Abstract
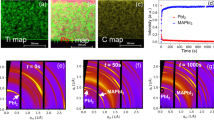
The extraordinary optoelectronic performance of hybrid organic–inorganic perovskites has resulted in extensive efforts to unravel their properties. Recently, observations of ferroic twin domains in methylammonium lead triiodide drew significant attention as a possible explanation for the current–voltage hysteretic behaviour in these materials. However, the properties of the twin domains, their local chemistry and the chemical impact on optoelectronic performance remain unclear. Here, using multimodal chemical and functional imaging methods, we unveil the mechanical origin of the twin domain contrast observed with piezoresponse force microscopy in methylammonium lead triiodide. By combining experimental results with first principles simulations we reveal an inherent coupling between ferroelastic twin domains and chemical segregation. These results reveal an interplay of ferroic properties and chemical segregation on the optoelectronic performance of hybrid organic–inorganic perovskites, and offer an exploratory path to improving functional devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Burschka, J. et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499, 316–319 (2013).
Dong, R. et al. High‐gain and low‐driving‐voltage photodetectors based on organolead triiodide perovskites. Adv. Mater. 27, 1912–1918 (2015).
Su, L. et al. Photoinduced enhancement of a triboelectric nanogenerator based on an organolead halide perovskite. J. Mater. Chem. C 4, 10395–10399 (2016).
Dou, L. et al. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 5, 5404 (2014).
Rashkeev, S. N., El-Mellouhi, F., Kais, S. & Alharbi, F. H. Domain walls conductivity in hybrid organometallic perovskites and their essential role in CH3NH3PbI3 solar cell high performance. Sci. Rep. 5, 11467 (2015).
Liu, S. et al. Ferroelectric domain wall induced band gap reduction and charge separation in organometal halide perovskites. J. Phys. Chem. Lett. 6, 693–699 (2015).
Bi, F. et al. Enhanced photovoltaic properties induced by ferroelectric domain structures in organometallic halide perovskites. J. Phys. Chem. C 121, 11151–11158 (2017).
Choi, K. J. et al. Enhancement of ferroelectricity in strained BaTiO3 thin films. Science 306, 1005–1009 (2004).
Li, Q. et al. Probing local bias-induced transitions using photothermal excitation contact resonance atomic force microscopy and voltage spectroscopy. ACS Nano 9, 1848–1857 (2015).
Balke, N. et al. Exploring local electrostatic effects with scanning probe microscopy: implications for piezoresponse force microscopy and triboelectricity. ACS Nano 8, 10229–10236 (2014).
Hermes, I. M. et al. Ferroelastic fingerprints in methylammonium lead iodide perovskite. J. Phys. Chem. C 120, 5724–5731 (2016).
Strelcov, E. et al. CH3NH3PbI3 perovskites: ferroelasticity revealed. Sci. Adv. 3, e1602165 (2017).
Röhm, H., Leonhard, T., Hoffmann, M. J. & Colsmann, A. Ferroelectric domains in methylammonium lead iodide perovskite thin-films. Energy Environ. Sci. 10, 950–955 (2017).
MacDonald, G. A. et al. Determination of the true lateral grain size in organic–inorganic halide perovskite thin films. ACS Appl. Mater. Interfaces 9, 33565–33570 (2017).
Huang, B. et al. Ferroic domains of alternating polar and nonpolar orders regulate photocurrent in single crystalline CH3NH3PbI3 films self-grown on FTO/TiO2 substrate. Preprint at http://arXiv/cond-mat.mtrl-sci/1801.08305 (2018).
Rothmann, M. U. et al. Direct observation of intrinsic twin domains in tetragonal CH3NH3PbI3. Nat. Commun. 8, 14547 (2017).
Nambu, S. & Sagala, D. A. Domain formation and elastic long-range interaction in ferroelectric perovskites. Phys. Rev. B 50, 5838–5847 (1994).
Salje, E. & Ishibashi, Y. Mesoscopic structures in ferroelastic crystals: needle twins and right-angled domains. J. Phys. Condens. Mat. 8, 8477–8495 (1996).
Mao, J. et al. High thermoelectric power factor in Cu–Ni alloy originate from potential barrier scattering of twin boundaries. Nano Energy 17, 279–289 (2015).
