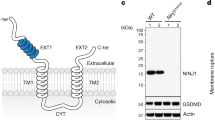
Abstract
Plasma membrane integrity is essential for the viability of eukaryotic cells. In response to bacterial pore-forming toxins, disrupted regions of the membrane are rapidly repaired. However, the pathways that mediate plasma membrane repair are unclear. Here we show that autophagy-related (ATG) protein ATG16L1 and its binding partners ATG5 and ATG12 are required for plasma membrane repair through a pathway independent of macroautophagy. ATG16L1 is required for lysosome fusion with the plasma membrane and blebbing responses that promote membrane repair. ATG16L1 deficiency causes accumulation of cholesterol in lysosomes that contributes to defective membrane repair. Cell-to-cell spread by Listeria monocytogenes requires membrane damage by the bacterial toxin listeriolysin O, which is restricted by ATG16L1-dependent membrane repair. Cells harbouring the ATG16L1 T300A allele associated with inflammatory bowel disease were also found to accumulate cholesterol and be defective in repair, linking a common inflammatory disease to plasma membrane integrity. Thus, plasma membrane repair could be an important therapeutic target for the treatment of bacterial infections and inflammatory disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Dal Peraro, M. & van der Goot, F. G. Pore-forming toxins: ancient, but never really out of fashion. Nat. Rev. Microbiol. 14, 77–92 (2016).
Babiychuk, E. B. & Draeger, A. Defying death: cellular survival strategies following plasmalemmal injury by bacterial toxins. Semin. Cell. Dev. Biol. 45, 39–47 (2015).
Cassidy, S. K. & O’Riordan, M. X. More than a pore: the cellular response to cholesterol-dependent cytolysins. Toxins (Basel) 5, 618–636 (2013).
Osborne, S. E. & Brumell, J. H. Listeriolysin O: from bazooka to Swiss army knife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160222 (2017).
Birmingham, C. L. et al. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature 451, 350–354 (2008).
Beauregard, K. E., Lee, K. D., Collier, R. J. & Swanson, J. A. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J. Exp. Med. 186, 1159–1163 (1997).
Lebreton, A., Stavru, F. & Cossart, P. Organelle targeting during bacterial infection: insights from Listeria. Trends. Cell Biol. 25, 330–338 (2015).
Czuczman, M. A. et al. Listeria monocytogenes exploits efferocytosis to promote cell-to-cell spread. Nature 509, 230–234 (2014).
Cassidy, S. K. et al. Membrane damage during Listeria monocytogenes infection triggers a caspase-7 dependent cytoprotective response. PLoS Pathog. 8, e1002628 (2012).
Pasternak, C. A. Effect of pore formers on intracellular calcium. Cell Calcium 7, 387–397 (1986).
Jimenez, A. J. & Perez, F. Plasma membrane repair: the adaptable cell life-insurance. Curr. Opin. Cell Biol. 47, 99–107 (2017).
Babiychuk, E. B., Monastyrskaya, K., Potez, S. & Draeger, A. Blebbing confers resistance against cell lysis. Cell Death Differ. 18, 80–89 (2011).
Hagmann, J., Burger, M. M. & Dagan, D. Regulation of plasma membrane blebbing by the cytoskeleton. J. Cell. Biochem. 73, 488–499 (1999).
Roy, D. et al. A process for controlling intracellular bacterial infections induced by membrane injury. Science 304, 1515–1518 (2004).
Levine, B., Mizushima, N. & Virgin, H. W. Autophagy in immunity and inflammation. Nature 469, 323–335 (2011).
Ktistakis, N. T. & Tooze, S. A. Digesting the expanding mechanisms of autophagy. Trends. Cell Biol. 26, 624–635 (2016).
Gerstenmaier, L. et al. The autophagic machinery ensures nonlytic transmission of mycobacteria. Proc. Natl Acad. Sci. USA 112, E687–E692 (2015).
Gonzalez, M. R. et al. Pore-forming toxins induce multiple cellular responses promoting survival. Cell Microbiol. 13, 1026–1043 (2011).
Marchiando, A. M. et al. A deficiency in the autophagy gene Atg16L1 enhances resistance to enteric bacterial infection. Cell. Host. Microbe. 14, 216–224 (2013).
Fadeel, B. & Xue, D. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 44, 264–277 (2009).
Hampe, J. et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 39, 207–211 (2007).
