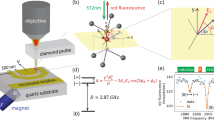
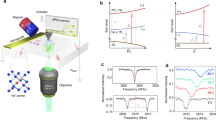
Abstract
Technologies that capture the complex electrical dynamics occurring in biological systems, across fluid membranes and at solid–liquid interfaces are important for furthering fundamental understanding and innovation in diverse fields from neuroscience to energy storage. However, the capabilities of existing voltage imaging techniques utilizing microelectrode arrays, scanning probes or optical fluorescence methods are limited by resolution, scan speed and photostability, respectively. Here we report an optoelectronic voltage imaging system that overcomes these limitations by using nitrogen-vacancy defects as charge-sensitive fluorescent reporters embedded within a transparent semiconducting diamond device. Electrochemical tuning of the diamond surface termination enables photostable optical voltage imaging with a quantitative linear response at biologically relevant voltages and timescales. This technology represents a major step towards label-free, large-scale and long-term voltage recording of physical and biological systems with sub-micrometre spatial resolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data used in the production of this work is available via Zenodo at https://doi.org/10.5281/zenodo.6717484.
Code availability
Custom data analysis and simulation code used in the production of this work is available via Zenodo at https://doi.org/10.5281/zenodo.6717484.
References
Knöpfel, T. & Song, C. Optical voltage imaging in neurons: moving from technology development to practical tool. Nat. Rev. Neurosci. 20, 719–727 (2019).
Piatkevich, K. D. et al. Population imaging of neural activity in awake behaving mice. Nature 574, 413–417 (2019).
Wang, W., Kim, C. K. & Ting, A. Y. Molecular tools for imaging and recording neuronal activity. Nat. Chem. Biol. 15, 101–110 (2019).
Abdelfattah, A. S. et al. Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science 365, 699–704 (2019).
Abbott, J. et al. Extracellular recording of direct synaptic signals with a CMOS-nanoelectrode array. Lab Chip 20, 3239–3248 (2020).
Emmenegger, V., Obien, M. E. J., Franke, F. & Hierlemann, A. Technologies to study action potential propagation with a focus on HD-MEAs. Front. Cell. Neurosci. 13, 159 (2019).
Castelletto, S. & Boretti, A. Color centers in wide-bandgap semiconductors for subdiffraction imaging: a review. Adv. Photon. 3, 054001 (2021).
Lühmann, T. et al. Screening and engineering of colour centres in diamond. J. Phys. D: Appl. Phys. 51, 483002 (2018).
Petrakova, V. et al. Charge-sensitive fluorescent nanosensors created from nanodiamonds. Nanoscale 7, 12307–12311 (2015).
Karaveli, S. et al. Modulation of nitrogen vacancy charge state and fluorescence in nanodiamonds using electrochemical potential. Proc. Natl Acad. Sci. USA 113, 3938–43 (2016).
Doherty, M. W. et al. The nitrogen-vacancy colour centre in diamond. Phys. Rep. 528, 1–45 (2013).
Schirhagl, R., Chang, K., Loretz, M. & Degen, C. L. Nitrogen-vacancy centers in diamond: nanoscale sensors for physics and biology. Annu. Rev. Phys. Chem. 65, 83–105 (2014).
Grotz, B. et al. Charge state manipulation of qubits in diamond. Nat. Commun. 3, 729 (2012).
Krečmarová, M. et al. A label-free diamond microfluidic DNA sensor based on active nitrogen-vacancy center charge state control. ACS Appl. Mater. Interfaces 13, 18500–18510 (2021).
Yang, K.-H. & Narayan, R. J. Biocompatibility and functionalization of diamond for neural applications. Curr. Opin. Biomed. Eng. 10, 60–68 (2019).
Hanlon, L. et al. Diamond nanopillar arrays for quantum microscopy of neuronal signals. Neurophotonics 7, 035002 (2020).
Momenzadeh, S. A. et al. Nanoengineered diamond waveguide as a robust bright platform for nanomagnetometry using shallow nitrogen vacancy centers. Nano Lett. 15, 165–169 (2015).
Kehayias, P. et al. Solution nuclear magnetic resonance spectroscopy on a nanostructured diamond chip. Nat. Commun. 8, 188 (2017).
McCloskey, D. J. et al. Enhanced widefield quantum sensing with nitrogen-vacancy ensembles using diamond nanopillar arrays. ACS Appl. Mater. Interfaces 12, 13421–13427 (2020).
Abbott, J. et al. A nanoelectrode array for obtaining intracellular recordings from thousands of connected neurons. Nat. Biomed. Eng. 4, 232–241 (2020).
Liu, R. et al. High density individually addressable nanowire arrays record intracellular activity from primary rodent and human stem cell derived neurons. Nano Lett. 17, 2757–2764 (2017).
