the pore size by various gas molecules with the sub-angstrom size discrepancy as a simplified interpretation.
Specifically, accurate quantification of small micropore orifices in PDA-Cx was resolved by isotherm measurements at 273 K using a series of probing gases (CO2, Ar, C2H4, C3H6, C2H6, C3H8, CF4, and i-C4H10) with molecular dimensions increasing from 3.3 to 5.0 Å (Fig. 3, Supplementary Figs. 16–20 and Tables 3–6)43,44. The pore volume obtained from different probes was estimated by Dubinin-Astakhov (D-A) equation based on the pore-filling adsorption mechanism45. A function of the logarithm of adsorbed capacity against the square of potential energy was plotted (Eqs. 2 and 3, Supplementary Figs. 17–22), and the micropore volume can be directly calculated from the intercept (Eq. 4)46. Based on the CO2 probe (3.3 Å), the micropore volume of PDA-Cx increased gradually with increased pyrolysis temperature, which reached up to 0.21 cm3 g−1 on PDA-C900 (Fig. 3c). Moreover, the difference in micropore volume can be reduced to sterical restrictions in pores smaller than the size of probes, resulting in a high-resolution PSD below 5 Å (Eq. 5). Apart from PDA-C300 with low porosity, the small micropore orifice size of PDA-C500 was determined to be falling in-between 4.1 and 4.7 Å. Higher temperature contributes to the evolution of micropores towards smaller size driven by thermal energy. Noticeably, small pore sizes of PDA-C800 and PDA-C900 fell in-between 4.1–4.3 Å and 3.7–4.0 Å, respectively (Fig. 3d). Such narrow size distributions are even comparable with typical crystalline adsorbents such as zeolites and MOFs3,4,47,48, promising great potential for size-sieving separation of olefin/paraffin. In distinct contrast to the commercial carbons, the major porosities of AC-1 were broadly distributed in the micro-mesopore region ranging from 5.8 to 31.4 Å, while that of CMS-1 were more narrowly distributed in the micropore region of 5.8–15.4 Å, according to N2 physisorption at 77 K (Supplementary Fig. 23). Meanwhile, bare small micropores below 5 Å were probed in CMS-1. A small portion of small micropores but below 3.7 Å was probed in AC-1 (Supplementary Fig. 24). The above encouraging results reveal that the pre-organized precursor and thermal-controlled route could introduce narrowly distributed small micropores below 5 Å in carbons, which was conventionally underestimated due to their non-crystalline nature and absence of molecular-level comprehensive assessment techniques. Furthermore, from the mercury porosimetry and small-angle X-ray scattering, a very small amount of macropores (~1 μm) in PDA-Cx are detected, which can be related to the interparticle voids that barely contributes to the adsorption of angstrom-size gases (Supplementary Figs. 25–26).
Sorption equilibrium isotherms of various gas probes with minimum molecular dimensions ranging from 3.3 Å (CO2) to 5.0 Å (i-C4H10) at 273 K and N2 at 77 K on a PDA-C800 and b PDA-C900 materials; c The pore volumes of PDA-Cx calculated from different probe gases based on the Dubinin-Astakhov (D-A) equation (the data points from left to right were calculated from probing gases of CO2, Ar, C2H4, C3H6, C2H6, C3H8, CF4, and i-C4H10 for PDA-Cx. While C3H6, C3H8, and i-C4H10 with high polarizability were excluded for heteroatom-rich PDA-C300 and PDA-C500 due to stronger host-guest interaction); d The pore size distributions of PDA-Cx (the differential pore volume (∆V) of y-axis was obtained from probes with successive sizes. VAr calculated by Ar was subtracted from VCO2, VC2H4 from VAr, and so on).
