Abstract
Developing artificial porous systems with high molecular recognition performance is critical but very challenging to achieve selective uptake of a particular component from a mixture of many similar species, regardless of the size and affinity of these competing species. A porous platform that integrates multiple recognition mechanisms working cooperatively for highly efficient guest identification is desired. Here, we designed a flexible porous coordination polymer (PCP) and realised a corrugated channel system that cooperatively responds to only target gas molecules by taking advantage of its stereochemical shape, location of binding sites, and structural softness. The binding sites and structural deformation act synergistically, exhibiting exclusive discrimination gating (EDG) effect for selective gate-opening adsorption of CO2 over nine similar gas molecules, including N2, CH4, CO, O2, H2, Ar, C2H6, and even higher-affinity gases such as C2H2 and C2H4. Combining in-situ crystallographic experiments with theoretical studies, it is clear that this unparalleled ability to decipher the CO2 molecule is achieved through the coordination of framework dynamics, guest diffusion, and interaction energetics. Furthermore, the gas co-adsorption and breakthrough separation performance render the obtained PCP an efficient adsorbent for CO2 capture from various gas mixtures.
Similar content being viewed by others
Introduction
Molecular recognition, one of the essential processes in chemical and biological systems, is the specific interaction between multicomponent molecular mixtures through non-covalent bonds for guest identification and selective binding1,2. Natural biological hosts (e.g., proteins and enzymes) typically perform the most efficient molecular recognition, which relies on their conformational dynamical complimentary dimensions with abundant binding interactions3. Integrating molecular recognition regimes into porous materials can help create materials with exceptional separation, selective transportation, catalysis, and sensing properties4,5,6. Although significant progress has been made, achieving the selective binding with only a target guest from multicomponent mixtures in artificial porous materials, regardless of the sizes and affinities of other competing species, is still particularly challenging. To develop such an intelligent molecular recognition mechanism, porous structures utilising sophisticated supramolecular interactions should be actively explored7,8,9.
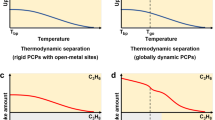
Porous coordination polymers (PCPs) or metal-organic frameworks (MOFs) are highly designable porous materials for constructing efficient recognisers10,11,12,13,14,15,16,17. Similar to zeolite and mesoporous silica, rigid PCPs can exhibit size-exclusion or diffusion-limited effects by tuning pore size and shape to sort different-sized molecules (Fig. 1a)18,19,20,21,22. The spatial organisation of interacting sites within PCPs contributes to their recognition properties by boosting target guests’ binding affinities (Fig. 1b)6,23,24,25,26,27,28. However, these PCPs show unsatisfactory selectivity for multicomponent mixtures of guests, which are smaller than the pore apertures or those with similar affinities. Recently, flexible PCPs exhibiting guest-triggered reversible structural transformations have attracted significant attention29,30,31,32,33. This structural transformation, called ‘gate-opening behaviour,’ endows soft PCPs with specific molecular discrimination capability34,35,36,37. The subtle energy balance among the thermodynamic interactions involving guest molecules and the framework deformation energy can explain this selective gate-opening mechanism (Fig. 1c)38,39,40,41. However, it is also challenging to selectively uptake guests with a weaker affinity to the soft PCP over ones with a higher affinity. Furthermore, the co-adsorption of undesired guests in flexible PCPs after gate opening is also problematic42.
a Rigid PCPs can exhibit size-exclusion or diffusion-limited effect for guest recognition. d2 is the size of the channel, which is between the size of the target gases (d1) and the size of the rest (d3). b Functional interacting sites within PCPs for guest recognition. The incorporated binding sites show a more robust interaction with the target gases than the rest. c In a soft PCP system, guest adsorption accompanied by structural transformations from closed phase (CP) to open phase (OP) occurs only when the interaction energy surpasses the structural deformation energy between the PCP and gas molecules. d PCPs with exclusive discrimination gating (EDG), which can efficiently utilise all the above mechanisms synergistically, exhibit precise molecular recognition performance (flexible framework with restricted narrow-corrugated channel structure is a suitable candidate).
We propose that synergistically utilising all these available recognition mechanisms through manipulating the energetic and stereochemical features of PCPs may bring unprecedented permeable recognition performance, for example, the specific selective adsorption of gases with moderate affinity from a mixture of components with similar structure and properties. In reality, such recognition property is highly desired in separation systems for mixtures, specifically for capturing only the target impurity molecules from a multicomponent mixture through a single energy-efficient adsorption step. Herein, we demonstrate that a soft PCP featuring an adaptable, narrow-corrugated channel is a versatile platform (Fig. 1d) for intelligent molecular recognition through the exclusive discrimination gating (EDG) effect. As a ‘smart gate,’ the dynamic bottleneck aperture in the corrugated channel can adjust its size and shape to regulate guest diffusion. Simultaneously, it can also thermodynamically distinguish the target gas from other competing gases with fewer host-guest interactions. Additionally, the interacting sites on the cavity surface in a corrugated channel can provide further specific interactions with a target molecule, synergistically improving recognition capability. In this research, a flexible 2-D interdigitated framework with relatively strong metal-ligand coordination bonds was designed to provide suitable pore geometry and structural softness for selective CO2 adsorption. CO2 is an essential greenhouse gas and a significant impurity in a mixture of various industrial gases43,44. Benefiting from the collaboration of the binding sites and soft corrugated-channel structures, this PCP exhibits a unique exclusive discrimination gating (EDG) effect for CO2 over the competing nine similar gaseous molecules, including N2, CH4, CO, O2, H2, Ar, C2H6, and even gases with similar molecular size but higher affinity, such as C2H4 and C2H2 (Supplementary Table 1). Such novel molecular recognition capability from the proposed structural design strategy yields porous materials with high performance in challenging recognition and separation systems.
