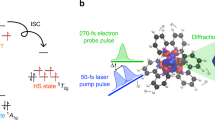
Abstract
It is well known that the solvent plays a critical role in ultrafast electron-transfer reactions. However, solvent reorganization occurs on multiple length scales, and selectively measuring short-range solute–solvent interactions at the atomic level with femtosecond time resolution remains a challenge. Here we report femtosecond X-ray scattering and emission measurements following photoinduced charge-transfer excitation in a mixed-valence bimetallic (FeiiRuiii) complex in water, and their interpretation using non-equilibrium molecular dynamics simulations. Combined experimental and computational analysis reveals that the charge-transfer excited state has a lifetime of 62 fs and that coherent translational motions of the first solvation shell are coupled to the back electron transfer. Our molecular dynamics simulations identify that the observed coherent translational motions arise from hydrogen bonding changes between the solute and nearby water molecules upon photoexcitation, and have an amplitude of tenths of ångströms, 120–200 cm−1 frequency and ~100 fs relaxation time. This study provides an atomistic view of coherent solvent reorganization mediating ultrafast intramolecular electron transfer.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The experimental data and the simulation results that support the findings of this study are available in Figshare with the identifier https://doi.org/10.6084/m9.figshare.13322975 (ref. 59).
Change history
24 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41557-021-00663-9
References
Marcus, R. & Sutin, N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta 811, 265–322 (1985).
Rossky, P. J. & Simon, J. D. Dynamics of chemical processes in polar solvents. Nature 370, 263–269 (1994).
Chen, P. & Meyer, T. J. Medium effects on charge transfer in metal complexes. Chem. Rev. 98, 1439–1478 (1998).
Carpenter, B. K., Harvey, J. N. & Orr-Ewing, A. J. The study of reactive intermediates in condensed phases. J. Am. Chem. Soc. 138, 4695–4705 (2016).
Kumpulainen, T., Lang, B., Rosspeintner, A. & Vauthey, E. Ultrafast elementary photochemical processes of organic molecules in liquid solution. Chem. Rev. 117, 10826–10939 (2017).
Raineri, F. O. & Friedman, H. L. Solvent Control of Electron Transfer Reactions 81–189 (John Wiley & Sons, 2007).
Barbara, P. F., Walker, G. C. & Smith, T. P. Vibrational modes and the dynamic solvent effect in electron and proton transfer. Science 256, 975–981 (1992).
Barbara, P. F., Meyer, T. J. & Ratner, M. A. Contemporary issues in electron transfer research. J. Phys. Chem. 100, 13148–13168 (1996).
Jimenez, R., Fleming, G. R., Kumar, P. V. & Maroncelli, M. Femtosecond solvation dynamics of water. Nature 369, 471–473 (1994).
Hsu, C.-P., Song, X. & Marcus, R. A. Time-dependent Stokes shift and its calculation from solvent dielectric dispersion data. J. Phys. Chem. B 101, 2546–2551 (1997).
de Boeij, W. P., Pshenichnikov, M. S. & Wiersma, D. A. Ultrafast solvation dynamics explored by femtosecond photon echo spectroscopies. Ann. Rev. Phys. Chem. 49, 99–123 (1998).
Underwood, D. F. & Blank, D. A. Ultrafast solvation dynamics: a view from the solvent’s perspective using a novel resonant-pump, nonresonant-probe technique. J. Phys. Chem. A 107, 956–961 (2003).
Petrone, A., Donati, G., Caruso, P. & Rega, N. Understanding THz and IR signals beneath time-resolved fluorescence from excited-state ab initio dynamics. J. Am. Chem. Soc. 136, 14866–14874 (2014).
Błasiak, B., Londergan, C. H., Webb, L. J. & Cho, M. Vibrational probes: from small molecule solvatochromism theory and experiments to applications in complex systems. Acc. Chem. Res. 50, 968–976 (2017).
Baiz, C. R. et al. Vibrational spectroscopic map, vibrational spectroscopy, and intermolecular interaction. Chem. Rev. 120, 7152–7218 (2020).