Molecular recognition of olefin/paraffin
To evaluate the adsorptive separation performance of PDA-Cx, single-component gas (C2H4, C2H6, C3H6, and C3H8) sorption isotherms were collected and satisfactorily described by model-fitted approach (Supplementary Figs. 27–37). Non-porous PDA and low-temperature pyrolyzed PDA-C300 exhibited negligible capacity of both C2- and C3- olefin/paraffin pairs (<0.2 mmol g−1 at 1.0 bar and 298 K) (Supplementary Figs. 27–29). The pyrolysis of PDA at higher temperatures is responsible for developing accessible micropores for adsorption. Specifically, PDA-C500 and PDA-C700 showed co-adsorption of both C2 and C3 components due to the enlarged size of the micropore orifice (Supplementary Fig. 30–33). As pyrolysis temperature increased further, more refined small micropores started to form. Narrowly distributed pore orifices of 4.1–4.3 Å in PDA-C800 fell in between the minimal molecular dimension of C3H6 and C3H8, allowing the entry of C3H6 with a capacity of 1.98 mmol g−1 at 1.0 bar and 298 K, and exclusion of larger C3H8 molecules with separation factor (S’) reaching up to 36.7 (Fig. 4a). On the other hand, the S’ value of C2H4/C2H6 for PDA-C900 with narrower micropore orifice (3.7–4.0 Å) could reach 24.7, with a high C2H4 uptake of 2.25 mmol g−1 at 1.0 bar and 298 K, and negligible C2H6 uptake (Fig. 4b). The superior selectivity outperforms previously reported carbonaceous materials (Fig. 4c), as well as most zeolites and MOFs (Supplementary Fig. 38 and Tables 7–8). Conversely, AC-1 and CMS-1 with widely distributed pores exhibited a similar capacity for both C2H4/C2H6 and C3H6/C3H8, and a low S’ value of <1.1 (Supplementary Figs. 39–40). The mainly reported approach to enhance S’ value to 3.8 for C2H4/C2H6 separation and 3.5 for C3H6/C3H8 separation is by introducing the pi-complexation function of monovalent copper or silver. However, such an approach is hindered by higher heat of adsorption requiring more energy for desorption and the undesired polymerization of adsorbent in long-runs49,50. The above results suggest that the tailorable narrow micropores in carbon are highly desirable for the selective separation of C2/C3 olefin/paraffin pairs.
Single-component gas adsorption isotherms of a C3H6 and C3H8 on PDA-C800 and b C2H4 and C2H6 on PDA-C900 at varied temperatures. The separation factor (S’) was calculated by the uptake ratio of olefin/paraffin at 298 K and 1.0 bar; c Comparison of the number of olefins adsorbed (Q) and olefin/paraffin separation factor of PDA-Cx with state-of-the-art porous carbon adsorbents (PDA-Cx marked as pentagram). The details are given in Supplementary Tables 7-8. d Comparison of the experimental INS of solid C3H6 and C3H6 in PDA-C800 (difference between PDA-C800 and PDA-C800 with C3H6 adsorbed); e Comparison of the experimental INS of solid C2H4 and C2H4 in PDA-C900 (difference between PDA-C900 and PDA-C900 with C2H4 adsorbed). Discrete phonon modes are highlighted by orange color, and three prominent vibrational modes are highlighted by blue, green and yellow colors.
Inelastic neutron scattering (INS) measurements were performed to probe the interaction between C3H6 and C2H4 and the pore surface in the PDA-C800 and PDA-C900 substrate (Fig. 4d, e)51. By comparing the vibrational spectrum of solid C3H6 with the spectrum of PDA-C800 with C3H6 after the PDA-C800 spectrum is subtracted, we can see that below 140 cm−1, discrete phonon modes are apparent in the solid C3H6 spectrum (Fig. 4d). However, these modes are less prominent in the adsorbed C3H6 sample, indicating no solid C3H6 formed inside the PDA-C800 sample. In addition, three prominent vibrational modes are observed in the vibrational spectrum around 225, 425, and 580 cm−1. Only the mode at around 580 cm−1 is shifted compared to solid C3H6, and this mode is also slightly broadened. This would indicate weak physisorption of C3H6 on the porous carbon framework and is likely related to the torsion of the methyl group of the guest molecules and deformation of the =CH2 group. Perturbation of the mode (shift and broadening) is consistent with the C3H6 molecule lying flat on the carbon surface. The modest shift would indicate a weak interaction with the carbon surface hence supporting that the selectivity in PDA-C800 is primarily a size effect rather than active, strong physisorption. The split modes around 225 cm−1 are the methyl torsions and are consistent with the solid C3H6, where the splitting is due to intermolecular interactions. Given the weak interaction of C3H6 with the carbon framework, it is likely that the splitting is due to intermolecular interactions of neighboring C3H6 molecules in the pores. Similar observations can be made for C2H4 adsorbed in PDA-C900, where the absence of prominent discrete phonon modes indicates weak interaction between C2H4 molecules and the carbon substrate (Fig. 4e). The broad phonon band indicates no bulk, solid C2H4 formed. However, the simplicity of the vibrational spectrum of C2H4 limits further observations.
