Abstract
The shieldin complex functions as the downstream effector of 53BP1–RIF1 to promote DNA double-strand break end-joining by restricting end resection. The SHLD2 subunit binds to single-stranded DNA ends and blocks end resection through OB-fold domains. Besides blocking end resection, it is unclear how the shieldin complex processes SHLD2-bound single-stranded DNA and promotes non-homologous end-joining. Here, we identify a downstream effector of the shieldin complex, ASTE1, as a structure-specific DNA endonuclease that specifically cleaves single-stranded DNA and 3′ overhang DNA. ASTE1 localizes to DNA damage sites in a shieldin-dependent manner. Loss of ASTE1 impairs non-homologous end-joining, leads to hyper-resection and causes defective immunoglobulin class switch recombination. ASTE1 deficiency also causes resistance to poly(ADP-ribose) polymerase inhibitors in BRCA1-deficient cells owing to restoration of homologous recombination. These findings suggest that ASTE1-mediated 3′ single-stranded DNA end cleavage contributes to the control of DSB repair choice by 53BP1, RIF1 and shieldin.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Gowen, L. C., Johnson, B. L., Latour, A. M., Sulik, K. K. & Koller, B. H. Brca1 deficiency results in early embryonic lethality characterized by neuroepithelial abnormalities. Nat. Genet. 12, 191–194 (1996).
Sharan, S. K. et al. Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature 386, 804–810 (1997).
Cao, L., Li, W., Kim, S., Brodie, S. G. & Deng, C. X. Senescence, aging, and malignant transformation mediated by p53 in mice lacking the Brca1 full-length isoform. Genes Dev. 17, 201–213 (2003).
Lowndes, N. F. The interplay between BRCA1 and 53BP1 influences death, aging, senescence and cancer. DNA Repair 9, 1112–1116 (2010).
Ward, I. M. et al. 53BP1 is required for class switch recombination. J. Cell Biol. 165, 459–464 (2004).
Manis, J. P. et al. 53BP1 links DNA damage-response pathways to immunoglobulin heavy chain class-switch recombination. Nat. Immunol. 5, 481–487 (2004).
McKinnon, P. J. DNA repair deficiency and neurological disease. Nat. Rev. Neurosci. 10, 100–112 (2009).
Narod, S. A. & Foulkes, W. D. BRCA1 and BRCA2: 1994 and beyond. Nat. Rev. Cancer 4, 665–676 (2004).
Zimmermann, M., Lottersberger, F., Buonomo, S. B., Sfeir, A. & de Lange, T. 53BP1 regulates DSB repair using Rif1 to control 5′ end resection. Science 339, 700–704 (2013).
Chapman, J. R. et al. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol. Cell 49, 858–871 (2013).
Di Virgilio, M. et al. Rif1 prevents resection of DNA breaks and promotes immunoglobulin class switching. Science 339, 711–715 (2013).
Ghezraoui, H. et al. 53BP1 cooperation with the REV7–shieldin complex underpins DNA structure-specific NHEJ. Nature 560, 122–127 (2018).
Mirman, Z. et al. 53BP1–RIF1–shieldin counteracts DSB resection through CST- and Polalpha-dependent fill-in. Nature 560, 112–116 (2018).
Noordermeer, S. M. et al. The shieldin complex mediates 53BP1-dependent DNA repair. Nature 560, 117–121 (2018).
Dev, H. et al. Shieldin complex promotes DNA end-joining and counters homologous recombination in BRCA1-null cells. Nat. Cell Biol. 20, 954–965 (2018).
Findlay, S. et al. SHLD2/FAM35A co-operates with REV7 to coordinate DNA double-strand break repair pathway choice. Embo J. 37, e100158 (2018).
Kim, W. K. et al. Identification and selective degradation of neopeptide-containing truncated mutant proteins in the tumors with high microsatellite instability. Clin. Cancer Res. 19, 3369–3382 (2013).