Results and discussion
Crystal structures and gases sorption selectivity
Pink-coloured single crystals of [Co(3,5-pdc)dpg]n (PCP-3,5-pdc; 3,5-pdc= 3,5-pyridinedicarboxylic acid; dpg=meso-α,β-di(4-pyridyl) glycol) were synthesised via the solvothermal reaction of Co(NO3)2·6H2O, dpg, and 3,5-pdc in mixed DMF/MeOH solutions (Supplementary Figs. 1 and 2). Single-crystal X-ray diffraction (SXRD) analysis revealed that as-synthesised PCP-3,5-pdc crystallised in the monoclinic I2/a space group (Supplementary Table 2). In the as-synthesised structure phase α, the Co centre was a complex with six coordinates exhibiting an octahedral geometry. Each Co(II) ion coordinates with three dpg ligands and three 3,5-pdc ligands to form a 3-D network (Fig. 2a and Supplementary Fig. 3). Activation of the as-synthesised samples at 120 °C under vacuum led to a change in the PXRD pattern, indicating the formation of guest-free PCP-3,5-pdc (phase β, Fig. 2b). Thermogravimetric analysis (TGA) demonstrated that the guest molecules were removed entirely and that the coordination framework was thermally stable up to ca. 270 °C (Supplementary Fig. 4). SXRD analysis of activated PCP-3,5-pdc revealed that the coordination environment of the Co(II) centre and global framework underwent a drastic recasting and distortion process (Supplementary Table 3). During the activation process, the original coordination bonds between the Co centre and N of the 3,5-pdc ligand and the O atom of the hydroxyl group of the dpg ligand were broken. Meanwhile, coordination bonds between the Co centre and the original uncoordinated O atom from the carboxylic group of the 3,5-pdc ligand were formed (Supplementary Fig. 5). Such rearrangement of the coordination bonding geometry enables the structural transformation of the framework from a 3-D network to a 2-D system (Fig. 2b). In phase β, each Co(II) ion is in a distorted octahedral geometry coordinated by two dpg ligands at the axial positions, two oxygen atoms from the chelating carboxylate end of the 3,5-pdc ligand, and two oxygen atoms from 3,5-pdc in the equatorial plane (Supplementary Fig. 3). Co(II) and 3,5-pdc give rise to 1-D double-chain structures along the axis, and further linkages of these chains via dpg ligands in the axial positions generate a dense interdigitated 2-D layer structure (Supplementary Fig. 6). The purity of phases α and β were confirmed by comparing the simulated and experimental synchrotron powder X-ray diffraction (PXRD) patterns (Supplementary Fig. 7). Coordination polymers with interdigitated (CID) 2-D sheet systems have long been studied as good motifs for dynamic structures31,45. The constituent sublayer networks may dislocate their mutual positions upon specific gas adsorption, thereby increasing the adequate pore size. Therefore, the structural nature of activated PCP-3,5-pdc may result in exciting gas adsorption and recognition behaviour.
a Overall framework of the as-synthesised 3-D network structure of PCP-3,5-pdc (phase α). b Overall framework of the activated 2-D layer structure of PCP-3,5-pdc (phase β). c CO2 adsorption induced structural transformation of PCP-3,5-pdc. The accessible 1-D channels in PCP-3,5-pdc (phase γ) are highlighted in yellow (Probe radius: 1.2 Å). Purple, red, blue, and grey in the PCP frameworks represent Co, O, N and C, respectively. The hydrogen atoms and guest molecules are omitted for clarity. d Adsorption isotherms of N2, CO2, CH4, CO, O2, H2, Ar, C2H2, C2H4, and C2H6 at low temperatures. The solid and open circles in red represent the adsorption and desorption of CO2, respectively. e In-situ synchrotron PXRD patterns of PCP-3,5-pdc accompanying CO2 adsorption at 195 K. f A view of the 1-D narrow-corrugated channel in phase γ structure of PCP-3,5-pdc.
To investigate the adsorption properties of the presented PCP, molecular probes of 10 small molecular gases, including N2, CH4, CO, CO2, O2, H2, Ar, C2H2, C2H4, and C2H6 were utilised. Unprecedently, the prepared PCP exclusively exhibited CO2 recognition. As shown in Fig. 2d, e, the uptake below the gate-opening pressure is attributed to the inclusion of CO2 in the intrinsic microporous cavities of phase β (Supplementary Table 4 and Supplementary Fig. 8). Then, the gate-opening behaviour was observed at the pressure of P/P0 = 0.05 at 195 K, leading to final maximum adsorption of 90 mL·g−1 at 1 bar (corresponding to 1.75 molecules per Co2+). The Hill coefficient (n), which is a measure of the degree of adsorption cooperativity for the gate-opening step in the CO2 adsorption isotherm at 195 K, was determined to be 4.1 (>1) (Supplementary Fig. 9), confirming the positive, cooperative adsorption phenomenon. Conversely, negligible adsorption of N2, CH4, CO, O2, H2, Ar, C2H2, C2H4, and C2H6 was observed at low temperatures, indicating that these nine gases could not unlock the open-phase structure of PCP-3,5-pdc. Notably, distinguishing between C2H2 and CO2 is one of the most challenging tasks due to the similarities in their boiling points (194.7 K for CO2 and 189.3 K for C2H2) and kinetic diameters (3.3 Å for both molecules)46. Additionally, the higher quadrupole moment of C2H2 (7.2 × 10−26 esu cm2) compared to that of CO2 (0.65 × 10−26 esu cm2) often results in more vital electrostatic interaction of C2H2 with the adsorbent, causing preferential adsorption of C2H2 over CO247. This inverse selectivity of CO2 over C2H2 harvested in this PCP is relatively rare. Isobar adsorption measurements further confirmed that the other nine gases, except CO2, did not induce the gate-opening behaviour of PCP-3,5-pdc (Supplementary Figs. 10 and 11).
Even under high-pressure conditions (Supplementary Fig. 12), PCP-3,5-pdc adsorbed CO2 selectively. As illustrated in Supplementary Fig. 13, the compound offered a two-step adsorption isotherm for CO2 at 25 bar and 298 K. The uptake in the first step (20 mL·g−1) is attributed to the inclusion of CO2 into the microporous cavities of PCP-3,5-pdc (phase β). The second step of CO2 uptake exhibits a characteristic sigmoidal hysteretic adsorption, which suggests gate opening in PCP-3,5-pdc, leading to a final maximum uptake of ca. 55 mL·g−1. The positive CO2 adsorption cooperativity in the structural transformation step was even stronger (Hill coefficient n = 6.6, Supplementary Fig. 14). The difference in cooperativity at different temperatures may be due to the varying diffusion and stabilising abilities of the CO2 molecules. At the same time, no apparent gate-opening type adsorption was observed for N2, CH4, CO, O2, H2, Ar, C2H4 and C2H6 at 298 K. Notably, the slopes of the C2H4 and CO2 adsorption isotherms before the gate-opening pressure (Supplementary Fig. 15) indicated that PCP-3,5-pdc (phase β) exhibits stronger C2H4-framework interactions than CO2 before the transformation of the framework structure. Even so, the lack of further gate-open adsorption indicates that C2H4 cannot provide the open structure of PCP-3,5-pdc. These gas adsorption results demonstrate the unprecedented CO2 molecular recognition capability of PCP-3,5-pdc.