Nibbering, E. T. J. & Elsaesser, T. Ultrafast vibrational dynamics of hydrogen bonds in the condensed phase. Chem. Rev. 104, 1887–1914 (2004).
Bakker, H. J. & Skinner, J. L. Vibrational spectroscopy as a probe of structure and dynamics in liquid water. Chem. Rev. 110, 1498–1517 (2010).
Kuharski, R. A. et al. Molecular model for aqueous ferrous–ferric electron transfer. J. Chem. Phys. 89, 3248–3257 (1988).
Fonseca, T. & Ladanyi, B. M. Solvation dynamics in methanol: solute and perturbation dependence. J. Mol. Liq. 60, 1–24 (1994).
Maroncelli, M. & Fleming, G. R. Computer simulation of the dynamics of aqueous solvation. J. Chem. Phys. 89, 5044–5069 (1988).
Martini, I. B., Barthel, E. R. & Schwartz, B. J. Optical control of electrons during electron transfer. Science 293, 462–465 (2001).
Kim, K. H. et al. Direct observation of bond formation in solution with femtosecond X-ray scattering. Nature 518, 385–389 (2015).
Biasin, E. et al. Femtosecond X-ray scattering study of ultrafast photoinduced structural dynamics in solvated [Co(terpy)2]2+. Phys. Rev. Lett. 117, 013002 (2016).
Haldrup, K. et al. Ultrafast X-ray scattering measurements of coherent structural dynamics on the ground-state potential energy surface of a diplatinum molecule. Phys. Rev. Lett. 122, 063001 (2019).
Kunnus, K. et al. Vibrational wavepacket dynamics in Fe carbene photosensitizer determined with femtosecond X-ray emission and scattering. Nat. Commun. 11, 634 (2020).
van Driel, T. B. et al. Atomistic characterization of the active-site solvation dynamics of a model photocatalyst. Nat. Commun. 7, 13678 (2016).
Courtney, T. L., Fox, Z. W., Estergreen, L. & Khalil, M. Measuring coherently coupled intramolecular vibrational and charge-transfer dynamics with two-dimensional vibrational-electronic spectroscopy. J. Phys. Chem. Lett. 6, 1286–1292 (2015).
Reid, P. J., Silva, C., Barbara, P. F., Karki, L. & Hupp, J. T. Electronic coherence, vibrational coherence, and solvent degrees of freedom in the femtosecond spectroscopy of mixed-valence metal dimers in H2O and D2O. J. Phys. Chem. 99, 2609–2616 (1995).
Wang, C., Mohney, B. K., Akhremitchev, B. B. & Walker, G. C. Ultrafast infrared spectroscopy of vibrational states prepared by photoinduced electron transfer in (CN)5FeCNRu(NH3)5-. J. Phys. Chem. A 104, 4314–4320 (2000).
Son, D. H., Kambhampati, P., Kee, T. W. & Barbara, P. F. Femtosecond multicolor pump–probe study of ultrafast electron transfer of [(NH3)5RuiiiNCRuii(CN)5]- in aqueous solution. J. Phys. Chem. A 106, 4591–4597 (2002).
Timpson, C. J., Bignozzi, C. A., Sullivan, B. P., Kober, E. M. & Meyer, T. J. Influence of solvent on the spectroscopic properties of cyano complexes of ruthenium(II). J. Phys. Chem. 100, 2915–2925 (1996).
Lay, P. A., McAlpine, N. S., Hupp, J. T., Weaver, M. J. & Sargeson, A. M. Solvent-dependent redox thermodynamics of metal amine complexes. Delineation of specific solvation effects. Inorg. Chem. 29, 4322–4328 (1990).
Wang, C. et al. Solvent control of vibronic coupling upon intervalence charge transfer excitation of (CN)5FeCNRu(NH3)5- as revealed by resonance Raman and near-infrared absorption spectroscopies. J. Am. Chem. Soc. 120, 5848–5849 (1998).
Slenkamp, K. M. et al. Investigating vibrational anharmonic couplings in cyanide-bridged transition metal mixed valence complexes using two-dimensional infrared spectroscopy. J. Chem. Phys. 140, 084505 (2014).