Kuballa, P., Huett, A., Rioux, J. D., Daly, M. J. & Xavier, R. J. Impaired autophagy of an intracellular pathogen induced by a Crohn’s disease associated ATG16L1 variant. PLoS ONE 3, e3391 (2008).
Raju, D. et al. Vacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote Helicobacter pylori infection in humans. Gastroenterology 142, 1160–1171 (2012).
Sampath, V. et al. A functional ATG16L1 (T300A) variant is associated with necrotizing enterocolitis in premature infants. Pediatr. Res. 81, 582–588 (2016).
Burada, F. et al. ATG16L1 T300A polymorphism is correlated with gastric cancer susceptibility. Pathol. Oncol. Res. 22, 317–322 (2016).
Fujita, N. et al. Differential involvement of Atg16L1 in Crohn disease and canonical autophagy: analysis of the organization of the Atg16L1 complex in fibroblasts. J. Biol. Chem. 284, 32602–32609 (2009).
Wolfmeier, H. et al. Active release of pneumolysin prepores and pores by mammalian cells undergoing a Streptococcus pneumoniae attack. Biochim. Biophys. Acta 1860, 2498–2509 (2016).
Maekawa, M. & Fairn, G. D. Complementary probes reveal that phosphatidylserine is required for the proper transbilayer distribution of cholesterol. J. Cell. Sci. 128, 1422–1433 (2015).
Boucher, E. & Mandato, C. A. Plasma membrane and cytoskeleton dynamics during single-cell wound healing. Biochim. Biophys. Acta 1853, 2649–2661 (2015).
Jimenez, A. J. et al. ESCRT machinery is required for plasma membrane repair. Science 343, 1247136 (2014).
Andrews, N. W., Almeida, P. E. & Corrotte, M. Damage control: cellular mechanisms of plasma membrane repair. Trends. Cell Biol. 24, 734–742 (2014).
Corrotte, M., Castro-Gomes, T., Koushik, A. B. & Andrews, N. W. Approaches for plasma membrane wounding and assessment of lysosome-mediated repair responses. Methods Cell Biol. 126, 139–158 (2015).
Tinevez, J. Y. et al. Role of cortical tension in bleb growth. Proc. Natl Acad. Sci. USA 106, 18581–18586 (2009).
Anishkin, A. & Kung, C. Stiffened lipid platforms at molecular force foci. Proc. Natl Acad. Sci. USA 110, 4886–4892 (2013).
Xu, J. et al. Mechanism of polarized lysosome exocytosis in epithelial cells. J. Cell. Sci. 125, 5937–5943 (2012).
Ouimet, M. et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell. Metab. 13, 655–667 (2011).
Lu, F. et al. Identification of NPC1 as the target of U18666A, an inhibitor of lysosomal cholesterol export and Ebola infection. eLife 4, e12177 (2015).
Zanotti, I. et al. The LXR agonist T0901317 promotes the reverse cholesterol transport from macrophages by increasing plasma efflux potential. J. Lipid Res. 49, 954–960 (2008).
Lamason, R. L. & Welch, M. D. Actin-based motility and cell-to-cell spread of bacterial pathogens. Curr. Opin. Microbiol. 35, 48–57 (2016).
Grundling, A., Gonzalez, M. D. & Higgins, D. E. Requirement of the Listeria monocytogenes broad-range phospholipase PC-PLC during infection of human epithelial cells. J. Bacteriol. 185, 6295–6307 (2003).
Zhao, Z. et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell. Host. Microbe. 4, 458–469 (2008).
Pavel, M. & Rubinsztein, D. C. Mammalian autophagy and the plasma membrane. FEBS J. 284, 672–679 (2017).
Murrow, L., Malhotra, R. & Debnath, J. ATG12-ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat. Cell Biol. 17, 300–310 (2015).
Maurer, K. et al. Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin. Cell Host Microbe. 17, 429–440 (2015).
DeSelm, C. J. et al. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev. Cell. 21, 966–974 (2011).
Mrschtik, M. & Ryan, K. M. Lysosomal proteins in cell death and autophagy. FEBS J. 282, 1858–1870 (2015).
Huynh, K. K., Gershenzon, E. & Grinstein, S. Cholesterol accumulation by macrophages impairs phagosome maturation. J. Biol. Chem. 283, 35745–35755 (2008).
Lebrand, C. et al. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 21, 1289–1300 (2002).
Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9, 125–138 (2008).
Meyer-Morse, N. et al. Listeriolysin O is necessary and sufficient to induce autophagy during Listeria monocytogenes infection. PLoS ONE 5, e8610 (2010).