High-pressure mixture gas co-adsorption (Supplementary Fig. 16) and breakthrough separation (Supplementary Fig. 17) tests were conducted at room temperature to evaluate further the CO2 recognition performance of PCP-3,5-pdc in mixed-gas systems. As shown in Fig. 3a–c, the mixed-gas adsorption of CO2/N2, CO2/CH4, and CO2/C2H4 (50.0/50.0 v/v) at 298 K was measured up to a total pressure of 20 bar for PCP-3,5-pdc. The volume of N2, CH4, and C2H4 adsorbed in the binary adsorption mixtures was 0.5, 2.8 and 3.3 mL·g−1, respectively. Still, the amount of CO2 adsorbed was similar to that observed in the single-component experiments, indicating that PCP-3,5-pdc maintained high selectivity for CO2 in gas mixtures. Notably, unlike N2 and CH4, C2H4 adsorption shows a higher uptake than CO2 in the low-pressure region (single gas pressure < 1 bar), indicating that PCP-3,5-pdc shows stronger interactions with C2H4 than CO2 in the initial state (Supplementary Fig. 18). Even so, only CO2 adsorption can further induce the gate opening of PCP-3,5-pdc and can be selectively adsorbed into the open framework at high pressure. These results suggest that the adsorption of CO2 in the open framework of PCP-3,5-pdc is strong enough to block the pore entry, preventing effective guest exchange in the pore network and resulting in high adsorption selectivity. The separation factors (S) calculated from the co-adsorption isotherms were up to 116.6, 16.0, and 10.9 CO2/N2, CO2/CH4, and CO2/C2H4, respectively (Fig. 3d), suggesting promising potential of PCP-3,5-pdc for trapping CO2 under dynamic conditions. To verify this hypothesis, a mixture of N2/CO2, CH4/CO2 or C2H4/CO2 (50:50, v/v) at a flow rate of 6 mL/min was passed through a fixed-bed column filled with activated PCP-3,5-pdc at room temperature. Remarkable CO2 capture performance was achieved for the N2/CO2 and CH4/CO2 mixtures (Supplementary Figs. 19 and 20). Pure N2 or CH4 gas was first eluted from the separation bed, whereas CO2 was captured as an impurity in a packed column until its saturated uptake. The separation performance of a CO2/C2H4 binary mixture in PCP-35-pdc was relatively weaker (Supplementary Fig. 21). Unlike N2 and CH4, C2H4 adsorption shows a higher uptake than CO2 before pore opening at room temperature, indicating that PCP-3,5-pdc shows stronger interactions with C2H4 than CO2 in the initial state (Fig. 3c). This observation is consistent with the calculated binding energies of these gas molecules at different pore-opening states for this PCP. The preferential adsorption of C2H4 in the closed phase could potentially affect the separation performance of the CO2/C2H4 mixture due to the insufficient adsorption equilibrium under dynamic conditions, in contrast to co-adsorption experiments.
a Volumetric high-pressure co-adsorption equilibria of a binary mixture N2/CO2 (50.0/50.0 v/v) at 298 K of PCP-3,5-pdc. b Volumetric high-pressure co-adsorption equilibria of a binary mixture CH4/CO2 (50.0/50.0 v/v) at 298 K of PCP-3,5-pdc. c Volumetric high-pressure co-adsorption equilibria of a binary mixture C2H4/CO2 (50.0/50.0 v/v) at 298 K of PCP-3,5-pdc. d Separation factors (S) calculated from the co-adsorption isotherms for CO2/N2, CO2/CH4, and CO2/C2H4 (50.0/50.0 v/v) gas mixtures.
An ideal adsorbent for practical applications should allow for recyclability and energy-efficient regeneration. Therefore, we performed cycling breakthrough experiments for N2/CO2 separation under the same conditions. Between each cycle, the PCPs were regenerated under an in-situ vacuum without heating. The results showed that both PCPs maintained the same retention time (Supplementary Fig. 22). Moreover, after the high-pressure adsorption tests, PCP-3,5-pdc retained its original crystal structure (Supplementary Fig. 23). PCP-3,5-pdc exhibits excellent stability making it conducive for practical applications, especially under high-pressure separation conditions, such as precombustion CO2 capture (20–30 bar) and natural gas processing (50 bar)27,43. To test the humidity stability, we exposed PCP-3,5-pdc to 75% relative humidity at room temperature for seven days. This PCP retained its CO2 adsorption capacity (Supplementary Fig. 24). In addition, the impact of humidity on the separation performance was investigated by comparing the CO2/N2 breakthrough curves of the sample exposed to humid air for more than one week, both before and after thermal activation (Supplementary Fig. 25). The results indicate that while the CO2/N2 separation ability was sustained, its performance degraded due to humidity. However, since the structure remained stable even under humid conditions, the separation performance was not affected after activation. The water moisture resistance performance of PCP may originate from the lack of strong hydrophilic sites, as suggested by the water vapour adsorption isotherm (Supplementary Fig. 26).
Gas-loaded crystal structure
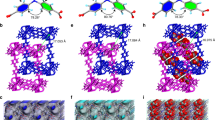
To monitor the structural transformation of PCP-3,5-pdc, we conducted in-situ synchrotron PXRD measurements during CO2 adsorption at 195 K. As shown in Fig. 2e, guest-free PCP-3,5-pdc starts to transform into a new phase structure (phase γ) at a CO2 pressure of P/P0 = 0.05. This structural change corresponds to the sudden opening of the pores of the framework from a dense structure (phase β) to a wide-pore structure (phase γ) during CO2 uptake (Fig. 2c). For comparison, in-situ synchrotron PXRD, accompanied by C2H2 sorption at 195 K, was also recorded (Supplementary Fig. 27). As expected, PCP-3,5-pdc retains its dense phase structure, suggesting no structural response to C2H2 adsorption. C2H2 has a similar kinetic diameter to that of CO2. Still, the adsorption behaviour of C2H2 differs from that of CO2, suggesting that some other factors lead to the difference between CO2 and C2H2, as discussed below. The crystal structure of phase γ was successfully determined by an ab initio charge-flipping method, and subsequent structural refinement was performed using Rietveld analyses of in-situ synchrotron PXRD data (Supplementary Fig. 28 and Supplementary Table 5). As shown in Fig. 2c, the open-phase structure is formed by the distortion and displacement of the original densely packed 2-D interdigitated sublayers in response to CO2 uptake, providing accessible 1-D narrow-corrugated channels (Fig. 2c,f). These infinite undulating channels in phase γ structure feature relatively large cavities (7.2 × 3.8 Å) connected by ultra-small windows (3.8 × 3.3 Å). The narrow-sized window perfectly matches the dimensions of CO2 for diffusion in the channel, potentially the key mechanism for CO2 adsorption selectivity. The in-situ synchrotron PXRD data during CO2 desorption at 195 K further indicates that the dense-to-open phase transformation is fully reversible (Supplementary Fig. 29). Additional in-situ PXRD measurements during CO2 adsorption at 298 K up to 10 bar further verified the corresponding elastic structure of PCP-3,5-pdc upon CO2 uptake at high pressure (Supplementary Fig. 30).
Visualisation of the binding positions of CO2 was also achieved through the Rietveld refinement of CO2-loaded crystals. In the refined crystal structure, the adsorbed CO2 molecules assemble into 1-D chains along the channel direction (Fig. 4a). These chains were stabilised by gas-gas interactions between CO2 molecules from two types of binding sites in a T-shaped geometry [C(δ+)…O(δ−) = 4.366 Å]. At site I, CO2 molecules diffused through the narrow windows. They were adsorbed in relatively large cavities, sandwiched by two pyrazine units of the 3,5-pdc ligand (Fig. 4b). At site II, CO2 molecules were adsorbed parallel to the narrow windows, sandwiched by the two pyrazine units of the dpg ligand. Such narrow window sizes can provide an unavoidable steric hindrance for the diffusion of other gases with larger sizes, leading to a high selectivity for CO2 adsorption. The presence of the negatively charged N atom from the 3,5-pdc ligand allows additional interactions with CO2 [CO2-I, C(δ+)…N(δ−) = 3.441 Å; CO2-II, C(δ+)…N(δ−) = 3.584 Å, Fig. 4b], providing an electrostatic CO2-framework interaction for recognition.
a The CO2 adsorption structure in PCP-3.5-pdc, showing two types of adsorption sites (CO2-I is highlighted in blue and CO2-II in orange). The CO2-I is shown as disordered, with occupancies of 50%. b CO2-framework interactions. Purple, red, blue, and grey in the PCP framework represent Co, O, N and C, respectively. The hydrogen atoms of molecules are omitted for clarity. The black dashed line represents interactions, and the value along the line represents the distance (in Å). c DFT-calculated energy diagram for CO2 adsorption into PCP-3,5-pdc, where CO2-I is highlighted in blue, CO2-IIA in orange and CO2-IIB in green. Energies are given in kcal mol−1.
