Abstract
Host–pathogen interactions impose recurrent selective pressures that lead to constant adaptation and counter-adaptation in both competing species. Here, we sought to study this evolutionary arms-race and assessed the impact of the innate immune system on viral population diversity and evolution, using Drosophila melanogaster as model host and its natural pathogen Drosophila C virus (DCV). We isogenized eight fly genotypes generating animals defective for RNAi, Imd and Toll innate immune pathways as well as pathogen-sensing and gut renewal pathways. Wild-type or mutant flies were then orally infected with DCV and the virus was serially passaged ten times via reinfection in naive flies. Viral population diversity was studied after each viral passage by high-throughput sequencing and infection phenotypes were assessed at the beginning and at the end of the evolution experiment. We found that the absence of any of the various immune pathways studied increased viral genetic diversity while attenuating virulence. Strikingly, these effects were observed in a range of host factors described as having mainly antiviral or antibacterial functions. Together, our results indicate that the innate immune system as a whole and not specific antiviral defence pathways in isolation, generally constrains viral diversity and evolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All raw data from high-throughput sequencing were deposited to NCBI BioProjects under accession number PRJNA782868. Source data are provided with this paper.
Code availability
Scripts are provided in Supplementary Data 1.
References
Morgan, A. D. & Koskella, B. in Genetics and Evolution of Infectious Diseases (ed. Tibayrenc, M.) 115–140 (Elsevier, 2017).
Daugherty, M. D. & Malik, H. S. Rules of engagement: molecular insights from host–virus arms races. Annu. Rev. Genet. 46, 677–700 (2012).
Barreiro, L. B. & Quintana-Murci, L. From evolutionary genetics to human immunology: how selection shapes host defence genes. Nat. Rev. Genet. 11, 17–30 (2010).
Thompson, J. N. & Burdon, J. J. Gene-for-gene coevolution between plants and parasites. 360, 121–125 (1992).
Buckling, A. & Rainey, P. B. Antagonistic coevolution between a bacterium and a bacteriophage. Proc. Biol. Sci. 269, 931–936 (2002).
Masri, L. et al. Host–pathogen coevolution: the selective advantage of Bacillus thuringiensis virulence and its Cry toxin genes. PLoS Biol. 13, e1002169 (2015).
Obbard, D. J., Gordon, K. H. J., Buck, A. H. & Jiggins, F. M. The evolution of RNAi as a defence against viruses and transposable elements. Philos. Trans. R. Soc. B 364, 99–115 (2009).
Lazzaro, B. P. Natural selection on the Drosophila antimicrobial immune system. Curr. Opin. Microbiol. 11, 284–289 (2008).
Lazzaro, B. P. Molecular population genetics of inducible antibacterial peptide genes in Drosophila melanogaster. Mol. Biol. Evol. 20, 914–923 (2003).
Sackton, T. B. et al. Dynamic evolution of the innate immune system in Drosophila. Nat. Genet. 39, 1461–1468 (2007).
Brackney, D. E., Beane, J. E. & Ebel, G. D. RNAi targeting of West Nile virus in mosquito midguts promotes virus diversification. PLoS Pathog. 5, e1000502 (2009).
Lin, S.-S. et al. Molecular evolution of a viral non-coding sequence under the selective pressure of amiRNA-mediated silencing. PLoS Pathog. 5, e1000312 (2009).
Lafforgue, G. et al. Tempo and mode of plant RNA virus escape from RNA interference-mediated resistance. J. Virol. 85, 9686–9695 (2011).
Martínez, F. et al. Ultradeep sequencing analysis of population dynamics of virus escape mutants in RNAi-mediated resistant plants. Mol. Biol. Evol. 29, 3297–3307 (2012).
Das, A. T. et al. Human immunodeficiency virus type 1 escapes from RNA interference-mediated inhibition. J. Virol. 78, 2601–2605 (2004).
Gitlin, L., Stone, J. K. & Andino, R. Poliovirus escape from RNA interference: short interfering RNA-target recognition and implications for therapeutic approaches. J. Virol. 79, 1027–1035 (2005).
Mondotte, J. A. & Saleh, M.-C. Antiviral immune response and the route of infection in Drosophila melanogaster. Adv. Virus Res. 100, 247–278 (2017).
Swevers, L., Liu, J. & Smagghe, G. Defense mechanisms against viral infection in Drosophila: RNAi and non-RNAi. Viruses 10, 230 (2018).
Galiana-Arnoux, D., Dostert, C., Schneemann, A., Hoffmann, J. A. & Imler, J.-L. Essential function in vivo for Dicer-2 in host defense against RNA viruses in Drosophila. Nat. Immunol. 7, 590–597 (2006).
van Rij, R. P. et al. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 20, 2985–2995 (2006).