Tougeron, D. et al. Tumor-infiltrating lymphocytes in colorectal cancers with microsatellite instability are correlated with the number and spectrum of frameshift mutations. Mod. Pathol. 22, 1186–1195 (2009).
Staffa, L. et al. Mismatch repair-deficient crypt foci in Lynch syndrome-molecular alterations and association with clinical parameters. PLoS ONE 10, e0121980 (2015).
Maby, P. et al. Correlation between density of CD8+ T-cell infiltrate in microsatellite unstable colorectal cancers and frameshift mutations: a rationale for personalized immunotherapy. Cancer Res. 75, 3446–3455 (2015).
Kuntz, K. & O’Connell, M. J. Initiation of DNA damage responses through XPG-related nucleases. EMBO J. 32, 290–302 (2013).
Escribano-Diaz, C. et al. A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Mol. Cell 49, 872–883 (2013).
Callen, E. et al. 53BP1 enforces distinct pre- and post-resection blocks on homologous recombination. Mol. Cell 77, 26–38 (2020).
Sartori, A. A. et al. Human CtIP promotes DNA end resection. Nature 450, 509–514 (2007).
Nicolette, M. L. et al. Mre11–Rad50–Xrs2 and Sae2 promote 5′ strand resection of DNA double-strand breaks. Nat. Struct. Mol. Biol. 17, 1478–1485 (2010).
Shim, E. Y. et al. Saccharomyces cerevisiae Mre11/Rad50/Xrs2 and Ku proteins regulate association of Exo1 and Dna2 with DNA breaks. EMBO J. 29, 3370–3380 (2010).
Cejka, P. et al. DNA end resection by Dna2–Sgs1–RPA and its stimulation by Top3–Rmi1 and Mre11–Rad50–Xrs2. Nature 467, 112–116 (2010).
Nimonkar, A. V., Özsoy, A. Z., Genschel, J., Modrich, P. & Kowalczykowski, S. C. Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair. Proc. Natl Acad. Sci. USA 105, 16906–16911 (2008).
Bouwman, P. et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat. Struct. Mol. Biol. 17, 688–695 (2010).
Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000).
Manis, J. P., Tian, M. & Alt, F. W. Mechanism and control of class-switch recombination. Trends Immunol. 23, 31–39 (2002).
Chang, H. H. Y., Pannunzio, N. R., Adachi, N. & Lieber, M. R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 18, 495–506 (2017).
Barazas, M. et al. The CST complex mediates end protection at double-strand breaks and promotes parp inhibitor sensitivity in BRCA1-deficient cells. Cell Rep. 23, 2107–2118 (2018).
Liang, L. et al. Molecular basis for assembly of the shieldin complex and its implications for NHEJ. Nat. Commun. 11, 1972 (2020).
Hohn, K. T. & Grosse, F. Processivity of the DNA polymerase α-primase complex from calf thymus. Biochemistry 26, 2870–2878 (1987).
Tang, J. et al. Acetylation limits 53BP1 association with damaged chromatin to promote homologous recombination. Nat. Struct. Mol. Biol. 20, 317–325 (2013).
Zhou, Y., Caron, P., Legube, G. & Paull, T. T. Quantitation of DNA double-strand break resection intermediates in human cells. Nucleic Acids Res. 42, e19 (2014).
Okamoto, K. et al. A two-step mechanism for TRF2-mediated chromosome-end protection. Nature 494, 502–505 (2013).
Shen, B. et al. Perturbation of base excision repair sensitizes breast cancer cells to APOBEC3 deaminase-mediated mutations. eLife 9, e51605 (2020).
Knijnenburg, T. A. et al. Genomic and molecular landscape of DNA damage repair deficiency across the cancer genome atlas. Cell Rep. 23, 239–254 (2018).
Telli, M. L. et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin. Cancer Res. 22, 3764–3773 (2016).