Flexible framework with a narrow-corrugated channel
To further verify the design strategy for a ‘flexible framework with a narrow-corrugated channel’ to enhance guest selectivity, we conducted theoretical calculations of CO2, N2, CH4, C2H2, and C2H4 adsorptions to PCP-3,5-pdc. The adsorption structures were obtained by classical Monte Carlo simulation48 followed by the geometry optimisation using the periodic density functional theory (DFT) calculation with the PBE-D3 functional49. The calculation finds the CO2 molecules at site I in the broader cavity and site II in the narrow window, similar to those determined by the experiment (Supplementary Fig. 31). In site II, there exist two possible adsorption positions, named IIA and IIB. The binding energy (BE) for CO2 adsorption at these sites decreases in the order of site I (−8.6 kcal mol−1) > site IIA (−2.4 kcal mol−1) > site IIB (−1.0 kcal mol−1), as shown in Supplementary Table 6, where the negative value of BE means adsorption is exothermic. This result suggests that CO2 molecules occupy site I first. Because the experimentally observed adsorption amount of CO2 was approximately 3.5 molecules per unit cell of PCP-3,5-pdc, which corresponds to 1.75 molecules per Co2+, we investigated the binding energies for the subsequent CO2 adsorption at the sites IIA and IIB (Supplementary Table 7). As shown in Fig. 4c, the BE decreases (less negative) when the second CO2 molecule is adsorbed at the site IIA (PCP∙2CO2) in the presence of one molecule at site I, but increases (more negative) when one more CO2 molecule is simultaneously adsorbed at the site IIA (PCP∙2CO2) even in the presence of one molecule at the site I. The increase in BE arises from the formation of T-shaped molecular clusters of CO2 between the adsorbed CO2 molecules stabilising gas-gas interactions, which stabilises gas-gas interactions, and the decrease in average deformation energy of PCP induced by CO2 adsorptions (Fig. 4a and Supplementary Table 7). However, the BE value decreases (less negative) when four CO2 molecules are adsorbed at all the adsorption sites II to afford PCP∙4CO2 (Fig. 4c) because of the congestion due to the ‘narrow-corrugated channel’, as suggested by the decreased interaction energy (Eint, H-G) between CO2 molecules and PCP framework (Supplementary Table 7). Interestingly, the total BE value is the largest (the most negative) when one CO2 molecule is adsorbed at site I, two CO2 molecules are adsorbed at site IIA, and one CO2 molecule is adsorbed at one site IIB of two unit cells, suggesting that simultaneous adsorption of more than one CO2 molecules can happen in PCP-3,5-pdc, which is a typical feature of gate-opening adsorption39.
The adsorption sites of other gaseous molecules are similar to those of CO2 (Supplementary Fig. 31). Among these positions, the adsorption at site I is the most stable; the BE value is the most negative (Supplementary Table 6). At the adsorption site I, N2 and CH4 exhibit less negative BE values (−6.1 and −7.6 kcal mol−1) than that of CO2 (−8.6 kcal mol−1), suggesting the weaker affinity of PCP-3,5-pdc to N2 and CH4 than to CO2. This is reasonable because the interaction of N2 and CH4 molecules with the PCP framework is usually weaker than that of CO2, probably since N2 and CH4 are much less polarised. This common feature is useful for selectively adsorbing CO2 over N2 and CH4. Therefore, the exclusion of N2 and CH4 from the gas mixture occurs through the thermodynamic mechanism. However, we should note that the BE values for C2H2 and C2H4 adsorptions at site I (−9.9 and −11.9 kcal mol−1, respectively) are more negative than for CO2 adsorption (Supplementary Table 6). The stronger binding affinity of PCP-3,5-pdc to C2H4 was also verified by high-pressure single-gas adsorption (Supplementary Fig. 15) and mixture gas co-adsorption measurements (Supplementary Fig. 18). The results are consistent with the observation that C2H4 loading is more significant than that of CO2 at high temperature before the gate-opening pressure for CO2 adsorption but seemingly against our experimental results indicating that PCP-3,5-pdc can selectively adsorb CO2 over C2H2 and C2H4 at low temperature. We also investigated the energy diagrams for the subsequent adsorption of C2H2 and C2H4 at site II (Supplementary Fig. 32) and found that the adsorption occurs similarly to that of CO2, as follows: The subsequent adsorption of one more C2H2 or C2H4 at the site II leads to a decrease (less negative) in BE, but the simultaneous adsorption of more than one C2H2 or C2H4 molecules at site II results in more negative BE values. Such changes in BE suggest that gate-opening adsorption of C2H2 or C2H4 is possible. These results suggest that the selective adsorption of CO2 over C2H2 and C2H4 at low temperatures was not thermodynamically but could be kinetically controlled, as discussed below.
To verify whether the kinetic factor contributes to the selective recognition of CO2 over C2H2 and C2H4 or not, we calculated diffusion barriers for these gas molecules moving through the narrow-corrugated channel (Fig. 5 and Supplementary Fig. 33). The DFT-calculated activation barrier (Ea) for diffusion decreases in the order of C2H4 (16.1 kcal mol−1) > C2H2 (12.9 kcal mol−1) > CO2 (10.6 kcal mol−1), suggesting that C2H4 and C2H2 are more challenging to enter into the pore of PCP-3,5-pdc than does CO2. To find the reason why the diffusion of C2H2 and C2H4 needs a larger barrier than CO2, we analysed the interaction energies (Eint) and deformation energies (Edef) at the adsorption structures (i.e., the initial state of diffusion and transition state of diffusion, named IS and TS, respectively). The Eint at the IS decreases in the order of C2H4 > C2H2 > CO2, which is a standard feature for the interaction energies of these gas molecules with PCP frameworks. In addition, the Edef is negligibly small for all the gas molecules because adsorption at site I induces minor structural deformation of the framework. As going from IS to TS, the structural expansion of the framework occurs, leading to an increase in Edef. In addition, the Eint decreases (less negative) because the gas molecule leaves its best position for interacting with the framework. Because C2H4 has a larger size than CO2, the structural expansion of the framework induced by C2H4 diffusion is larger, leading to a larger Edef at TS for C2H4 than for CO2. Thus, the diffusion of C2H4 needs a much larger barrier than that of CO2. On the other hand, this feature is not observed for comparison between CO2 and C2H2 because their sizes are similar. However, these two gas molecules have different electronic structures50 and thus interact with the framework differently. As shown in Supplementary Fig. 34, the C2H2 molecule interacts with the framework via the C-H···O interaction, where the positively charged H atom of C2H2 approaches the negatively charged O atoms of the 3,5-pdc ligand, whereas CO2 does not form such an interaction. Such an interaction yields a more negative Eint for C2H2 at both IS and TS than CO2. However, to create the C-H···O interaction, the C2H2 molecule must keep its orientation to be perpendicular to the channel wall, which gives rise to larger structural deformation because that C2H2 must exist at a narrow channel at TS. As a result, the Edef for C2H2 at TS is much larger than that for CO2. Thus, despite the Eint less decreases the Edef increases much more at TS than at IS in the C2H2 case as going from IS to TS, leading to a larger Ea in the C2H2 diffusion. These results indicate that the ideal gate size, adsorption structure (or intermolecular interaction manner), and limited framework flexibility in PCP-3,5-pdc is vital to the limited diffusion of C2H2 and C2H4. Therefore, because of the restricted flexible framework with a narrow-corrugated channel structure, PCP-3,5-pdc can achieve unusual selective adsorption of CO2 through structural response to gas diffusion despite weaker binding affinity of CO2 than those of C2H2 and C2H4. In other words, the selective adsorption of CO2 over C2H2 and C2H4 occurs through kinetics. The measured adsorption isobars illustrate that C2H2 and C2H4 molecules can slightly enter the pores. In contrast, diffusion is facilitated at elevated temperatures (Supplementary Fig. 35). Additional analyses of C2H2 and C2H4 adsorption at different exposure times and temperatures further confirmed that the observed recognition of CO2 over C2H2 and C2H4 is the result of kinetic factors (Supplementary Figs. 36–38).