Dynamic separation of olefin/paraffin
Besides static adsorption, single-component diffusional kinetics is another important parameter related to practical application52,53. PDA-C900 reached 80% of the saturated C2H4 uptake and equilibrium within 27 and 85 min, respectively, with the calculated diffusion rate (D’ = D r−2) of 4.13 × 10−3 min−1 (Fig. 5a, Supplementary Fig. 41). Bare C2H6 adsorption (<0.01 mmol g−1) was found within the whole 100 min. Such molecular-recognition behavior is consistent with static isotherms. As expected, PDA-C800 reached 80% of the C3H6 saturated uptake and equilibrium within about 12 and 36 min, respectively, with a diffusion rate of 7.57 × 10−3 min−1. The C3H8 uptake is negligible in the process (<0.01 mmol g−1) (Fig. 5a). The moderate adsorption rate of olefins is beneficial for actual dynamic olefin/paraffin separation.
a Time-dependent gas uptake profiles of C3H6 on PDA-C800 and C2H4 on PDA-C900 at 308 K and 0.5 bar. The time required to reach 80% of the saturated olefin uptake and equilibrium is depicted as dashed lines. Experimental column breakthrough curves for the mixtures of b C3H6/C3H8 (50/50, v/v) on PDA-C800 and c C2H4/C2H6 (50/50, v/v) on PDA-C900 at 298 K with a constant flow rate of 1.5 mL/min; d Desorption curves following a column breakthrough experiment under 10 mL/min flow of He at 353 K with PDA-C800 and PDA-C900, and the corresponding cumulative purity of olefins. C and C0 are the concentrations of each gas at the outlet and inlet, respectively; e Cycling uptake tests for pure-component C3H6 on PDA-C800 and C2H4 on PDA-C900; f Heat of adsorption (Qst) of C3H6 in PDA-C800 and C2H4 in PDA-C900.
Breakthrough experiments were conducted to validate the feasibility of separating binary mixtures under dynamic conditions (Supplementary Figs. 42–47, Tables 9–10). The molecular-recognition carbons, including PDA-C800 and PDA-C900, were chosen as targeted materials for the systematic study. A clean and sharp sieving separation of the C3H6/C3H8 mixture was realized over PDA-C800 with a C3H6 breakthrough time of around 26 min (Fig. 5b). The calculated dynamic C3H6 uptake was 1.5 mmol g−1. As expected, a clean separation of the challenging C2H4/C2H6 mixture was observed by the fixed bed packed with PDA-C900. C2H6 eluted out at the beginning owing to complete exclusion, and smaller C2H4 was retained until around 30 min (Fig. 5c). The calculated dynamic uptakes of C2H4 reached 1.8 mmol g−1; Subsequent desorption of eluted gas by heating under helium flow yielded C2H4 and C3H6 purity larger than 97.2% and 98.1%, respectively, before the period of 60 min (Fig. 5d). In contrast, though AC-1 and CMS-1 provided a longer C2H4 or C3H6 retaining time, the similar breakthrough time led to a lower separation efficiency (Supplementary Figs. 43–46). Above results further highlight the importance of refined small micropores for highly efficient molecular recognition of gas mixture. Multiple adsorption-desorption cycles demonstrated the excellent stability of PDA-Cx (Fig. 5e). The easier renewability can be quantitatively demonstrated by the low isosteric heat of adsorption (Qst) of 32.3 and 41.5 kJ mol−1 for C2H4 in PDA-C900 and C3H6 in PDA-C800 at zero loading, respectively (Fig. 5f, and Supplementary Table 11).