Goldman, M. et al. The UCSC Xena Platform for cancer genomics data visualization and interpretation. Preprint at bioRxiv https://doi.org/10.1101/326470 (2018).
Marquard, A. M. et al. Pan-cancer analysis of genomic scar signatures associated with homologous recombination deficiency suggests novel indications for existing cancer drugs. Biomark. Res. 3, 9 (2015).
Martí-Renom, M. A. et al. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 29, 291–325 (2000).
Sali, A. & Blundell, T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Hildebrand, A., Remmert, M., Biegert, A. & Soding, J. Fast and accurate automatic structure prediction with HHpred. Proteins 77, 128–132 (2009).
Soding, J. Protein homology detection by HMM-HMM comparison. Bioinformatics 21, 951–960 (2005).
Acknowledgements
We thank R.A. Greenberg for providing the ER-mCherry-LacI-FokI-DD U2OS cell line and G. Legube for providing the AsiSI-ER U2OS cell line. This work was supported by the National Natural Science Foundation of China (nos 32090032, 32070713, 82002985, 81872298 and 81802754) to J.Y. and the Mayo foundation to Z.L.
Author information
Authors and Affiliations
Contributions
F.Z. and W.K. designed and conducted experiments. H.G., S.Q. and C.Z. conducted bioinformatics data. C.L., F.Z., M.D., S.-H.C. and Y.L. conducted microirradiation experiments and analysed data. F.Z., W.K., Y.Z., P.Y., B.Q. and X.T. conducted mouse work. F.Z., W.K., Q.H., M.D. and Q.Z. conducted protein purification and nuclease assays. Q.H. conducted the protein structure analysis. Y.C., J.H., G.G. and M.G. helped with plasmid construction. S.N. and J.A.K. helped with writing and proofreading the paper. F.Z., W.K., K.L., J.Y. and Z.L. initiated the study, designed experiments, analysed data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Cell Biology thanks Dipanjan Chowdhury, Peter McHugh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 ASTE1 plays a role in maintaining genomic stability.
a, Schematic representation of gene-trapped alleles in mouse Aste1 genomic locus. b, PCR analysis of mice genotypes using the indicated primers. c, Western blot analysis of mASTE1 expression in Aste1+/+ and Aste1−/− MEFs. Data are representative of two independent experiments in b and c. d-e, The body weight of 2-month or 18-month old mice of different genotype (d, male; e, female). The graph represents mean ± s.d., two-tailed, unpaired t-tests. f, Survival analysis of Aste1+/+ or Aste1−/− mice. Two-sided log-rank (Mantel-Cox) test was applied. n represents the number of mice analyzed as indicated in the panels in d-f. g, Aste1−/− MEFs show increased genomic instability following 2-Gy IR treatment. The arrows indicate chromosomal breaks. The blue boxes are enlarged representative breaks. n indicates the cell number analyzed in each group, pooled from three independent experiments. Statistical analysis was performed using two-tailed unpaired t-tests. h, Representative micrographs and quantification of 53BP1 foci in the indicated MEFs following IR treatment. The quantification graph represents mean ± 95% CI. Statistical analysis was performed using two-tailed unpaired t-tests. 90 cells quantified in each group were obtained from one experiment. Data are representative of three independent experiments. Scale bars, 10 µm. i-p, Cell survival in response to IR (i), etoposide (j), neocarzinostatin (k), cisplatin (l), methyl methanesulfonate (m), olaparib (n), ultraviolet light (o) or camptothecin (p) of wild-type or Aste1−/− MEFs. The graph represents mean ± s.d., n = 3 independent experiments. q, Representative micrographs of GFP-ASTE1 and 53BP1 accumulation at DSB sites induced by laser-microirradiation in U2OS cells. Scale bars, 5 µm. Data are representative of three independent experiments. r, Assessment of ASTE1 knockdown efficiency for experiments in Fig. 1f–g. Data are representative of two independent experiments. s, Cell cycle analysis of the indicated U2OS cells. The graphs represent mean ± s.d., two-tailed, paired t-test; n = 3 independent experiments. t, Gating strategy to identify micronucleus in normochromic erythrocytes in Fig. 1d.