a CO2 molecule at the initial state (IS), transition state (TS) for the diffusion of CO2 through the narrow window, and the diffusion pathway of CO2 in the 1-D corrugated channel. b C2H2 molecule at IS, TS for the diffusion of C2H2 through the narrow window, and the diffusion pathway of C2H2 in the 1-D corrugated channel. c C2H4 molecule at IS, TS for the diffusion of C2H4 through the narrow window, and the diffusion pathway of C2H4 in the 1-D corrugated channel. Purple, red, blue, grey and pink in the PCP frameworks and gas molecules represent Co, O, N, C and H, respectively. The diffusion barrier was calculated using the computational model with one adsorbed gas molecule. We considered a diffusion process in which gas molecules move from site I to its neighbouring site II. Interaction energies (Eint), deformation energies (Edef), and diffusion barriers (Eb) are given in kcal mol−1.
We successfully demonstrated precise CO2 recognition over nine similar, small gaseous molecules using a designed flexible PCP with exclusive discrimination gating behaviour. Compared with known molecule recognition strategies (Supplementary Table 8), including molecular sieving and functional interacting sites, the optimal cooperation of stereochemical shape, location of binding sites, and structural softness through designing narrow-corrugated channel structures in soft porous materials bring unprecedented recognition efficiency, especially for obtaining the desired selectivity in multicomponent mixtures containing molecules of various sizes and affinities. This strategy offers a promising blueprint for designing high-performance porous materials with high performance in challenging recognition and separation systems.
Methods
Materials
Cobalt nitrate hexahydrate (Co(NO3)2·6H2O), methanol (MeOH) and dimethylformamide (DMF) were purchased from FUJIFILM Wako Pure Chemical Corporation Co., Ltd. 3,5-Pyridinedicarboxylic Acid (3,5-pdc) was purchased from Tokyo Chemical Industry Co., Ltd. All chemicals and solvents were used without further purification. Deionized water was used throughout this work. N2, CO, CO2, C2H2, O2, H2, Ar, CH4, C2H4, C2H6 and He were purchased from TAIYO NIPPON SANSO Company (Japan) with a purity of 99.999%.
Synthesis of PCP-3,5-pdc
First, 194 mg (0.6 mmol) dpg was dissolved in DMF/MeOH (1:1, 60 mL). Then, 150 mg (0.6 mmol) 3,5-pdc and 261 mg (0.6 mmol) Co(NO3)2·6H2O were added to the above solution. Then the mixture was heated at 60 °C for 24 h to yield as-synthesised single crystals of PCP-3,5-pdc.
Activation of PCP-3,5-pdc
To obtain fully desolvated PCP-3,5-pdc, the as-synthesised samples were washed with methanol three times. Finally, the samples were dried under vacuum at 120 °C for 24 h. TGA analysis results indicated that all guest solvents were completely removed (Supplementary Fig. 4).
General methods
The TGA curves were obtained from a Rigaku TG 8120 analyzer (EVO2 TG/S-SL) using a heating rate of 5 °C min−1 in flowing N2. Synchrotron single-crystal X-ray direction (PXRD) patterns were recorded using a RIGAKU RAXIS IV diffractometer (Rigaku, Japan) equipped with a PILATUS3 X CdTe 1M photon counting detector installed in the BL02B1 beamline of SPring-8 (λ = 0.41220 Å). Single-component N2, CO, CO2, C2H2, O2, H2, Ar, CH4, C2H4, C2H6 sorptions were measured by BEL-mini, BEL-max and BEL-18 (MicrotracBEL Corp., Japan) gas adsorption instruments. The lowest measurement temperature is 60 K for H2 sorption due to the measurement limitation. The pressure of isobar measurement is 100 kPa. Water vapour sorption was measured by Multi-station Gravimetry Vapor Sorption Analyzer 3H-2000PW (BeiShiDe Instrument, Beijing, China).
High-pressure sorption experiments
High-pressure sorption experiments were carried out by the volumetric BELSORP HP (MicrotracBEL Corp.) instrument (Flow diagram is shown in Supplementary Fig. 12). Prior to the measurement, the blank sample cell weight was measured. The sample was then loaded into the sample cell and heated to 373 K for activation, after which the pretreated sample was weighted. The adsorption measurement method was the volumetric method, wherein the volume of the measurement system was precisely determined to calculate the volume of adsorption. Then, the volume of adsorption was calculated from the gas pressure change in the measurement system using the gas equation. The dead volume of the sample cell was measured using helium gas of 99.9999% purity. Non-ideal corrections were made by applying virial coefficients at respective measurement temperatures.
High-pressure co-sorption experiments
High-pressure co-sorption experiments were carried out by the volumetric BELSORP VC (MicrotracBEL Corp.) instrument connected to an Agilent 490 Micro gas chromatographic (GC) system equipped with a thermal conductivity detector (TCD). The flow diagram in Supplementary Fig. 16a51 provides a visual representation of the experimental setup. In a typical experiment, the sample was first loaded into a pre-weighed stainless-steel sample tube, and activated under dynamic vacuum at 373 K overnight. After activation, the exact sample weight was determined. Then, the sample tube was connected to the instrument and sealed with a metal gasket. Prior to the measurements, the samples were re-heated to 373 K for activation through a removable heater. During measurements, the temperature of the sample was set to 298 K using a removable temperature control unit. Ultra-high purity helium gas (99.9999%) was used to measure the dead volume of the sample tube. The measurement principle is depicted shown in Supplementary Fig. 16b–f. Before the co-adsorption experiment, both gases are stored separately (Supplementary Fig. 16b). To begin the co-adsorption, the gases are introduced into the manifold at the targeted partial pressure and then mixed by the equipped circulation pump (Supplementary Fig. 16c, d). The resulting gas mixture is then introduced into the sample cell (Supplementary Fig. 16e) and adsorbed by the sample (Supplementary Fig. 16f). After a certain equilibration time, the total amount of adsorbed gas is calculated using a constant volume method, and the composition ratio of the adsorbed gas mixture is analysed using GC. The non-adsorbed gas phase over the sample is used to estimate the ratio of the adsorbed gas (Supplementary Fig. 16g). Based on this data, the adsorbed amounts and partial pressures of each gas were calculated.
