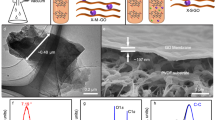
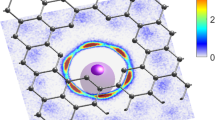
Abstract
Ion transport in nanoconfinement differs from that in bulk and has been extensively researched across scientific and engineering disciplines1,2,3,4. For many energy and water applications of nanoporous materials, concentration-driven ion diffusion is simultaneously subjected to a local electric field arising from surface charge or an externally applied potential. Due to the uniquely crowded intermolecular forces under severe nanoconfinement (<2 nm), the transport behaviours of ions can be influenced by the interfacial electrical double layer (EDL) induced by a surface potential, with complex implications, engendering unusual ion dynamics5,6,7. However, it remains an experimental challenge to investigate how such a surface potential and its coupling with nanoconfinement manipulate ion diffusion. Here, we exploit the tunable nanoconfinement in layered graphene-based nanoporous membranes to show that sub-2 nm confined ion diffusion can be strongly modulated by the surface potential-induced EDL. Depending on the potential sign, the combination and concentration of ion pairs, diffusion rates can be reversibly modulated and anomalously enhanced by 4~7 times within 0.5 volts, across a salt concentration gradient up to seawater salinity. Modelling suggests that this anomalously enhanced diffusion is related to the strong ion–ion correlations under severe nanoconfinement, and cannot be explained by conventional theoretical predictions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bocquet, L. & Charlaix, E. Nanofluidics, from bulk to interfaces. Chem. Soc. Rev. 39, 1073–1095 (2010).
Sparreboom, W., van den Berg, A. & Eijkel, J. C. T. Principles and applications of nanofluidic transport. Nat. Nanotech. 4, 713–720 (2009).
Duan, C. & Majumdar, A. Anomalous ion transport in 2-nm hydrophilic nanochannels . Nat. Nanotech. 5, 848–852 (2010).
Maier, J. Nanoionics: ion transport and electrochemical storage in confined systems. Nat. Mater. 4, 805–815 (2005).
Feng, J. et al. Observation of ionic Coulomb blockade in nanopores. Nat. Mater. 15, 850–855 (2016).
Griffin, J. M. et al. In situ NMR and electrochemical quartz crystal microbalance techniques reveal the structure of the electrical double layer in supercapacitors. Nat. Mater. 14, 812–819 (2015).
Kondrat, S., Wu, P., Qiao, R. & Kornyshev, A. A. Accelerating charging dynamics in subnanometre pores. Nat. Mater. 13, 387–393 (2014).
Koltonow, A. R. & Huang, J. Two-dimensional nanofluidics. Science 351, 1395–1396 (2016).
Gao, J., Feng, Y., Guo, W. & Jiang, L. Nanofluidics in two-dimensional layered materials: inspirations from nature. Chem. Soc. Rev. 46, 5400–5424 (2017).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752–754 (2014).
Morelos-Gomez, A. et al. Effective NaCl and dye rejection of hybrid graphene oxide/graphene layered membranes. Nat. Nanotech. 12, 1083–1088 (2017).
Chen, L. et al. Ion sieving in graphene oxide membranes via ationic control of interlayer spacing. Nature 550, 380–383 (2017).
Yang, X. et al. Ordered gelation of chemically converted graphene for next-generation electroconductive hydrogel films. Angew. Chem. Int. Ed. 50, 7325–7328 (2011).
Cheng, C. et al. Ion transport in complex layered graphene-based membranes with tuneable interlayer spacing. Sci. Adv. 2, e1501272 (2016).
Karnik, R. et al. Electrostatic control of ions and molecules in nanofluidic transistors. Nano Lett. 5, 943–948 (2005).
Yang, X., Cheng, C., Wang, Y., Qiu, L. & Li, D. Liquid-mediated dense integration of graphene materials for compact capacitive energy storage. Science 341, 534–537 (2013).
Jiang, G., Cheng, C., Li, D. & Liu, J. Z. Molecular dynamics simulations of the electric double layer capacitance of graphene electrodes in mono-valent aqueous electrolytes. Nano Res. 9, 174–186 (2016).
Surwade, S. P. et al. Electrochemical control of ion transport through a mesoporous carbon membrane. Langmuir 30, 3606–3611 (2014).
Borukhov, I., Andelman, D. & Orland, H. Steric effects in electrolytes: a modified Poisson–Boltzmann equation. Phys. Rev. Lett. 79, 435–438 (1997).
Schaefer, A., Fane, A. G. & Waite, T. Nanofiltration: Principles and Applications 2nd edn (Elsevier, 2017).