Curtis, J. C., Sullivan, B. P. & Meyer, T. J. Hydrogen-bonding-induced solvatochromism in the charge-transfer transitions of ruthenium(II) and ruthenium(III) ammine complexes. Inorg. Chem. 22, 224–236 (1983).
Ross, M. et al. Comprehensive experimental and computational spectroscopic study of hexacyanoferrate complexes in water: from infrared to X-ray wavelengths. J. Phys. Chem. B 122, 5075–5086 (2018).
Kjær, K. S. et al. Finding intersections between electronic excited state potential energy surfaces with simultaneous ultrafast X-ray scattering and spectroscopy. Chem. Sci. 10, 5749–5760 (2019).
Glatzel, P. & Bergmann, U. High resolution 1s core hole X-ray spectroscopy in 3d transition metal complexes—electronic and structural information. Coord. Chem. Rev. 249, 65–95 (2005).
Dohn, A. O. et al. On the calculation of X-ray scattering signals from pairwise radial distribution functions. J. Phys. B 48, 244010 (2015).
Ihee, H. et al. Ultrafast X-ray diffraction of transient molecular structures in solution. Science 309, 1223–1227 (2005).
Kjær, K. S. et al. Introducing a standard method for experimental determination of the solvent response in laser pump, X-ray probe time-resolved wide-angle X-ray scattering experiments on systems in solution. Phys. Chem. Chem. Phys. 15, 15003–15016 (2013).
Barthel, E. R., Martini, I. B. & Schwartz, B. J. How does the solvent control electron transfer? Experimental and theoretical studies of the simplest charge transfer reaction. J. Phys. Chem. B 105, 12230–12241 (2001).
Aherne, D., Tran, V. & Schwartz, B. J. Nonlinear, nonpolar solvation dynamics in water: the roles of electrostriction and solvent translation in the breakdown of linear response. J. Phys. Chem. B 104, 5382–5394 (2000).
Fonseca, T. & Ladanyi, B. M. Breakdown of linear response for solvation dynamics in methanol. J. Phys. Chem. 95, 2116–2119 (1991).
Castner, E. W., Chang, Y. J., Chu, Y. C. & Walrafen, G. E. The intermolecular dynamics of liquid water. J. Chem. Phys. 102, 653–659 (1995).
Bragg, A. E., Cavanagh, M. C. & Schwartz, B. J. Linear response breakdown in solvation dynamics induced by atomic electron-transfer reactions. Science 321, 1817–1822 (2008).
Chapman, C. F., Fee, R. S. & Maroncelli, M. Measurements of the solute dependence of solvation dynamics in 1-propanol: the role of specific hydrogen-bonding interactions. J. Phys. Chem. 99, 4811–4819 (1995).
Sajadi, M., Weinberger, M., Wagenknecht, H.-A. & Ernsting, N. P. Polar solvation dynamics in water and methanol: search for molecularity. Phys. Chem. Chem. Phys. 13, 17768–17774 (2011).
Bellissent-Funel, M.-C. et al. Water determines the structure and dynamics of proteins. Chem. Rev. 116, 7673–7697 (2016).
Schirò, G. et al. Translational diffusion of hydration water correlates with functional motions in folded and intrinsically disordered proteins. Nat. Commun. 6, 6490 (2015).
Minitti, M. P. et al. Optical laser systems at the Linac Coherent Light Source. J. Synchrotron Radiat. 22, 526–531 (2015).
Philipp, H. T., Hromalik, M., Tate, M., Koerner, L. & Gruner, S. M. Pixel array detector for X-ray free electron laser experiments. Nucl. Instrum. Methods Phys. Res. A 649, 67–69 (2011).
Biasin, E. et al. Anisotropy enhanced X-ray scattering from solvated transition metal complexes. J. Synchrotron Radiat. 25, 306–315 (2018).