Discussion
In conclusion, precise probing and manipulating narrowed small micropores in carbons unveiled the existing blind spot of pores below 5 Å and verified their efficiency in important industrial gas separations. PDA-Cx possesses refined sub-5 Å small micropore orifices together with 4.68–5.25 Å microvoids from thermal-controlled pyrolysis of polydopamine. Though undetectable by the standard N2 adsorption at 77 K, the small micropores below 5 Å were accurately probed and defined by a series of gas molecules ranging from 3.3 to 5.0 Å at sub-angstrom size discrepancies. Results show that the small micropore orifices of 3.7–4.0 Å in PDA-C900 and 4.1–4.3 Å in PDA-C800 fall in-between the molecule size of C2H4/C2H6 and C3H6/C3H8 pairs, respectively, allowing the entrance of olefins while entirely exclude larger paraffin counterparts. The larger voids allow high C2H4 and C3H6 uptakes of 2.25 and 1.98 mmol g−1 at 298 K and 1.0 bar, respectively. Binary breakthrough experiments have further demonstrated ideal molecular recognition behavior. We identified the weak physisorption of olefins in carbon through INS measurement, supporting the size-sieving effect rather than strong enthalpic interaction. This work provides important indications for designing and probing carbon-based materials with finely tunable small micropores below 5 Å that can also be extended to other challenging separations, as well as catalysis, energy storage, and conversion.
Methods
Synthesis of PDA-Cx
In a typical synthesis, 1.5 ml NH4OH, 180 ml deionized water (DI) and 80 ml C2H5OH were mixed thoroughly to form solution A. Then, 2 g dopamine hydrochloride was dissolved in 20 ml DI water as solution B. Next, solution B was added dropwise to solution A under continuous stirring and reacted for 30 h at room temperature. Next, the black polydopamine product (named PDA) was collected by vacuum filtration, followed by washing with DI water three times and drying at 60 °C overnight. Afterward, the PDA product was placed in a tube furnace, and carbonized at different temperatures (300–900 °C) for 1 h under nitrogen atmosphere with a heating rate of 5 °C min−1. The resulting products were PDA-Cx (where x stands for the final carbonization temperature).
Positron annihilation lifetime spectroscopy (PALS) experiment
PALS was performed to analyze the free-volume of the samples. The positron source (22Na) with an activity of 30 μCi was used and sandwiched between two same disk-shaped pieces. The sample-source-sample set was kept at room temperature in a vacuum chamber (vacuum better than 1 × 10−3 Pa) during the PALS measurements. One gamma detector is regarded as “start” to detect the 1.275 MeV gamma from the 22Na nucleus that is emitted simultaneously with the beta decay positron. Another gamma detector is “stop” for detecting one of the two subsequent 0.511 MeV annihilation photons. Electronics measure the “start” - “stop” time intervals between two γ rays, and then lots of annihilation events were recorded for a positron lifetime spectrum for eventual computer fitting. Each lifetime spectrum contains about 5 × 106 accumulated counts. The time resolution of the digital PALS spectrometer (TechnoAP, Japan) was 190 picosecond (ps) and the count rate was around 200 cps. The PALS spectra were analyzed using the LTv9 program.
In our work, the o-Ps intensity (I3, formation probability of o-Ps) in each PDA-Cx sample is lower than 1% (Supplementary Table 2). Thus, the average diameter (2 R) of free-volumes was estimated from τ2 based on an empirically linear equation, that exhibits a decent correlation coefficient of 0.9268 for τ2 -R data on polymers, zeolites, and molecular sieves with R < 5 Å38. The equation is formally written as:
Where, the units of τ2 and R are expressed in ns and Å, respectively.
Pore size distribution calculation
This part used various sorbates (CO2, Ar, C2H4, C2H6, C3H6, C3H8, CF4, i-C4H10) with different molecular dimensions probe molecules to determine the pore size distribution and pore volume54,55. The physical property of molecular probes was listed in Supplementary Table 3. Their isotherms were measured at 273 K volumetrically by using a Micromeritics 3Flex. The adsorption data were correlated by the Dubinin-Astakhov (D-A) equation, which modified the Dubinin-Radushkevich (D-R) equation. The D-R equation predicted adsorption isotherms on homogeneous pore surfaces, while the D-A equation evaluated the adsorption isotherms on micropore surfaces with some degree of heterogeneity56. D-A equation is formally written as57:
Here, W refers to the adsorbed capacity (mmol g−1), W0 is the saturation capacity (mmol g−1), β is termed the similarity affinity coefficient of the characteristic curves, E0 is the micropore characteristic energy of adsorption, and n is an empirical constant related to surface heterogeneity. If n = 2, the D-A equation reduces to the D-R form. A is the adsorption potential by Polanyi, which can be defined as:
P0 and P represent saturated vapor pressure and adsorption pressure (bar), respectively. T is the temperature (K), and R is the gas constant. Therefore, W0 can be calculated from the intercept of the line plot of ln W vs lnn (P0 P−1). Furthermore, W0 is defined as:
V0 is the micropore volume (cm3 g−1) and ρM is the liquid molar density (mmol cm−3). Thus, by combing Eqs. (2–4), a series of micropore volumes (V0) calculated from different gas probes can be obtained. After that, the V0 calculated from gas A was subtracted from V0 calculated from gas B. In this way, the differential pore volumes can be obtained, as shown below:
∆V is the pore volume with a size falling in-between the molecular size of gas A and gas B.