Column breakthrough experiments
The breakthrough experiments were carried out using a custom-build dynamic mixed-gas breakthrough setup (Supplementary Fig. 17). In a typical experiment, 1 g of PCP-3,5-pdc sample was packed into a stainless-steel column with inner dimensions of ϕ = 8 mm. The mixed-gas flow and pressure were controlled by using pressure-control valves (Swagelok) and mass flow controllers (Brooks instrument). Outlet effluent from the column was continuously monitored using a quadrupole-type mass spectrometer, BEL Mass (MicrotracBEL Corp.). The column packed with powder sample was first purged with a flow of He (20 mL·min−1) for 1 h at room temperature. The mixed-gas flow rate during the breakthrough process is 6 mL·min−1 using 50/50 (v/v) CO2/other gas at room temperatures. The total pressure of the mixture gases was 20 bar. After the breakthrough experiment, the sample was regenerated under vacuum for 24 h for cycling measurement.
Separation factor calculation
The separation factor is calculated based on the mixture gases’ co-adsorption results. The separation factor (S) is defined as Eq. (1):
where X1 and X2 are the concentration of gas 1 and gas 2 in the adsorbed phase and Y1 and Y2 are the concentration of gas 1 and gas 2 in the feed phase.
Hill analysis
The Hill coefficient, n, is recognised as an indicator of cooperative interactions because it describes the number of molecules bound per receptor52,53. The Hill coefficient is the slope of the Hill plot {log [Y/(1 − Y)] versus log P}, where the Y-axis is the CO2 fractional unloading and P is the gas pressure54,55. Generally, n < 1 corresponds to negatively cooperative systems, while n > 1 corresponds to positively cooperative systems. To evaluate the degree of cooperativity for the gate-opening step in CO2 adsorption, we applied Hill’s model analysis to the CO2 adsorption isotherm at 195 and 298 K.
In-situ PXRD/adsorption measurements
The synchrotron PXRD data for the structural analyses of CO2-loaded PCP-3,5-pdc was collected using a synchrotron X-ray and multiple MYTHEN detectors of the BL02B2 beam line at SPring-8 in Japan56,57. The powder sample was put in glass capillaries of 0.5 mm diameter under vacuum. X-ray of wavelength 0.799671(1) Å was selected by a double crystal monochromator using Si(111) plane. The data was taken for 10 min. The lattice parameters and space group were determined by the N-TREOR09 program of EXPO2014 software58, which was further refined by the Le Bail fit using RIETAN-FP software59. A structural starting model for Rietveld refinement was subsequently found with the charge-flipping method using the Superflip and EDMA programs included in RIETAN-FP software based on the extracted lattice parameters and peak intensities from the previous Le Bail fit in space group P–1 (No. 2). The Rietveld refinement was carried out using the RIETAN-FP software using slack soft constraints for bond lengths, angles and planar groups. The final structure of CO2-loaded PCP-3,5-pdc was obtained with reliability factors Rwp = 3.783, Rp = 2.711, S = 4.1301, RB = 3.480 and RF = 9.099, respectively. Crystallographic data in CIF format have been deposited in the Cambridge Crystallographic Data Centre (CCDC) under deposition number CCDC-2219713. The data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, U.K.).
Single-crystal X-ray crystallography
Suitable crystals of as-synthesised and activated PCP-3,5-pdc were selected for single-crystal X-ray data collection. Intensities were collected on a Rigaku XtaLAB P200 diffractometer using a VariMax Mo Optic with Mo-Kα (λ = 0.71073 Å) equipped with PILATUS 200K detector. These structures were solved by direct methods and refined on F2 by full-matrix least-squares methods with SHELXTL version 2018/3. Some thermal and structural restraints (ISO, REGU, SIMU, SADI, FLAT) and constraint (EADP) on disordered solvent and framework atoms were also used. Hydrogen atoms within the ligand backbones were fixed geometrically and allowed to ride on the parent non-hydrogen atoms in this study. Crystallographic data in CIF format have been deposited in the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC-2219711 to CCDC-2219712. The data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, U.K.).
Computational details
Adsorption energies for several gas species (CO2, N2, CH4, C2H2, and C2H4) were carried out to understand the preferential CO2 adsorption over other gas species in PCP-3,5-pdc. Because only CO2 adsorption positions were determined and those of other gas species were unclear in the experiment, we carried out canonical Monte Carlo (MC) simulation60 to locate their positions in PCP-3,5-pdc in both open and activated phases, as implemented in RASPA61. The Lennard–Jones (LJ) potentials were used to describe the Van der Waals interaction of gas molecules with the PCP framework and the electrostatic interaction was evaluated with the Ewald summation method. The LJ parameters for the PCP framework were taken from the standard universal force field (UFF)62 and the DDEC atomic charges63,64 of the PCP framework were used in the evaluation of electrostatic interaction. The LJ parameters and atomic charges of CO2, N2, CH4, and C2H4 were taken from the TraPPE force field65. Because there are no reported parameters for C2H2 in the TraPPE force field, we used those values for the CH moiety in 2-butene66. In the MC simulation, the first 1 × 105 cycles were employed for obtaining equilibration and then 3 × 105 cycles were used for obtaining a distribution of guest molecules at room temperature. The final gas adsorption configuration obtained by the above MC simulation was used to construct the initial structure for performing geometry optimisation with density functional theory (DFT).
The binding energy for gas adsorption was calculated using the DFT method with periodic boundary conditions as implemented in the Vienna Ab initio Simulation Package (VASP 5.4.4)67,68. The Perdew–Burke–Ernzerhof functional69 with Grimme’s semi-empirical “D3” dispersion term49 (PBE-D3) was employed in these calculations. The plane wave basis sets with an energy cutoff of 500 eV were used to describe valence electrons and the projector-augmented-wave pseudopotentials70,71 were used to describe core electrons. The threshold for atomic force convergence was set to be 0.005 eV/Å in geometry optimisation. The Brillouin zone was sampled by a Γ-point in geometry optimisations and 3×3×3 Monkhorst-Pack72 k-point meshes for energy calculations.
The binding energies (BE) of gas molecules (G, G = CO2, N2, CH4, C2H2, and C2H4) with PCP-3,5-pdc were calculated with Eq. (2);
where E(PCP·nG)eq is the total energy of PCP-3,5-pdc with n gas molecules per unit cell, E(PCP)eq and E(G)eq are the total energies of empty PCP-3,5-pdc and one free gas molecule, respectively, and the subscript “eq” represents the equilibrium structure. The BE was further decomposed into the interaction energies (Eint) between gas molecules and PCP framework and the deformation energy (Edef) of the framework induced by gas adsorption. These two energy terms were calculated with Eqs. (3) and (4), respectively;
where E(PCP)dis is the energy of PCP in its distorted structure, which was taken from the equilibrium structure of PCP-3,5-pdc with adsorbed gas molecules. Host-guest and guest-guest interaction energies (Eint,H-G and Eint,G-G) were calculated with Eqs. (5) and (6), respectively;
where E(nG)dis is the energy of n adsorbed gas molecules taken from their equilibrium adsorption structure.