Bishop, D. K. & Hinrichs, D. J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139, 2005–2009 (1987).
Jones, S. & Portnoy, D. A. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect. Immun. 62, 5608–5613 (1994).
Skoble, J., Portnoy, D. A. & Welch, M. D. Three regions within ActA promote Arp2/3 complex-mediated actin nucleation and Listeria monocytogenes motility. J. Cell. Biol. 150, 527–538 (2000).
Shen, A. & Higgins, D. E. The 5’ untranslated region-mediated enhancement of intracellular listeriolysin O production is required for Listeria monocytogenes pathogenicity. Mol. Microbiol. 57, 1460–1473 (2005).
Gelber, S. E., Aguilar, J. L., Lewis, K. L. & Ratner, A. J. Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J. Bacteriol. 190, 3896–3903 (2008).
Wolfmeier, H. et al. -dependent repair of pneumolysin pores: a new paradigm for host cellular defense against bacterial pore-forming toxins. Biochim. Biophys. Acta 1853, 2045–2054 (2015).
Rescher, U., Zobiack, N. & Gerke, V. Intact -binding sites are required for targeting of annexin 1 to endosomal membranes in living HeLa cells. J. Cell. Sci. 113(Pt 22), 3931–3938 (2000).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000).
Puri, C., Renna, M., Bento, C. F., Moreau, K. & Rubinsztein, D. C. Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell 154, 1285–1299 (2013).
Munsie, L. N., Caron, N., Desmond, C. R. & Truant, R. Lifeact cannot visualize some forms of stress-induced twisted F-actin. Nat. Methods 6, 317 (2009).
Conway, K. L. et al. Atg16l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology 145, 1347–1357 (2013).
Acknowledgements
We are grateful to E.A. Creasey, A. Draeger, V. Gerke, S. Grinstein, A. Hostettles, N. Mizushima, D. Portnoy, A. Ratner, D. Rubinsztein and R. Truant for providing reagents and advice, and to the SickKids Hospital Flow and Mass Cytometry Facility for help with flow cytometry. J.H.B. holds the Pitblado Chair in Cell Biology. Infrastructure for the Brumell Laboratory was provided by a John Evans Leadership Fund grant from the Canadian Foundation for Innovation and the Ontario Innovation Trust. S.E.O. and N.M. were supported by a fellowship from the Research Training Centre at the Hospital for Sick Children. S.E.O. and J.H. were supported by CIHR fellowships. D.B. was supported by the Dutch Digestive Foundation. D.A.A. and M.A.C. were supported by a studentship from the Research Training Committee at the Hospital for Sick Children and an NSERC CGS-M scholarship. M.A.C. was supported by a University of Toronto Open Fellowship. M.C. was supported by an NSERC PGS-D scholarship and a CIHR Training Fellowship (no. TGF-53877). This work was supported by operating grants from The Arthritis Society of Canada (grant no. RG11/013) and the Canadian Institutes of Health Research (grant nos. MOP#97756, PJT#148668 and FDN154329).
Author information
Authors and Affiliations
Contributions
J.H.B., J.M.J.T., N.M., S.E.O. J.H. and D.E.H. designed the experiments. J.M.J.T., N.M., S.E.O., D.A.A., D.D., R.L., D.B., J.M.v.R, M.A.C., M.C., E.C. and A.M.W. performed the experiments. C.M.Y., R.J.X., D.M., F.R., T.Y., J.D., G.D.F., B.R., P.K.K. and A.M.M. contributed reagents and consultations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–9, Supplementary Methods, Supplementary References.
Rights and permissions
About this article
Cite this article
Tan, J.M.J., Mellouk, N., Osborne, S.E. et al. An ATG16L1-dependent pathway promotes plasma membrane repair and limits Listeria monocytogenes cell-to-cell spread. Nat Microbiol 3, 1472–1485 (2018). https://doi.org/10.1038/s41564-018-0293-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0293-5
This article is cited by
-
Autophagy restricts Mycobacterium tuberculosis during acute infection in mice
Nature Microbiology (2023)
-
Intracellular cholesterol transport inhibition Impairs autophagy flux by decreasing autophagosome–lysosome fusion
Cell Communication and Signaling (2022)
-
Plasma membrane integrity: implications for health and disease
BMC Biology (2021)
-
Listeria exploits IFITM3 to suppress antibacterial activity in phagocytes
Nature Communications (2021)
-
Decoy exosomes provide protection against bacterial toxins
Nature (2020)