Spira, M. E. & Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 8, 83–94 (2013).
Stotter, J., Show, Y., Wang, S. & Swain, G. Comparison of the electrical, optical, and electrochemical properties of diamond and indium tin oxide thin-film electrodes. Chem. Mater. 17, 4880–4888 (2005).
Kempaiah, R., Vasudevamurthy, G. & Subramanian, A. Scanning probe microscopy based characterization of battery materials, interfaces, and processes. Nano Energy 65, 103925 (2019).
Liu, D. et al. Evolution of solid electrolyte interface on TiO2 electrodes in an aqueous Li-ion battery studied using scanning electrochemical microscopy. J. Phys. Chem. C 123, 30958–30971 (2019).
Schreyvogel, C., Polyakov, V., Wunderlich, R., Meijer, J. & Nebel, C. E. Active charge state control of single NV centres in diamond by in-plane Al-Schottky junctions. Sci. Rep. 5, 12160 (2015).
Stacey, A. et al. Depletion of nitrogen‐vacancy color centers in diamond via hydrogen passivation. Appl. Phys. Lett. 100, 071902 (2012).
Findler, C., Lang, J., Osterkamp, C., Nesládek, M. & Jelezko, F. Indirect overgrowth as a synthesis route for superior diamond nano sensors. Sci. Rep. 10, 22404 (2020).
Ristein, J. Surface transfer doping of diamond. J. Phys. D: Appl. Phys. 39, R71–R81 (2006).
Maier, F., Riedel, M., Mantel, B., Ristein, J. & Ley, L. Origin of surface conductivity in diamond. Phys. Rev. Lett. 85, 3472–3475 (2000).
Broadway, D. A. et al. Spatial mapping of band bending in semiconductor devices using in situ quantum sensors. Nat. Electron. 1, 502–507 (2018).
Chaplin, B. P., Hubler, D. K. & Farrell, J. Understanding anodic wear at boron doped diamond film electrodes. Electrochim. Acta 89, 122–131 (2013).
Petráková, V. et al. Luminescence of nanodiamond driven by atomic functionalization: towards novel detection principles. Adv. Funct. Mater. 22, 812–819 (2012).
Ristein, J., Zhang, W. & Ley, L. Hydrogen-terminated diamond electrodes. I. Charges, potentials, and energies. Phys. Rev. E 78, 041602 (2008).
Licht, S., Cammarata, V. & Wrighton, M. S. Direct measurements of the physical diffusion of redox active species: microelectrochemical experiments and their simulation. J. Phys. Chem. 94, 6133–6140 (1990).
Dankerl, M. et al. Hydrophobic interaction and charge accumulation at the diamond-electrolyte interface. Phys. Rev. Lett. 106, 196103 (2011).
Spira, M. E., Shmoel, N., Huang, S.-H. M. & Erez, H. Multisite attenuated intracellular recordings by extracellular multielectrode arrays, a perspective. Front. Neurosci. 12, 212 (2018).
He, G. et al. Nanoneedle platforms: the many ways to pierce the cell membrane. Adv. Funct. Mater. 30, 1909890 (2020).
Ogi, J. et al. A 4.8-μVr.m.s.-noise CMOS-microelectrode array with density-scalable active readout pixels via disaggregated differential amplifier implementation. Front. Neurosci. 13, 234 (2019).
Viswam, V., Obien, M. E. J., Franke, F., Frey, U. & Hierlemann, A. Optimal electrode size for multi-scale extracellular-potential recording from neuronal assemblies. Front. Neurosci. 13, 385 (2019).
Müller, J. et al. High-resolution CMOS MEA platform to study neurons at subcellular, cellular, and network levels. Lab Chip 15, 2767–2780 (2015).
Bazant, M. Z., Thornton, K. & Ajdari, A. Diffuse-charge dynamics in electrochemical systems. Phys. Rev. E 70, 021506 (2004).
Collins, L., Kilpatrick, J. I., Kalinin, S. V. & Rodriguez, B. J. Towards nanoscale electrical measurements in liquid by advanced KPFM techniques: a review. Rep. Prog. Phys. 81, 086101 (2018).
Collins, L. et al. Full data acquisition in Kelvin probe force microscopy: mapping dynamic electric phenomena in real space. Sci. Rep. 6, 30557 (2016).
Salerno, M. & Dante, S. Scanning Kelvin probe microscopy: challenges and perspectives towards increased application on biomaterials and biological samples. Materials 11, 951 (2018).
Garrett, D. J., Tong, W., Simpson, D. A. & Meffin, H. Diamond for neural interfacing: a review. Carbon 102, 437–454 (2016).