Wang, X.-H. et al. RNA interference directs innate immunity against viruses in adult Drosophila. Science 312, 452–454 (2006).
Zambon, R. A., Vakharia, V. N. & Wu, L. P. RNAi is an antiviral immune response against a dsRNA virus in Drosophila melanogaster. Cell. Microbiol. 8, 880–889 (2006).
Costa, A., Jan, E., Sarnow, P. & Schneider, D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS ONE 4, e7436 (2009).
Sansone, C. L. et al. Microbiota-dependent priming of antiviral intestinal immunity in Drosophila. Cell Host Microbe 18, 571–581 (2015).
Ferreira, Á. G. et al. The Toll–Dorsal pathway is required for resistance to viral oral infection in Drosophila. PLoS Pathog. 10, e1004507 (2014).
Zambon, R. A., Nandakumar, M., Vakharia, V. N. & Wu, L. P. The Toll pathway is important for an antiviral response in Drosophila. Proc. Natl Acad. Sci. USA 102, 7257–7262 (2005).
Dostert, C. et al. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat. Immunol. 6, 946–953 (2005).
Merkling, S. H. et al. The epigenetic regulator G9a mediates tolerance to RNA virus infection in Drosophila. PLoS Pathog. 11, e1004692 (2015).
Christian, P. D. & Johnson, K. N. The novel genome organization of the insect picorna-like virus Drosophila C virus suggests this virus belongs to a previously undescribed virus family. J. Gen. Virol. 79, 191–203 (1998).
Jousset, F. X. & Plus, N. Study of the vertical transmission and horizontal transmission of Drosophila melanogaster and Drosophila immigrans picornavirus (author’s translation). Ann. Microbiol. 126, 231–249 (1975).
Mondotte, J. A. et al. Immune priming and clearance of orally acquired RNA viruses in Drosophila. Nat. Microbiol. 3, 1394–1403 (2018).
Torri, A., Mongelli, V., Mondotte, J. A. & Saleh, M.-C. Viral infection and stress affect protein levels of Dicer 2 and Argonaute 2 in Drosophila melanogaster. Front. Immunol. 11, 362 (2020).
Deddouche, S. et al. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in Drosophila. Nat. Immunol. 9, 1425–1432 (2008).
Gomariz-Zilber, E., Jeune, B. & Thomas-Orillard, M. Limiting conditions of the horizontal transmission of the Drosophila C virus in its host (D. melanogaster). Acta Oecol. 19, 125–137 (1998).
Stevanovic, A. L. & Johnson, K. N. Infectivity of Drosophila C virus following oral delivery in Drosophila larvae. J. Gen. Virol. 96, 1490–1496 (2015).
Royet, J. Epithelial homeostasis and the underlying molecular mechanisms in the gut of the insect model Drosophila melanogaster. Cell. Mol. Life Sci. 68, 3651–3660 (2011).
Buchon, N., Broderick, N. A., Poidevin, M., Pradervand, S. & Lemaitre, B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5, 200–211 (2009).
Buchon, N., Broderick, N. A., Chakrabarti, S. & Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 23, 2333–2344 (2009).
Buchon, N., Broderick, N. A., Kuraishi, T. & Lemaitre, B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 8, 152 (2010).
Xu, J. et al. ERK signaling couples nutrient status to antiviral defense in the insect gut. Proc. Natl Acad. Sci. USA 110, 15025–15030 (2013).
Lauring, A. S. & Andino, R. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog. 6, e1001005 (2010).
Isakov, O. et al. Deep sequencing analysis of viral infection and evolution allows rapid and detailed characterization of viral mutant spectrum. Bioinformatics 31, 2141–2150 (2015).
Hartl, D. L. & Clark, A. G. Principles of Population Genetics 4th edn (Sinauer Associates, 2006).
Travisano, M., Mongold, J. A., Bennett, A. F. & Lenski, R. E. Experimental tests of the roles of adaptation, chance, and history in evolution. Science 267, 87–90 (1995).
Desai, M. M. & Fisher, D. S. Beneficial mutation–selection balance and the effect of linkage on positive selection. Genetics 176, 1759–1798 (2007).
Miralles, R. Clonal interference and the evolution of RNA viruses. Science 285, 1745–1747 (1999).
Miralles, R., Moya, A. & Elena, S. F. Diminishing returns of population size in the rate of RNA virus adaptation. J. Virol. 74, 3566–3571 (2000).
Pepin, K. M. & Wichman, H. A. Experimental evolution and genome sequencing reveal variation in levels of clonal interference in large populations of bacteriophage φX174. BMC Evol. Biol. 8, 85 (2008).