Measurement of statistic adsorption isotherms
The system is connected to a vacuum station that can generate a vacuum up to 10−7 Pa. N2 and H2 physisorptions at 77 K were performed on a Micromeritics ASAP 2020 with liquid nitrogen as a coolant. CO2 physisorption at 273 K and 195 K were performed on a Micromeritics ASAP 2020 with ice water and dry ice/isopropanol as coolants, respectively. Olefins and paraffin physisorption at different temperatures (273, 286, and 298 K) were determined using a Micromeritics 3Flex and ice water or a recycle water bath was used to control the temperature. Before the measurements, about 100 mg of the samples were degassed under a vacuum at 150 °C for at least 6 h.
Measurement of adsorption kinetic curves
Time-dependent adsorption measurements of C3H6, C3H8, C2H4, and C2H6 were performed using a TGA 55 thermogravimetric analyzer (TA Instruments, USA). Before the measurement, the sample was degassed at 423 K for 6 h under the N2 flow. After that, single-component gas of C2H4, C2H6, C3H6, or C3H8 flowed through the electrobalance system at 0.5 bar. The analyzer temperature was controlled at 308 K. Adsorption kinetic curves were done until the adsorption capacity reached saturation. Diffusional time constants (D’, D r−2) were calculated by the short-time solution of the diffusion equation58:
Where qt is the adsorbed capacity at time t, q∞ is the adsorbed capacity at equilibrium, D is the diffusivity and r is the radius of the equivalent spherical particle. The slopes of qt/q∞ versus t1/2 are derived from the fitting of the plots in the low gas uptake range, and then D’ can be calculated from the square of the slope multiplied by π/36.
Double-site Langmuir isotherms model
The adsorption isotherms data of olefins and paraffin with high capacity (>0.15 mmol g−1 at 1.0 bar) were fitted with the double-site Langmuir isotherms model. The model is shown as follows:
Where, Q is the adsorbed capacity (mmol g−1), bA and bB refer to the corresponding adsorption equilibrium constants reflecting the affinity coefficients of sites A and B, Pa-v, respectively. qA,sat and qB,sat are the saturation adsorbed capacity of sites A and B; P corresponds to an equilibrium pressure (bar). The fits are of good accuracy (R2 > 0.971).
Langmuir-Freundlich isotherms model
The adsorption isotherms data of olefins and paraffin with very low capacity (<0.15 mmol g−1 at 1.0 bar) were fitted with the Langmuir-Freundlich isotherms model. The model is shown as follows:
Where, Q is the adsorbed capacity (mmol g−1), bA refers to the corresponding adsorption equilibrium constants reflecting the affinity coefficients, Pa-v. qA,sat is the saturation adsorbed capacity; P corresponds to an equilibrium pressure (bar); n represents the deviation from the ideal homogeneous surface. The fits are of good accuracy (R2 > 0.991).
Inelastic neutron scattering (INS) measurements
INS data was collected on the VISION Beamline at Oak Ridge National Laboratory. Firstly, 1 g of each sample was activated by heating to 423 K for 2 h under a vacuum. Next, each sample was measured empty (absent of guest molecules) and subsequently dosed with 1 mmol (C3H6 for PDA-C800 and C2H4 for PDA-C900) at room temperature. After equilibrating the samples for 1 h, INS data were obtained for the samples in the presence of the guest molecules. Data collection for the empty and dosed samples took place at 5 K to reduce thermal effects. Finally, the data from the empty materials was subtracted from that obtained from the dosed samples to remove the porous carbon contribution and investigate the signal from the adsorbed gases.