Extended Data Fig. 2 The structure of ASTE1 catalytic domain is predicated to be similar to EXO1.
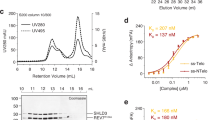
a, The predicted 3D structure of ASTE1 catalytic domain shares similarity with that of EXO1 as shown by the superposition of the two structures. b, Close-up view of an arginine and lysine-rich loop in the vicinity of DNA. Residue R252 is located in the loop and in close proximity to DNA. c, The enzyme activity profile of ASTE1 eluted from the gel filtration column. 1 unit defines as the amount of enzyme needed to totally digest 0.1 pmol ssDNA in 30 min, 37 °C. Two biologically independent experiments were performed, with similar results obtained.
Extended Data Fig. 3 ASTE1 knockdown has no effect on 53BP1-RIF1 -shieldin or BRCA1-CtIP recruitment to DNA damage sites.
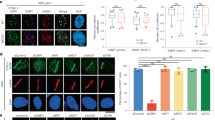
a and b, Representative micrographs and quantification data for 53BP1 (a) or RIF1 (b) foci formation in the indicated U2OS cell lines with or without irradiation treatment (1 h after irradiation with 2 Gy X-ray). c-f, Representative micrographs and quantification data for GFP-SHLD3 (c), REV7 (d), GFP-SHLD2 (e) or GFP-SHLD1 (f) foci formation in the indicated U2OS cell lines with or without irradiation treatment (2 h after 5 Gy X-ray irradiation). g and h, Representative micrographs and quantification data for BRCA1 (g) or CtIP (h) foci formation in the indicated U2OS cell lines with or without irradiation treatment (2 h after 5 Gy X-ray irradiation). The quantification graphs represent mean ± 95% CI. Statistical analysis was performed using two-tailed unpaired t-tests. Scale bars, 10 µm. Each point represents a cell. The cells used for analyzed in each experiment were from a single replicate. n indicates the cell number used for quantification in each group. Data are representative of three independent experiments.
Extended Data Fig. 4 The overexpression, knockdown or knockout efficiency of DSB pathway genes.
a, Western blot analysis of Flag-tagged wild-type or R252A mutant ASTE1 overexpression in Fig. 3. Western blot analysis of 53BP1 (b), RIF1 (c), SHLD3 (d), REV7 (e), SHLD2 (f), SHLD1 (g) and BRCA1 (h) shRNAs or siRNAs used in Fig. 4. i-k, Western blot analysis of human or mouse BRCA1, 53BP1 and ASTE1 knockdown or knockout in Fig. 6. Data are representatives of three biologically independent experiments, with similar results obtained.
Extended Data Fig. 5 The kinetic recruitment of ASTE1 by the shieldin complex.
a, The workflow for studying the kinetic recruitment of ASTE1. b-e, The indicated AID-AsiSI-ER U2OS cells were pre-treated with or without 4-OHT for 4 h to induce DSB. Then the cells were further treated Auxin to induce the degradation of AsiSI enzyme. Flag-ASTE1 accumulation at DNA damage sites was quantified using ChIP-qPCR. The graphs represent mean ± S.D, n = 3 biologically independent experiments. f, Representative micrographs and laser strips intensity quantification of GFP-53BP1, GFP-RIF1, GFP-SHLD3 or GFP-ASTE1 accumulation at DNA damage sites induced by laser micro-irradiation in U2OS cells. The graph represents mean ± s.d.; n = 10-12 cells were captured from 3 biologically independent experiments in each group.
Extended Data Fig. 6 ASTE1 is a SHLD2 binding protein.