Navarro, R., Ambrós, S., Martínez, F. & Elena, S. F. Diminishing returns of inoculum size on the rate of a plant RNA virus evolution. Europhys. Lett. 120, 38001 (2017).
Pandit, A. & de Boer, R. J. Reliable reconstruction of HIV-1 whole genome haplotypes reveals clonal interference and genetic hitchhiking among immune escape variants. Retrovirology 11, 56 (2014).
Strelkowa, N. & Lässig, M. Clonal interference in the evolution of influenza. Genetics 192, 671–682 (2012).
Gerrish, P. J. & Lenski, R. E. in Mutation and Evolution (eds Woodruff, R. C. & Thompson, J. N.) 127–144 (Springer, 1998).
Held, T., Klemmer, D. & Lässig, M. Survival of the simplest in microbial evolution. Nat. Commun. 10, 2472 (2019).
Novella, I. S., Zárate, S., Metzgar, D. & Ebendick-Corpus, B. E. Positive selection of synonymous mutations in vesicular stomatitis virus. J. Mol. Biol. 342, 1415–1421 (2004).
Zanini, F. & Neher, R. A. Quantifying selection against synonymous mutations in HIV-1 env evolution. J. Virol. 87, 11843–11850 (2013).
Martínez, M. A., Jordan-Paiz, A., Franco, S. & Nevot, M. Synonymous virus genome recoding as a tool to impact viral fitness. Trends Microbiol. 24, 134–147 (2016).
Kieft, J. S., Zhou, K., Jubin, R. & Doudna, J. A. Mechanism of ribosome recruitment by hepatitis C IRES RNA. RNA 7, 194–206 (2001).
Zhang, L. et al. lncRNA sensing of a viral suppressor of RNAi activates non-canonical innate immune signaling in Drosophila. Cell Host Microbe 27, 115–128 (2020).
Navarro, R. et al. Defects in plant immunity modulate the rates and patterns of RNA virus evolution. Preprint at bioRxiv https://doi.org/10.1101/2020.10.13.337402 (2021).
Merkling, S. H. & van Rij, R. P. Analysis of resistance and tolerance to virus infection in Drosophila. Nat. Protoc. 10, 1084–1097 (2015).
Lee, Y. S. et al. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117, 69–81 (2004).
Okamura, K. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 18, 1655–1666 (2004).
Levashina, E. A., Ohresser, S., Lemaitre, B. & Imler, J. L. Two distinct pathways can control expression of the gene encoding the Drosophila antimicrobial peptide metchnikowin. J. Mol. Biol. 278, 515–527 (1998).
Rutschmann, S. et al. The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity 12, 569–580 (2000).
Hedengren, M. et al. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol. Cell 4, 827–837 (1999).
Díaz-Benjumea, F. J. & García-Bellido, A. Behaviour of cells mutant for an EGF receptor homologue of Drosophila in genetic mosaics. Proc. Biol. Sci. 242, 36–44 (1990).
Reed, L. J. & Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 27, 493–497 (1938).
Hothorn, T., Bretz, F. & Westfall, P. Simultaneous inference in general parametric models. Biom. J. 50, 346–363 (2008).
Fox, J. & Weisberg S. An R Companion to Applied Regression 3rd edn (Sage, 2019).
Lenth, R. V. et al. Estimated Marginal Means, aka Least-Squares Means. R package version 1.5.5 (2021).
Wickham, H. et al. Welcome to the Tidyverse. J. Open Source Softw. 4, 1686 (2019).
Cornman, R. S. et al. Population-genomic variation within RNA viruses of the western honey bee, Apis mellifera, inferred from deep sequencing. BMC Genomics 14, 154 (2013).
Lequime, S., Fontaine, A., Ar Gouilh, M., Moltini-Conclois, I. & Lambrechts, L. Genetic drift, purifying selection and vector genotype shape Dengue virus intra-host genetic diversity in mosquitoes. PLoS Genet. 12, e1006111 (2016).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Myers, M. A., Satas, G. & Raphael, B. J. CALDER: inferring phylogenetic trees from longitudinal tumor samples. Cell Syst. 8, 514–522 (2019).
Smith, M. A. et al. E-scapescape: interactive visualization of single-cell phylogenetics and cancer evolution. Nat. Methods 14, 549–550 (2017).