Breakthrough experiments
The breakthrough experiments were performed by using a laboratory-built setup (Supplementary Fig. 47). The composition of the feed gas stream is as follows: C3H6/C3H8 (50/50, v/v) and C2H4/C2H6 (50/50, v/v). 950 mg of activated samples were packed into a stainless-steel column (4.6 mm inner diameter), and the gas flow rate was controlled at 1.5 ml min−1 by mass flow meter. The gas stream at the outlet gas was monitored by using gas chromatography (GC-9560) with a flame ionization detector (FID). Desorption data were collected under 10 mL min−1 flow of He at 353 K with adsorb saturated samples.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that all data supporting the conclusions of this work are available in the article and its Supplementary Information. Further source data will be made available at request from the corresponding authors.
References
Li, L. et al. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites. Science 362, 443–446 (2018).
Zeng, H. et al. Orthogonal-array dynamic molecular sieving of propylene/propane mixtures. Nature 595, 542–548 (2021).
Liang, B. et al. An ultramicroporous metal–organic framework for high sieving separation of propylene from propane. J. Am. Chem. Soc. 142, 17795–17801 (2020).
Lin, R.-B. et al. Molecular sieving of ethylene from ethane using a rigid metal-organic framework. Nat. Mater. 17, 1128–1133 (2018).
Bereciartua, P. J. et al. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 358, 1068–1071 (2017).
Wang, J. et al. Fine pore engineering in a series of isoreticular metal-organic frameworks for efficient C2H2/CO2 separation. Nat. Commun. 13, 200 (2022).
Du, S., Huang, J., Anjum, A. W., Xiao, J. & Li, Z. A novel mechanism of controlling ultramicropore size in carbons at sub-angstrom level for molecular sieving of propylene/propane mixtures. J. Mater. Chem. A 9, 23873–23881 (2021).
Wang, H. et al. Tailor-made microporous metal–organic frameworks for the full separation of propane from propylene through selective size exclusion. Adv. Mater. 30, 1805088 (2018).
Yang, Y. et al. Ethylene/ethane separation in a stable hydrogen-bonded organic framework through a gating mechanism. Nat. Chem. 13, 933–939 (2021).
Sholl, D. S. & Lively, R. P. Seven chemical separations to change the world. Nature 532, 435–437 (2016).
Ren, Y. et al. Membrane-based olefin/paraffin separations. Adv. Sci. 7, 2001398 (2020).
Zhang, P. et al. Ultramicroporous material based parallel and extended paraffin nano-trap for benchmark olefin purification. Nat. Commun. 13, 4928 (2022).
Wang, Q. et al. One-step removal of alkynes and propadiene from cracking gases using a multi-functional molecular separator. Nat. Commun. 13, 2955 (2022).
Li, L. et al. Discrimination of xylene isomers in a stacked coordination polymer. Science 377, 335–339 (2022).
Zhou, Y. et al. Self-assembled iron-containing mordenite monolith for carbon dioxide sieving. Science 373, 315–320 (2021).
Zhang, X.-W., Zhou, D.-D. & Zhang, J.-P. Tuning the gating energy barrier of metal-organic framework for molecular sieving. Chem 7, 1006–1019 (2021).
Walczak, R. et al. Template- and metal-free synthesis of nitrogen-rich nanoporous “Noble” carbon materials by direct pyrolysis of a preorganized hexaazatriphenylene precursor. Angew. Chem. Int. Ed. 57, 10765–10770 (2018).
Du, S. et al. Facile synthesis of ultramicroporous carbon adsorbents with ultra-high CH4 uptake by in situ ionic activation. AIChE J. 66, e16231 (2020).
Xu, S. et al. Beyond the selectivity-capacity trade-off: ultrathin carbon nanoplates with easily accessible ultramicropores for high-efficiency propylene/propane separation. Nano Lett. 22, 6615–6621 (2022).
Wang, H., Luo, D., Velasco, E., Yu, L. & Li, J. Separation of alkane and alkene mixtures by metal–organic frameworks. J. Mater. Chem. A 9, 20874–20896 (2021).