The climbing-image nudged elastic band (CI-NEB) method73 was used to evaluate the diffusion barrier of CO2, C2H2, and C2H4 in PCP-3,5-pdc. Because the adsorption amount of C2H2 and C2H4 is less than 1 molecule per unit cell (0.5 molecule per Co2+), we considered only the adsorption with one gas molecule. The convergence criterion for geometry optimisation of the transition state was chosen to be 0.02 eV/Å. Vibrational frequency calculations were carried out to confirm that the initial and transition states have no and one imaginary frequency, respectively.
Data availability
The crystallographic data for the structures of as-synthesised PCP-3,5-pdc, activated PCP-3,5-pdc and CO2-loaded PCP-3,5-bdc at 195 K in this work have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 2219711, 2219713 and 2219714, respectively. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk. The datasets generated during and/or analysed during the current study are available from the corresponding author upon request.
References
Persch, E., Dumele, O. & Diederich, F. Molecular recognition in chemical and biological systems. Angew. Chem. Int. Ed. Engl. 54, 3290–3327 (2015).
Gellman, S. H. Introduction: molecular recognition. Chem. Rev. 97, 1231–1232 (1997).
Csermely, P., Palotai, R. & Nussinov, R. Induced fit, conformational selection and independent dynamic segments: an extended view of binding events. Trends Biochem. Sci. 35, 539–546 (2010).
Tashiro, S. & Shionoya, M. Novel porous crystals with macrocycle-based well-defined molecular recognition sites. Acc. Chem. Res. 53, 632–643 (2020).
Yuan, Y., Yang, Y. & Zhu, G. Molecularly imprinted porous aromatic frameworks for molecular recognition. ACS Cent. Sci. 6, 1082–1094 (2020).
Chen, B., Xiang, S. & Qian, G. Metal-organic frameworks with functional pores for recognition of small molecules. Acc. Chem. Res. 43, 1115–1124 (2010).
Lehn, J.-M. Perspectives in supramolecular chemistry—from molecular recognition towards molecular information processing and self-organization. Angew. Chem. Int. Ed. Engl. 29, 1304–1319 (1990).
Dong, J. & Davis, A. P. Molecular recognition mediated by hydrogen bonding in aqueous media. Angew. Chem. Int. Ed. Engl. 60, 8035–8048 (2021).
Ariga, K., Ito, H., Hill, J. P. & Tsukube, H. Molecular recognition: from solution science to nano/materials technology. Chem. Soc. Rev. 41, 5800–5835 (2012).
Li, J.-R., Kuppler, R. J. & Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 38, 1477–1504 (2009).
Li, J., Bhatt, P. M., Li, J., Eddaoudi, M. & Liu, Y. Recent progress on microfine design of metal-organic frameworks: structure regulation and gas sorption and separation. Adv. Mater. 32, 2002563 (2020).
Zhao, X., Wang, Y., Li, D. S., Bu, X. & Feng, P. Metal–organic frameworks for separation. Adv. Mater. 30, 1705189 (2018).
Idrees, K. B. et al. Tailoring pore aperture and structural defects in zirconium-based metal-organic frameworks for krypton/xenon separation. Chem. Mater. 32, 3776–3782 (2020).
Yan, Y. et al. Non-interpenetrated metal-organic frameworks based on copper(II) paddlewheel and oligoparaxylene-isophthalate linkers: synthesis, structure, and gas adsorption. J. Am. Chem. Soc. 138, 3371–3381 (2016).
Liao, P.-Q., Huang, N.-Y., Zhang, W.-X., Zhang, J.-P. & Chen, X.-M. Controlling guest conformation for efficient purification of butadiene. Science 356, 1193–1196 (2017).
Nakagawa, K. et al. Enhanced selectivity of CO2 from a ternary gas mixture in an interdigitated porous framework. Chem. Commun. 46, 4258–4260 (2010).
Inubushi, Y. et al. Modification of flexible part in Cu2+ interdigitated framework for CH4/CO2 separation. Chem. Commun. 46, 9229–9231 (2010).
Lin, R.-B. et al. Molecular sieving of ethylene from ethane using a rigid metal-organic framework. Nat. Mater. 17, 1128–1133 (2018).
Ma, S., Sun, D., Yuan, D., Wang, X.-S. & Zhou, H.-C. Preparation and gas adsorption studies of three mesh-adjustable molecular sieves with a common structure. J. Am. Chem. Soc. 131, 6445–6451 (2009).
Pan, L., Olson, D. H., Ciemnolonski, L. R., Heddy, R. & Li, J. Separation of hydrocarbons with a microporous metal-organic framework. Angew. Chem. Int. Ed. Engl. 45, 616–619 (2006).
Cadiau, A., Adil, K., Bhatt, P., Belmabkhout, Y. & Eddaoudi, M. A metal-organic framework–based splitter for separating propylene from propane. Science 353, 137–140 (2016).
Zhang, Z. et al. Sorting of C4 olefins with interpenetrated hybrid ultramicroporous materials by combining molecular recognition and size‐sieving. Angew. Chem. Int. Ed. Engl. 56, 16282–16287 (2017).
Cui, X. et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 353, 141–144 (2016).
Yoon, J. W. et al. Selective nitrogen capture by porous hybrid materials containing accessible transition metal ion sites. Nat. Mater. 16, 526–531 (2017).
Kim, E. J. et al. Cooperative carbon capture and steam regeneration with tetraamine-appended metal–organic frameworks. Science 369, 392–396 (2020).
Dutcher, B., Fan, M. & Russell, A. G. Amine-based CO2 capture technology development from the beginning of 2013—a review. ACS Appl. Mater. Interfaces 7, 2137–2148 (2015).
Qazvini, O. T., Babarao, R. & Telfer, S. G. Selective capture of carbon dioxide from hydrocarbons using a metal-organic framework. Nat. Commun. 12, 197 (2021).
Du, M. et al. Divergent kinetic and thermodynamic hydration of a porous Cu(II) coordination polymer with exclusive CO2 sorption selectivity. J. Am. Chem. Soc. 136, 10906–10909 (2014).
Elsaidi, S. K., Mohamed, M. H., Banerjee, D. & Thallapally, P. K. Flexibility in metal–organic frameworks: a fundamental understanding. Coord. Chem. Rev. 358, 125–152 (2018).
Chang, Z., Yang, D.-H., Xu, J., Hu, T.-L. & Bu, X.-H. Flexible metal-organic frameworks: recent advances and potential applications. Adv. Mater. 27, 5432–5441 (2015).
Schneemann, A. et al. Flexible metal–organic frameworks. Chem. Soc. Rev. 43, 6062–6096 (2014).
Horike, S., Shimomura, S. & Kitagawa, S. Soft porous crystals. Nat. Chem. 1, 695–704 (2009).
Katsoulidis, A. P. et al. Chemical control of structure and guest uptake by a conformationally mobile porous material. Nature 565, 213–217 (2019).