Petrakova, V. et al. Imaging of transfection and intracellular release of intact, non-labeled DNA using fluorescent nanodiamonds. Nanoscale 8, 12002–12012 (2016).
Kitagawa, H. et al. Activity-dependent dynamics of the transcription factor of cAMP-response element binding protein in cortical neurons revealed by single-molecule imaging. J. Neurosci. 37, 1–10 (2017).
Wu, B., Eliscovich, C., Yoon, Y. J. & Singer, R. H. Translation dynamics of single mRNAs in live cells and neurons. Science 352, 1430–1435 (2016).
Lukinavičius, G. et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat. Methods 11, 731–733 (2014).
Zhang, Z., Chen, W., Zhao, Y. & Yang, Y. Spatiotemporal imaging of cellular energy metabolism with genetically-encoded fluorescent sensors in brain. Neurosci. Bull. 34, 875–886 (2018).
Barral, J. & D Reyes, A. Synaptic scaling rule preserves excitatory–inhibitory balance and salient neuronal network dynamics. Nat. Neurosci. 19, 1690–1696 (2016).
Werley, C. A., Chien, M.-P. & Cohen, A. E. Ultrawidefield microscope for high-speed fluorescence imaging and targeted optogenetic stimulation. Biomed. Opt. Express 8, 5794–5813 (2017).
Kim, S.-W., Takaya, R., Hirano, S. & Kasu, M. Two-inch high-quality (001) diamond heteroepitaxial growth on sapphire (11\(\bar {2}\)0) misoriented substrate by step-flow mode. Appl. Phys. Express 14, 115501 (2021).
Chia, C., Machielse, B., Shams-Ansari, A. & Lončar, M. Development of hard masks for reactive ion beam angled etching of diamond. Opt. Express 30, 14189–14201 (2022).
Smereka, M. & Dulęba, I. Circular object detection using a modified Hough transform. Int. J. Appl. Math. Comput. Sci. 18, 85–91 (2008).
Hall, L. T. et al. Detection of nanoscale electron spin resonance spectra demonstrated using nitrogen-vacancy centre probes in diamond. Nat. Commun. 7, 10211 (2016).
Acknowledgements
We thank W. Tong for providing poly-d-lysine and assisting with the coating process. We thank G. Berecki and S. Petrou for helpful discussions. D.A.S. and L.T.H. were supported by the Australian Research Council (ARC) through Discovery Project 200103712. L.T.H. was supported by the ARC through DECRA 2001011785. A.S. was supported by the ARC through DECRA 190100336. A.N. was supported by the ARC through Linkage 190100528. L.C.L.H. was supported by the ARC Centre of Excellence for Quantum Computation and Communication Technology. D.J.M. was supported by an Australian Government Graduate Research Training Scholarship. This work was performed in part at the Melbourne Centre for Nanofabrication (MCN) in the Victorian Node of the Australian National Fabrication Facility (ANFF).
Author information
Authors and Affiliations
Contributions
D.J.M., N.D. and D.A.S. developed the technological concepts and designed the experiments with input from A.S., S.P. and L.C.L.H. The devices were designed and fabricated by D.J.M. and N.D. with input from A.N. Hydrogen termination was performed by A.S. with input from D.J.M. The electrochemical oxidation procedure was performed by C.P. with input from D.J.M. and N.D. Microelectrode measurements and corresponding data analysis were performed by D.J.M. and N.D. with input from L.T.H. The equivalent circuit model was developed by D.J.M. Optrode array measurements and data analysis were performed by D.J.M. with input from N.D. and L.T.H. The original manuscript draft was written by D.J.M., N.D. and D.A.S. All the authors contributed to reviewing and editing the manuscript. L.C.L.H., S.P. and D.A.S. supervised the work.
Corresponding authors
Ethics declarations
Competing interests
D.J.M., N.D., A.S. and D.A.S. are authors on a provisional patent granted to The University of Melbourne covering the fabrication of the NV0/+ ensemble chip and its use in voltage sensing applications (IP Australia patent no. 2021901331). S.P. is a director and shareholder of Carbon Cybernetics, a company developing a diamond-based neural implant. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Photonics thanks Fedor Jelezko and Milos Nesladek for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Tables 1–4, Notes 1–4, Video 1, Methods 1 and references.
Supplementary Video 1
DVIM recording of a spatiotemporal voltage transient.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McCloskey, D.J., Dontschuk, N., Stacey, A. et al. A diamond voltage imaging microscope. Nat. Photon. 16, 730–736 (2022). https://doi.org/10.1038/s41566-022-01064-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41566-022-01064-1
This article is cited by
-
The diamond voltage microscope
Nature Photonics (2022)
-
A diamond sensor shines at ‘seeing’ voltages
Nature (2022)