Tsuru, T., Nakao, S. I. & Kimura, S. Calculation of ion rejection by extended Nernst–Planck equation with charged reverse osmosis membranes for single and mixed electrolyte solutions. J. Chem. Eng. Jap. 24, 511–517 (1991).
Nishizawa, M., Menon, V. P. & Martin, C. R. Metal nanotubule membranes with electrochemically switchable ion-transport selectivity. Science 268, 700–702 (1995).
Yan, L. Electrostatic correlations: from plasma to biology. Rep. Progr. Phys. 65, 1577 (2002).
Bazant, M. Z., Storey, B. D. & Kornyshev, A. A. Double layer in ionic liquids: overscreening versus crowding. Phys. Rev. Lett. 106, 046102 (2011).
Grosberg, A. Y., Nguyen, T. & Shklovskii, B. Colloquium: the physics of charge inversion in chemical and biological systems. Rev. Mod. Phys. 74, 329–345 (2002).
Quesada-Pérez, M., González-Tovar, E., Martín-Molina, A., Lozada-Cassou, M. & Hidalgo-Álvarez, R. Overcharging in colloids: beyond the poisson–boltzmann approach. ChemPhysChem 4, 234–248 (2003).
van der Vegt, N. F. A. et al. Water-mediated ion pairing: occurrence and relevance. Chem. Rev. 116, 7626–7641 (2016).
Ballenegger, V. & Hansen, J. P. Dielectric permittivity profiles of confined polar fluids. J. Chem. Phys. 122, 114711 (2005).
Prakash, S. & Conlisk, A. T. Field effect nanofluidics. Lab Chip 16, 3855–3865 (2016).
Li, D., Muller, M. B., Gilje, S., Kaner, R. B. & Wallace, G. G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotech. 3, 101–105 (2008).
Qiu, L. et al. Controllable corrugation of chemically converted graphene sheets in water and potential application for nanofiltration. Chem. Commun. 47, 5810–5812 (2011).
Kornyshev, A. A. Double-layer in ionic liquids: paradigm change? J. Phys. Chem. B 111, 5545–5557 (2007).
Ghoufi, A., Szymczyk, A., Renou, R. & Ding, M. Calculation of local dielectric permittivity of confined liquids from spatial dipolar correlations. Europhys. Lett. 99, 37008 (2012).
Daiguji, H., Yang, P. & Majumdar, A. Ion transport in nanofluidic channels. Nano Lett. 4, 137–142 (2003).
Daiguji, H. Ion transport in nanofluidic channels. Chem. Soc. Rev. 39, 901–911 (2010).
Horn, H. W. et al. Development of an improved four-site water model for biomolecular simulations: TIP4P-Ew. J. Chem. Phys. 120, 9665–9678 (2004).
Liu, J. L. Numerical methods for the Poisson–Fermi equation in electrolytes. J. Comput. Phys. 247, 88–99 (2013).
Xie, D., Liu, J. L. & Eisenberg, B. Nonlocal Poisson-Fermi model for ionic solvent. Phys. Rev. E 94, 012114 (2016).
Acknowledgements
We would like to acknowledge financial support from the Australian Research Council. This work made use of the facilities at the Monash Centre for Electron Microscopy (MCEM).
Author information
Authors and Affiliations
Contributions
C.C. conceived, designed and carried out the experiments under the guidance of D.L. and G.P.S. D.L. and C.C. formulated the concept of using a nano-confined electrical double layer for ion modulation. G.J. designed and carried out the theoretical modelling under the guidance of J.Z.L. All authors discussed and interpreted the results. C.C. and G.J. wrote the manuscript with contributions from all the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–10
Rights and permissions
About this article
Cite this article
Cheng, C., Jiang, G., Simon, G.P. et al. Low-voltage electrostatic modulation of ion diffusion through layered graphene-based nanoporous membranes. Nature Nanotech 13, 685–690 (2018). https://doi.org/10.1038/s41565-018-0181-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0181-4
This article is cited by
-
Light-responsive and ultrapermeable two-dimensional metal-organic framework membrane for efficient ionic energy harvesting
Nature Communications (2024)
-
Regulating ion affinity and dehydration of metal-organic framework sub-nanochannels for high-precision ion separation
Nature Communications (2024)
-
Two-dimensional MXene membranes with biomimetic sub-nanochannels for enhanced cation sieving
Nature Communications (2023)
-
Review of the role of ionic liquids in two-dimensional materials
Frontiers of Physics (2023)
-
Water’s motions in x-y and z directions of 2D nanochannels: Entirely different but tightly coupled
Nano Research (2023)