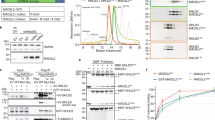
a, Co-immunoprecipitation analysis of Flag-ASTE1 with BRCA2, CtIP, RPA32, RAD51 and KU80 in 293T cells. Blots were probed with the indicated antibodies. b, Co-immunoprecipitation analysis of ASTE1 with SHLD2. The whole cell lysates of U2OS cells were incubated with anti-ASTE1 antibody in NETN buffer with increased NaCl concentration, and bound proteins were eluted before analysis by SDS–PAGE. Blots were probed with the indicated antibodies. c and d, Mapping the binding region of SHLD2 in ASTE1. 293T cells were transfected with GFP-tagged ASTE1 and full-length Flag-SHLD2 or truncated Flag-SHLD2. Bound proteins were eluted before analysis by SDS–PAGE. Blots were probed with the indicated antibodies. e and f, Mapping the binding region of ASTE1 in SHLD2. 293T cells were transfected with HA-tagged SHLD2 and full-length Flag-ASTE1 or truncated Flag-ASTE1. Whole cell lysates were incubated with Flag resin and bound proteins were eluted before analysis by SDS-PAGE. Blots were probed with the indicated antibodies. The blots data (a-f) are representative of three biologically independent experiments, with similar results obtained. g, Detection of Flag-ASTE1 full-length and 351-400 a.a. truncation accumulation at DSB sites induced by mCherry-LacR-FokI in U2OS cells using ChIP-qPCR. The graphs represent mean ± s.d. Statistical analysis was performed using two-tailed unpaired t-tests; n = 3 biologically independent experiments. h, AsiSI-ER U2OS cells depleted of ASTE1 were complemented with the indicated Flag-tagged ASTE1 constructs and treated with 300 nM 4-OHT for 4 h or mock-treated. DNA end resection was measured by qPCR. The graph represents mean ± s.d. Statistical analysis was performed using two-tailed unpaired t-tests; n = 3 biologically independent experiments. i, Coomassie brilliant blue staining of purified recombinant His-ASTE1, GST, GST-SHLD1, GST-SHLD2, GST-SHLD3 and GST-REV7 used in His pull-down assay in Fig. 4i. The black arrows indicate the recombinant protein bands. Data are representative of two biologically independent experiments, with similar results obtained.
Extended Data Fig. 7 ASTE1 promotes SHLD2 release and KU80 recruitment.
a-c, Representative micrographs and quantification data for of GFP-SHLD2, GFP-SHLD3 or REV7 IRIF in the indicated U2OS cell lines with or without irradiation treatment (4 h after irradiation with 5 Gy X-ray). The graph represents mean ± 95% CI. Statistical analysis was performed using two-tailed unpaired t-tests (n = 90 cells in a, n = 95 cells in b, and n = 93 cells in c in each group). Each point represents a cell. The cells used for analyzed in each experiment were from a single replicate. n indicates the cell number used for quantification in each group. Data are representative of three independent experiments. Scale bars, 10 µm. d-i, FLAG-MRE11 (d and g), KU80 (e and h), or Flag-SHLD2 (f and i) accumulation at DSB sites induced by mCherry-LacR-FokI was examined using ChIP-qPCR in the indicated U2OS cells, respectively. The graphs represent mean ± s.d. Statistical analysis was performed using two-tailed unpaired t-tests; n = 3 biologically independent experiments. The blots data (g-i) are representative of three biologically independent experiments, with similar results obtained.
Extended Data Fig. 8 ASTE1 affects the resolution of the shieldin complex.
Representative micrographs and quantification of GFP-SHLD3 (a), REV7 (b), GFP-SHLD2 (c) and GFP-SHLD1 (d) IRIF at different time points in the indicated U2OS cell lines following irradiation treatment (5 Gy X-ray). The quantification graphs represent mean ± 95% CI. Statistical analysis was performed using two-tailed unpaired t-tests. 60 cells analyzed in each group were obtained from a single replicate. Data are representative of three independent experiments. Scale bars, 10 µm.