Vasudevan, R. K., Balke, N., Maksymovych, P., Jesse, S. & Kalinin, S. V. Ferroelectric or non-ferroelectric: why so many materials exhibit ‘ferroelectricity’ on the nanoscale. Appl. Phys. Rev. 4, 021302 (2017).
Xie, W., Tang, X., Yan, Y., Zhang, Q. & Tritt, T. M. High thermoelectric performance BiSbTe alloy with unique low-dimensional structure. J. Appl. Phys. 105, 113713 (2009).
Lee, K. & Baik, S. Ferroelastic domain structure and switching in epitaxial ferroelectric thin films. Annu. Rev. Mater. Res. 36, 81–116 (2006).
Dong, Q. et al. Electron–hole diffusion lengths >175 μm in solution-grown CH3NH3PbI3 single crystals. Science 347, 967–970 (2015).
Jesse, S., Mirman, B. & Kalinin, S. V. Resonance enhancement in piezoresponse force microscopy: mapping electromechanical activity, contact stiffness, and Q factor. Appl. Phys. Lett. 89, 022906 (2006).
Jesse, S., Kalinin, S. V., Proksch, R., Baddorf, A. & Rodriguez, B. The band excitation method in scanning probe microscopy for rapid mapping of energy dissipation on the nanoscale. Nanotechnology 18, 435503 (2007).
Jesse, S. et al. Band excitation in scanning probe microscopy: recognition and functional imaging. Ann. Rev. Phys. Chem. 65, 519–536 (2014).
Ahmadi, M. et al. Exploring anomalous polarization dynamics in organometallic halide perovskites. Adv. Mater. 30, 1705298 (2018).
Gannepalli, A., Yablon, D., Tsou, A. & Proksch, R. Mapping nanoscale elasticity and dissipation using dual frequency contact resonance AFM. Nanotechnology 22, 355705 (2011).
Collins, L. et al. Breaking the limits of structural and mechanical imaging of the heterogeneous structure of coal macerals. Nanotechnology 25, 435402 (2014).
Labuda, A. & Proksch, R. Quantitative measurements of electromechanical response with a combined optical beam and interferometric atomic force microscope. Appl. Phys. Lett. 106, 253103 (2015).
Hawash, Z. et al. Interfacial modification of perovskite solar cells using an ultrathin MAI layer leads to enhanced energy level alignment, efficiencies, and reproducibility. J. Phys. Chem. Lett. 8, 3947–3953 (2017).
Wirtz, T. et al. Towards secondary ion mass spectrometry on the helium ion microscope: An experimental and simulation based feasibility study with He+ and Ne+ bombardment. Appl. Phys. Lett. 101, 041601 (2012).
Dowsett, D. & Wirtz, T. Co-registered in situ secondary electron and mass spectral imaging on the helium ion microscope demonstrated using lithium titanate and magnesium oxide nanoparticles. Anal. Chem. 89, 8957–8965 (2017).
Gratia, P. et al. Intrinsic halide segregation at nanometer scale determines the high efficiency of mixed cation/mixed halide perovskite solar cells. J. Am. Chem. Soc. 138, 15821–15824 (2016).
Gratia, P. et al. The many faces of mixed ion perovskites: unraveling and understanding the crystallization process. ACS Energy Lett. 2, 2686–2693 (2017).
Glaser, T. et al. Infrared spectroscopic study of vibrational modes in methylammonium lead halide perovskites. J. Phys. Chem. Letters 6, 2913–2918 (2015).
Watson, B. R. et al. Elucidation of perovskite film micro-orientations using two-photon total internal reflectance fluorescence microscopy. J. Phys. Chem. Lett. 6, 3283–3288 (2015).
Täuber, D., Dobrovolsky, A., Camacho, R. & Scheblykin, I. G. Exploring the electronic band structure of organometal halide perovskite via photoluminescence anisotropy of individual nanocrystals. Nano Lett. 16, 5087–5094 (2016).
Motta, C. et al. Revealing the role of organic cations in hybrid halide perovskite CH3NH3PbI3. Nat. Commun. 6, 7026 (2015).
Ziębińska, A., Rytz, D., Szot, K., Górny, M. & Roleder, K. Birefringence above T c in single crystals of barium titanate. J. Phys. Condens. Mat. 20, 142202 (2008).