Shen, J., Liu, G., Han, Y. & Jin, W. Artificial channels for confined mass transport at the sub-nanometre scale. Nat. Rev. Mater. 6, 294–312 (2021).
Benzigar, M. R. et al. Recent advances in functionalized micro and mesoporous carbon materials: synthesis and applications. Chem. Soc. Rev. 47, 2680–2721 (2018).
Wang, Y. S. et al. Recent advances in carbon-based adsorbents for adsorptive separation of light hydrocarbons. Research 2022, 9780864 (2022).
Du, S. et al. Ultramicroporous carbons featuring sub-Ångstrom tunable apertures for the selective separation of light hydrocarbon. AIChE J. 67, e17285 (2021).
Blankenship, L. S., Balahmar, N. & Mokaya, R. Oxygen-rich microporous carbons with exceptional hydrogen storage capacity. Nat. Commun. 8, 1545 (2017).
Xu, F. et al. Facile synthesis of ultrahigh-surface-area hollow carbon nanospheres for enhanced adsorption and energy storage. Nat. Commun. 6, 7221 (2015).
Chen, F. et al. Molecular sieving of propylene from propane in metal–organic framework-derived ultramicroporous carbon adsorbents. ACS Appl. Mater. Interfaces 14, 30443–30453 (2022).
Shao, H., Wu, Y.-C., Lin, Z., Taberna, P.-L. & Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 49, 3005–3039 (2020).
Beda, A., Vaulot, C. & Matei Ghimbeu, C. Hard carbon porosity revealed by the adsorption of multiple gas probe molecules (N2, Ar, CO2, O2 and H2). J. Mater. Chem. A 9, 937–943 (2021).
Liu, R. et al. Dopamine as a carbon source: the controlled synthesis of hollow carbon spheres and yolk-structured carbon nanocomposites. Angew. Chem. Int. Ed. 50, 6799–6802 (2011).
Qu, K. et al. Polydopamine-inspired, dual heteroatom-doped carbon nanotubes for highly efficient overall water splitting. Adv. Energy Mater. 7, 1602068 (2017).
Zhang, W. et al. Direct pyrolysis of supermolecules: an ultrahigh edge-nitrogen doping strategy of carbon anodes for potassium-ion batteries. Adv. Mater. 32, 2000732 (2020).
Qiu, W., Leisen, J. E., Liu, Z., Quan, W. & Koros, W. J. Key features of polyimide-derived carbon molecular sieves. Angew. Chem. Int. Ed. 60, 22322–22331 (2021).
Mao, H. et al. Designing hierarchical nanoporous membranes for highly efficient gas adsorption and storage. Sci. Adv. 6, eabb0694 (2020).
Pope, C. G. X-ray diffraction and the Bragg equation. J. Chem. Educ. 74, 129 (1997).
Lei, L. et al. Carbon hollow fiber membranes for a molecular sieve with precise-cutoff ultramicropores for superior hydrogen separation. Nat. Commun. 12, 268 (2021).
Zhang, H. J. et al. Effect of free-volume holes on dynamic mechanical properties of epoxy resins for carbon-fiber-reinforced polymers. Macromolecules 50, 3933–3942 (2017).
Liao, K.-S. et al. Determination of free-volume properties in polymers without orthopositronium components in positron annihilation lifetime spectroscopy. Macromolecules 44, 6818–6826 (2011).
Shang, J. et al. Discriminative separation of gases by a “Molecular Trapdoor” mechanism in chabazite zeolites. J. Am. Chem. Soc. 134, 19246–19253 (2012).
Fitzer, E. & Schäfer, W. The effect of crosslinking on the formation of glasslike carbons from thermosetting resins. Carbon 8, 353–364 (1970).
Fitzer, E., Schaefer, W. & Yamada, S. The formation of glasslike carbon by pyrolysis of polyfurfuryl alcohol and phenolic resin. Carbon 7, 643–648 (1969).
Cornette, V. et al. Insensitivity in the pore size distribution of ultramicroporous carbon materials by CO2 adsorption. Carbon 168, 508–514 (2020).
Sing, K. S. W. & Williams, R. T. The use of molecular probes for the characterization of nanoporous adsorbents. Part Part Syst. Char. 21, 71–79 (2004).