Extended Data Fig. 9 ASTE1 suppresses the extension of DNA end resection.
a, c and e, Representative micrographs and quantification of BrdU focus formation in the indicated U2OS cell lines with or without irradiation treatment (1 h after 10 Gy X-ray irradiation). The quantification graph represents mean ± 95% CI. Statistical analysis was performed using two-tailed unpaired t-tests. 90 cells analyzed in each group were obtained from a single replicate. Data are representative of three independent experiments. Scale bars, 10 µm. b, d and f, The indicated AsiSI-ER U2OS cells were treated with 300 nM 4-OHT for 4 h or mock-treated; DNA end resection was measured by qPCR. The graph represents mean ± s.d. Statistical analysis was performed using two-tailed unpaired t-tests. g, The knockdown efficiency of siRNAs or shRNAs used in a-f. The blots data are representative of three biologically independent experiments, with similar results obtained. h, Model of ASTE1 function. We propose that ASTE1 is recruited to ssDNA at DSB sites by the shieldin complex. Then ASTE1 cleaves the 3’ overhang ssDNA to suppress resection and favors NHEJ.
Extended Data Fig. 10 ASTE1 has no effect on B cells or T cells population.
a and b, Representative and quantification graphs of spleen (a) or bone marrow (b) CD19+B220+ B cell population of Aste1+/+ or Aste1−/− mice. The quantification graphs represent mean ± s.d., two-tailed, unpaired t-tests; n = 4 mice. c and d, Representative and quantification graphs of spleen (c) or bone marrow (d) CD4+ T cell population of Aste1+/+ or Aste1−/− mice. The quantification graphs represent mean ± s.d., two-tailed, unpaired t-tests; n = 4 mice. e and f, Representative and quantification graphs of spleen (e) or bone marrow (f) CD8+ T cell population of Aste1+/+ or Aste1−/− mice. The quantification graphs represent mean ± s.d., two-tailed, unpaired t-tests; n = 4 mice. g, Gating strategy to identify spleen CD45+CD4+ T cells. Similar gating strategy was applied to Extended Data Fig. 10a–f. h, Representative graphs of Aste1+/+ and Aste1−/− mice thymus. i, The weight of Aste1+/+ and Aste1−/− mice thymus (12-week old). The graph represents mean ± s.d., two-tailed, unpaired t-tests; n = 3 mice. j, Representative graphs of Aste1+/+ and Aste1−/− mice spleen. k, The weight of Aste1+/+ and Aste1−/− mice spleen (12-week old). The graph represents mean ± s.d., two-tailed, unpaired t-tests; n = 3 mice. l, Gating strategy to quantify cell cycle in Fig. 7f,g.
Supplementary information
Supplementary Tables
Supplementary Table 1: antibodies used for this study. Supplementary Table 2: shRNAs, siRNAs and sgRNAs used for this study.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Zhao, F., Kim, W., Gao, H. et al. ASTE1 promotes shieldin-complex-mediated DNA repair by attenuating end resection. Nat Cell Biol 23, 894–904 (2021). https://doi.org/10.1038/s41556-021-00723-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-021-00723-9
This article is cited by
-
Transmembrane nuclease NUMEN/ENDOD1 regulates DNA repair pathway choice at the nuclear periphery
Nature Cell Biology (2023)
-
Dynamics of the DYNLL1–MRE11 complex regulate DNA end resection and recruitment of Shieldin to DSBs
Nature Structural & Molecular Biology (2023)
-
Genomic instability genes in lung and colon adenocarcinoma indicate organ specificity of transcriptomic impact on Copy Number Alterations
Scientific Reports (2022)
-
METTL16 antagonizes MRE11-mediated DNA end resection and confers synthetic lethality to PARP inhibition in pancreatic ductal adenocarcinoma
Nature Cancer (2022)
-
LKB1 cooperates with Sox17 to drive metastasis
Nature Cell Biology (2021)