Acknowledgements
We thank members of the Saleh Lab, M. Vignuzzi and J. Pfeiffer for fruitful discussions. We thank C. Meignin for RelE20 and VagoΔM10 flies. This work was supported by the European Research Council (FP7/2013–2019 ERC CoG 615220) and the French Government’s Investissement d’Avenir programme, Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases (grant no. ANR-10-LABX-62-IBEID) to M.-C.S. Work in S.F.E.’s laboratory was supported by grant nos BFU2015-65037-P and PID2019-103998GB-I00 (Spain Agencia Estatal de Investigación—FEDER) and PROMETEU2019/012 (Generalitat Valenciana).
Author information
Authors and Affiliations
Contributions
V.M. and M.-C.S. conceived the study. V.M., M.-C.S., A.K. and L.Q.-M. established the experimental design. V.M., V.G., H.B and J.N. performed the investigations. S.L. and S.F.E. performed the formal analyses. V.M., S.F.E. and M.-C.S. wrote the paper and acquired funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Guan-Zhu Han and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
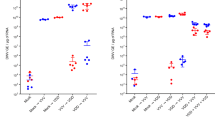
Extended Data Fig. 1 Viral load and prevalence across the DCV evolution experiment.
Viral load of 10 individual flies coming from DCV inoculated cages and four individual flies coming from mock inoculated cages was determined by TCID50. a) Prevalence, calculated as the percentage of flies positive by TCID50. b) Viral load determined by TCID50 in each genotype across the 10 DCV passages. c) DCV replication assessed by negative-strand RT–qPCR. Left panel: standard curve produced from a tenfold dilution series over a range from 108 to 103 copies per reaction of in vitro transcribed RNA corresponding to a portion of the full-length negative-strand DCV RNA (slope = −3.644, R2 = 0.990, efficiency = 88.25%). Right panel: amount of negative-strand DCV RNA present in the viral stocks produced from each fly genotype in P10, S2 DCV stock and DCV stock. Mock-infected flies were added as controls. LOD: Limit of detection of DCV negative stranded RNA. d) Average viral loads per individual fly of each genotype estimated from the GLM fitted to the data shown in panel b. Error bars represent ±1 SD.
Extended Data Fig. 2 Grouping of DCV population swarms by similarity and increasing nucleotide diversity (π).
Viral nucleotide diversity (π) was determined in each condition and grouped using a post hoc Bonferroni test based on the pairwise comparisons from Supplementary Table 1. SE: standard error. asymp.LCL: asymptomatic lower confidence level; asymp.UCL: asymptomatic upper confidence level.
Extended Data Fig. 3 Evolution of DCV variants.
a) Trajectories of DCV variants across passages, N: total number of SPNs found above the estimated frequency threshold (≥ 0.0028). Trajectories of viral variants found significant after FDR correction are show in green (p ≤ 0.006) and yellow (0,047 ≤ p ≤ 0.006) (based on data from Table 2). b) to k) Heatmaps showing the Pearson correlation coefficients between mutations’ frequencies along evolutionary time, ranging from blue, where no linkage between the SNPs was found, to red, where the SNPs were linked in a same viral haplotype.
Extended Data Fig. 4 SNPs on the DCV genome with significant estimates of fitness effects.
Green triangles represent synonymous mutations, pink triangles non-synonymous mutations and grey triangles mutations in non-coding sequences. Cases significant after FDR correction (p ≤ 0.006) are marked with an asterisk (based on data from Table 2).
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Tables 1 and 2.
Supplementary Data 1
R scripts used to estimate mutational fitness effects and their linkage (measured as correlation coefficients along the time-series data and as LVAFFP).
Source data
Source Data Fig. 2
Contains the DCV variants detected above the allele frequency threshold of 0.0028 determined.
Source Data Fig. 3
Contains the DCV variants detected above the allele frequency threshold of 0.0028 determined.
Source Data Fig. 4
Contains the raw data of the survival curves of the flies after DCV infection.
Source Data Extended Data Fig. 1
Contains the viral load and prevalence values, as well as qPCR results.
Source Data Extended Data Fig. 2
Contains the DCV variants detected above the allele frequency threshold of 0.0028 determined.
Source Data Extended Data Fig. 3
Contains the DCV variants detected above the allele frequency threshold of 0.0028 determined.
Rights and permissions
About this article
Cite this article
Mongelli, V., Lequime, S., Kousathanas, A. et al. Innate immune pathways act synergistically to constrain RNA virus evolution in Drosophila melanogaster. Nat Ecol Evol 6, 565–578 (2022). https://doi.org/10.1038/s41559-022-01697-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01697-z
This article is cited by
-
Origins and diversification of animal innate immune responses against viral infections
Nature Ecology & Evolution (2023)
-
Emergence of a deletion mutant of GFP-expressing plantago asiatica mosaic virus that has overcome acibenzolar-S-methyl-induced defense response against its long-distance movement
Journal of General Plant Pathology (2023)