Miura, K., Hayashi, J. & Hashimoto, K. Production of molecular sieving carbon through carbonization of coal modified by organic additives. Carbon 29, 653–660 (1991).
Stoeckli, H. F., Kraehenbuehl, F., Ballerini, L. & De Bernardini, S. Recent developments in the Dubinin equation. Carbon 27, 125–128 (1989).
Hu, Z., Maes, N. & Vansant, E. F. Molecular probe technique for the assessment of the carbon molecular sieve structure. J. Porous Mat. 2, 19–23 (1995).
Bao, Z. et al. Molecular sieving of ethane from ethylene through the molecular cross-section size differentiation in gallate-based metal–organic frameworks. Angew. Chem. Int. Ed. 57, 16020–16025 (2018).
Aguado, S., Bergeret, G., Daniel, C. & Farrusseng, D. Absolute molecular sieve separation of ethylene/ethane mixtures with silver zeolite A. J. Am. Chem. Soc. 134, 14635–14637 (2012).
Gao, F., Wang, Y., Wang, X. & Wang, S. Ethylene/ethane separation by CuCl/AC adsorbent prepared using CuCl2 as a precursor. Adsorption 22, 1013–1022 (2016).
Stephenson, A. et al. Efficient separation of propane and propene by a hypercrosslinked polymer doped with Ag (i). J. Mater. Chem. A 7, 25521–25525 (2019).
Armstrong, J., O’Malley, A. J., Ryder, M. R. & Butler, K. T. Understanding dynamic properties of materials using neutron spectroscopy and atomistic simulation. J. Phys. Commun. 4, 072001 (2020).
Yuan, Y.-F. et al. Wiggling mesopores kinetically amplify the adsorptive separation of propylene/propane. Angew. Chem. Int. Ed. 60, 19063–19067 (2021).
Du, S. et al. Application of thermal response measurements to investigate enhanced water adsorption kinetics in ball-milled C2N-type materials. ChemistryOpen 11, e202200193 (2022).
Dubinin, M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 60, 235–241 (1960).
Webster, C. E., Cottone, A. & Drago, R. S. Multiple equilibrium analysis description of adsorption on Na-mordenite and H-mordenite. J. Am. Chem. Soc. 121, 12127–12139 (1999).
Dobruskin, V. K. Micropore volume filling. A condensation approximation approach as a foundation to the Dubinin−Astakhov equation. Langmuir 14, 3840–3846 (1998).
Dubinin, M. M. & Stoeckli, H. F. Homogeneous and heterogeneous micropore structures in carbonaceous adsorbents. J. Colloid Interface Sci. 75, 34–42 (1980).
Lee, C. Y. et al. Kinetic separation of propene and propane in metal-organic frameworks: controlling diffusion rates in plate-shaped crystals via tuning of pore apertures and crystallite aspect ratios. J. Am. Chem. Soc. 133, 5228–5231 (2011).
Acknowledgements
Funding: J.X., S.-J.D., J.H., and C.Y. gratefully acknowledge the financial support from the National Natural Science Foundation of China (22022806). M.R.R. and S.D. acknowledge the U.S. Department of Energy (DOE) Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division (Separation Sciences) for research funding to support the neutron scattering experiments and data analysis. This research used resources at the Spallation Neutron Source (SNS), a DOE Office of Science User Facility operated by Oak Ridge National Laboratory.
Note This manuscript has been authored by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The US Government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Author information
Authors and Affiliations
Contributions
J.X. conceived the project. J.X., S.D., and B.C. co-supervised the project. S.-J. D. and J.H. designed the experiments. S.-J. D. conducted the breakthrough experiments, analyzed the data, and wrote the manuscript. J.H. prepared the samples and performed the initial experiments. M.R.R. and L.L.D. carried out the INS experiments and analyzed the results. H.Z. conducted the PALS experiments. P.Y. and Y.L. conducted the SAXS and WAXS experiments. C.Y. helped in drawing figures and adsorption data analyses. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Alexander Knebel, Zhiping Lai, and Radoslaw Zaleski for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Du, S., Huang, J., Ryder, M.R. et al. Probing sub-5 Ångstrom micropores in carbon for precise light olefin/paraffin separation. Nat Commun 14, 1197 (2023). https://doi.org/10.1038/s41467-023-36890-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-36890-6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.