Silverstein, D. W., Govind, N., van Dam, H. J. & Jensen, L. Simulating one-photon absorption and resonance Raman scattering spectra using analytical excited state energy gradients within time-dependent density functional theory. J. Chem. Theory Comput. 9, 5490–5503 (2013).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Aprà, E. et al. NWChem: past, present, and future. J. Chem. Phys. 152, 184102 (2020).
Horn, H. W. et al. Development of an improved four-site water model for biomolecular simulations: TIP4P-Ew. J. Chem. Phys. 120, 9665 (2004).
Jorgensen et al., W. L. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Biasin, E., Schoenlein, R. W., Govind, N. & Khalil, M. Time-resolved X-ray scattering and spectroscopy data and MD simulations reveal solvent motions coupled to intramolecular elecron transfer. Figshare https://doi.org/10.6084/m9.figshare.13322975 (2020).
Acknowledgements
This work was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division under award nos DE-SC0012450 (Z.W.F., J.M.C., J.D.G., Y.Z., S.M. and M.K.), DE-SC0019277 (C.L.-S. and M.K.), DE-FG02-04ER15571 (S.M.), KC-030105066418 (N.G.), KC-030105172685 (N.G.), DE-AC02-76SF00515 (E.B., K.L., K.S.K, K.H., J.H.L., M.R., K.J.G., R.W.S. and A.A.C) and DE-AC02-06CH11357 (G.D., A.M.M. and S.H.S.). J.D.G. acknowledges support by the National Science Foundation Graduate Research Fellowship Program (no. DGE-1256082). Use of the Linac Coherent Light Source, SLAC National Accelerator Laboratory, is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-76SF00515. This research used resources of the Advanced Photon Source, a US Department of Energy Office of Science User Facility operated for the US Department of Energy Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. This research benefited from computational resources provided by the Environmental Molecular Sciences Laboratory, a US Department of Energy Office of Science User Facility sponsored by the Office of Biological and Environmental Research and located at the Pacific Northwest National Laboratory. The Pacific Northwest National Laboratory is operated by Battelle Memorial Institute for the US Department of Energy under US Department of Energy contract no. DE-AC05-76RL1830. This research also used resources from the National Energy Research Scientific Computing Center, a US Department of Energy Office of Science User Facility operated under contract no. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
Z.W.F., K.S.K., R.A.-M., M.C., J.D.G., J.M.G., K.H., T.K., J.H.L., M.R., D.S., R.W.S., N.G., A.A.C. and M.K. prepared and conducted the experiment at the Linac Coherent Light Source. Z.W.F., K.H., J.H.L., G.D., A.M.M., S.H.S. and A.A.C. measured the reference iron spectra at the Advanced Photon Source. J.M.C. synthesized the sample. E.B. analysed the experimental data and performed the MD simulations. E.B and K.L. analysed the results from the MD simulations. A.A., Y.Z., S.M. and N.G. performed the quantum-mechanics/molecular-mechanics, density functional theory and TDDFT calculations. E.B., K.L., C.L.-S., K.J.G., R.W.S., N.G., A.A.C. and M.K. interpreted the results. E.B. and A.A.C. wrote the article with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Mark Maroncelli, Simone Techert and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussions 1–9, Figs. 1–24 and Tables 1–2.
Rights and permissions
About this article
Cite this article
Biasin, E., Fox, Z.W., Andersen, A. et al. Direct observation of coherent femtosecond solvent reorganization coupled to intramolecular electron transfer. Nat. Chem. 13, 343–349 (2021). https://doi.org/10.1038/s41557-020-00629-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00629-3
This article is cited by
-
Observing the primary steps of ion solvation in helium droplets
Nature (2023)
-
Ferricyanide photo-aquation pathway revealed by combined femtosecond Kβ main line and valence-to-core x-ray emission spectroscopy
Nature Communications (2023)
-
Revealing core-valence interactions in solution with femtosecond X-ray pump X-ray probe spectroscopy
Nature Communications (2023)
-
Determining the charge distribution and the direction of bond cleavage with femtosecond anisotropic x-ray liquidography
Nature Communications (2022)
-
Filming ultrafast roaming-mediated isomerization of bismuth triiodide in solution
Nature Communications (2021)