Banfi, G., Calvi, P. & Giulotto, E. Spontaneous and field-assisted transition in K0.984Li0.016TaO3: the polar pattern by birefringence and second-harmonic generation. Phys. Rev. B 51, 6231–6236 (1995).
Hu, Y. H., Chan, H. M., Wen, Z. X. & Harmer, M. P. Scanning electron microscopy and transmission electron microscopy study of ferroelectric domains in doped BaTiO3. J. Am. Ceram. Soc. 69, 594–602 (1986).
Zhao, J. et al. Single crystalline CH3NH3PbI3 self-grown on FTO/TiO2 substrate for high efficiency perovskite solar cells. Sci. Bull. 62, 1163–1226 (2017).
Wirtz, T., Philipp, P., Audinot, J., Dowsett, D. & Eswara, S. High-resolution high-sensitivity elemental imaging by secondary ion mass spectrometry: from traditional 2D and 3D imaging to correlative microscopy. Nanotechnology 26, 434001 (2015).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 41, 7892–7895 (1990).
Kresse, G. & Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Mat 6, 8245–8257 (1994).
Klimeš, J., Bowler, D. R. & Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 83, 195131 (2011).
Dion, M., Rydberg, H., Schröder, E., Langreth, D. C. & Lundqvist, B. I. Van der Waals density functional for general geometries. Phys. Rev. Lett. 92, 246401 (2004).
Zhang, L. & Sit, P. H.-L. Ab initio study of interaction of water, hydroxyl radicals, and hydroxide ions with CH3NH3PbI3 and CH3NH3PbBr3 surfaces. J. Phys. Chem. C 119, 22370–22378 (2015).
Stoumpos, C. C., Malliakas, C. D. & Kanatzidis, M. G. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 52, 9019–9038 (2013).
Acknowledgements
This research was supported by the Laboratory Directed Research and Development Program of Oak Ridge National Laboratory, managed by UT-Battelle, LLC, for the US Department of Energy (Y.L., A.I., B.D. and O.S.O.). The research was partially sponsored by the Air Force Office of Scientific Research (AFOSR) under grant no. FA 9550-15-1-0064, AOARD (FA2386-15-1-4104), and the National Science Foundation CBET-1438181 (M.A. and B.H.) and supported by the University of Tennessee, Knoxville (B.R.W. and T.R.C.). This research was conducted at the Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility.
Author information
Authors and Affiliations
Contributions
O.S.O., S.V.K., B.H., S.T.R, K.X., M.A., L.C. and Y.L. conceived the project and O.S.O. directed the experiments. Y.L. prepared the samples and performed the SEM measurements. Y.L. performed the scanning probe microscopy measurements with help from L.C. and the NSIR measurements with help from S.K. and A.I. R.P. developed the LDV–PFM and S.J. developed the band excitation scanning probe microscopy and analysis tools. S.K. performed the HIM–SIMS measurements with help from A.B. B.D. performed the TIRFM measurements and T.R.C. and B.R.W. helped to develop the TIRFM technique. J.H. and B.G.S. performed the DFT simulations. Y.L., L.C. and O.S.O. wrote the manuscript. All the authors contributed to the discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary Figures 1–10
Rights and permissions
About this article
Cite this article
Liu, Y., Collins, L., Proksch, R. et al. Chemical nature of ferroelastic twin domains in CH3NH3PbI3 perovskite. Nature Mater 17, 1013–1019 (2018). https://doi.org/10.1038/s41563-018-0152-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-018-0152-z
This article is cited by
-
Crystal structures, phase transitions, thermodynamics, and molecular dynamics of organic–inorganic hybrid crystal [NH(CH3)3]2ZnCl4
Scientific Reports (2024)
-
Suppressing non-radiative recombination in metal halide perovskite solar cells by synergistic effect of ferroelasticity
Nature Communications (2023)
-
Photocarrier-induced persistent structural polarization in soft-lattice lead halide perovskites
Nature Nanotechnology (2023)
-
Ferroelectric order in hybrid organic-inorganic perovskite NH4PbI3 with non-polar molecules and small tolerance factor
npj Computational Materials (2023)
-
Evidence for polarization-induced phase transformations and degradation in CH3NH3PbI3
Nano Research (2023)