Krause, S., Hosono, N. & Kitagawa, S. Chemistry of soft porous crystals: structural dynamics and gas adsorption properties. Angew. Chem. Int. Ed. Engl. 59, 15325–15341 (2020).
Matsuda, R. Selectivity from flexibility. Nature 509, 434–435 (2014).
Yan, Y. et al. Amino acid residues determine the response of flexible metal-organic frameworks to guests. J. Am. Chem. Soc. 142, 14903–14913 (2020).
Zhou, D.-D. et al. Intermediate-sized molecular sieving of styrene from larger and smaller analogs. Nat. Mater. 18, 994–998 (2019).
Gu, Y. et al. Structural‐deformation‐energy‐modulation strategy in a soft porous coordination polymer with an interpenetrated framework. Angew. Chem. Int. Ed. Engl. 59, 15517–15521 (2020).
Zheng, J.-J., Kusaka, S., Matsuda, R., Kitagawa, S. & Sakaki, S. Theoretical insight into gate-opening adsorption mechanism and sigmoidal adsorption isotherm into porous coordination polymer. J. Am. Chem. Soc. 140, 13958–13969 (2018).
Fraux, G. & Coudert, F.-X. Recent advances in the computational chemistry of soft porous crystals. Chem. Commun. 53, 7211–7221 (2017).
Vanduyfhuys, L. et al. Thermodynamic insight into stimuli-responsive behaviour of soft porous crystals. Nat. Commun. 9, 204 (2018).
Zhou, D.-D. & Zhang, J.-P. On the role of flexibility for adsorptive separation. Acc. Chem. Res. 55, 2966–2977 (2022).
Hu, Z., Wang, Y., Shah, B. B. & Zhao, D. CO2 capture in metal–organic framework adsorbents: an engineering perspective. Adv. Sustain. Syst. 3, 1800080 (2019).
Siegelman, R. L., Kim, E. J. & Long, J. R. Porous materials for carbon dioxide separations. Nat. Mater. 20, 1060–1072 (2021).
Horike, S., Tanaka, D., Nakagawa, K. & Kitagawa, S. Selective guest sorption in an interdigitated porous framework with hydrophobic pore surfaces. Chem. Commun. 32, 3395–3397 (2007).
Chen, K.-J. et al. Benchmark C2H2/CO2 and CO2/C2H2 separation by two closely related hybrid ultramicroporous materials. Chem 1, 753–765 (2016).
Fan, W. et al. Optimizing multivariate metal–organic frameworks for efficient C2H2/CO2 separation. J. Am. Chem. Soc. 142, 8728–8737 (2020).
Frenkel, D. & Smit, B. Understanding Molecular Simulation: From Algorithms to Applications, Vol. 1 (Elsevier, 2001).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Gu, Y. et al. Host–guest interaction modulation in porous coordination polymers for inverse selective CO2/C2H2 separation. Angew. Chem. Int. Ed. Engl. 60, 11688–11694 (2021).
Vervoorts, P. et al. Coordinated water as new binding sites for the separation of light hydrocarbons in metal–organic frameworks with open metal sites. ACS Appl. Mater. Interfaces 12, 9448–9456 (2020).
Swenson, H. & Stadie, N. P. Langmuir’s theory of adsorption: a centennial review. Langmuir 35, 5409–5426 (2019).
Weiss, J. N. The Hill equation revisited: uses and misuses. FASEB J. 11, 835–841 (1997).
Wang, W., Wang, L., Huang, Y., Xie, Z. & Jing, X. Nanoscale metal–organic framework–hemoglobin conjugates. Chem. Asian J. 11, 750–756 (2016).
Sen, S. et al. Cooperative bond scission in a soft porous crystal enables discriminatory gate opening for ethylene over ethane. J. Am. Chem. Soc. 139, 18313–18321 (2017).
Nishibori, E. et al. The large Debye–Scherrer camera installed at SPring-8 BL02B2 for charge density studies. J. Phys. Chem. Solids 62, 2095–2098 (2001).
Takata, M. et al. High resolution Debye-Scherrer camera installed at SPring-8. Adv. X-ray Anal. 45, 377–384 (2002).
Altomare, A. et al. EXPO2013: a kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 46, 1231–1235 (2013).
Izumi, F. & Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenomena 130, 15–20 (2007).
Adcock, S. A. & McCammon, J. A. Molecular dynamics: survey of methods for simulating the activity of proteins. Chem. Rev. 106, 1589–1615 (2006).
Dubbeldam, D., Calero, S., Ellis, D. E. & Snurr, R. Q. RASPA: molecular simulation software for adsorption and diffusion in flexible nanoporous materials. Mol. Simul. 42, 81–101 (2016).
Rappé, A. K., Casewit, C. J., Colwell, K., Goddard, W. A. III & Skiff, W. M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 114, 10024–10035 (1992).
Manz, T. A. & Limas, N. G. Introducing DDEC6 atomic population analysis: part 1. Charge partitioning theory and methodology. RSC Adv. 6, 47771–47801 (2016).
Limas, N. G. & Manz, T. A. Introducing DDEC6 atomic population analysis: part 2. Computed results for a wide range of periodic and nonperiodic materials. RSC Adv. 6, 45727–45747 (2016).
Potoff, J. J. & Siepmann, J. I. Vapor–liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen. AlChE J. 47, 1676–1682 (2001).
Jorgensen, W. L., Madura, J. D. & Swenson, C. J. Optimized intermolecular potential functions for liquid hydrocarbons. J. Am. Chem. Soc. 106, 6638–6646 (1984).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFE0110500) to F.L. and S.Ki., Shanghai Pujiang Program (NO.21PJ1412600) and the Fundamental Research Funds for the Central Universities of China to Y.G., KAKENHI Grant-in-Aid for Scientific Research (S) (JP18H05262, JP22H05005) and (C) (JP22K05128) from the Japan Society of the Promotion of Science (JSPS). K.O. acknowledges “Yazaki Memorial Foundation for Science and Technology” for the financial support. Synchrotron X-ray measurements were supported by the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2020A1469, 2020A0617, 2021A1104, 2021A1682). We thank the iCeMS Analysis Center for access to the analytical instruments. We also thank Dr. Mickaele Bonneau for the high-pressure gas sorption measurements.
Author information
Authors and Affiliations
Contributions
Y.G., F.L. and S.Ki. conceived the idea. Y.G., K.O. and S.Ki. designed the experiments. Y.G. and K.O. did the sample preparation, characterisations, gas sorption, and breakthrough measurements. Y.G. and K.O. analysed data. J.Z. and S.S. carried out calculation studies. K.O., H.A., Y.K. and S.Ka. contributed to the synchrotron in-situ PXRD measurements in BL02B2, SPring-8. M.Y. and P.W. helped with the characterisations. Y.G., J.Z., S.S., Y.W., F.L., K.O. and S.Ki. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gu, Y., Zheng, JJ., Otake, Ki. et al. Soft corrugated channel with synergistic exclusive discrimination gating for CO2 recognition in gas mixture. Nat Commun 14, 4245 (2023). https://doi.org/10.1038/s41467-023-39470-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-39470-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.