Abstract
The development of immune-checkpoint inhibitors (ICIs) has heralded a new era in cancer treatment, enabling the possibility of long-term survival in patients with metastatic disease, and providing new therapeutic indications in earlier-stage settings. As such, characterizing the long-term implications of receiving ICIs has grown in importance. An abundance of evidence exists describing the acute clinical toxicities of these agents, although chronic effects have not been as well catalogued. Nonetheless, emerging evidence indicates that persistent toxicities might be more common than initially suggested. While generally low-grade, these chronic sequelae can affect the endocrine, rheumatological, pulmonary, neurological and other organ systems. Fatal toxicities also comprise a diverse set of clinical manifestations and can occur in 0.4–1.2% of patients. This risk is a particularly relevant consideration in light of the possibility of long-term survival. Finally, the effects of immune-checkpoint blockade on a diverse range of immune processes, including atherosclerosis, heart failure, neuroinflammation, obesity and hypertension, have not been characterized but remain an important area of research with potential relevance to cancer survivors. In this Review, we describe the current evidence for chronic immune toxicities and the long-term implications of these effects for patients receiving ICIs.
Key points
-
Immune-checkpoint inhibitors (ICIs) produce durable responses in a growing number of patients with metastatic cancer, and are being used increasingly in (neo)adjuvant settings.
-
Although acute toxicities are more common, chronic immune-related adverse events (irAEs) are increasingly recognized, and can affect up to 40% of patients.
-
Chronic irAEs are mostly classed as endocrine or rheumatological, but can affect a diverse array of organs.
-
Other issues with long-term relevance include fatal irAEs (which can occur in 0.4–1.2% of patients), and rechallenge after severe irAEs.
-
ICIs could also affect other immune-mediated processes (such as atherosclerosis or neuroinflammation), although more studies are needed.
Similar content being viewed by others
Introduction
Immune-checkpoint inhibitors (ICIs) have emerged as a core pillar of cancer therapy. Nearly half of all patients with metastatic cancer in economically developed countries are eligible to receive ICIs, with eight approved agents available for 17 different malignancies as of December 2021, with increasing use of these agents seen in several (neo)adjuvant and maintenance settings1. ICIs are also often being used in combination regimens, including those involving other classes of ICI, cytotoxic chemotherapy, and biological and/or targeted therapies2. Moreover, durable responses are becoming increasingly common, even in the metastatic setting3; thus, characterizing the long-term physiological implications of treatment with ICIs is growing in importance.
Immune checkpoints are receptors expressed by immune cells that enable dynamic regulation of immune homeostasis and are particularly relevant to T cell functionality. PD-1 and its primary ligand PD-L1 are expressed on T cells, and on tumour cells and tumour-infiltrating myeloid cells, respectively. Interaction of these two proteins results in T cell exhaustion, a potentially permanent state of dysfunction characterized by reduced or absent effector function (cytotoxicity or cytokine production), lack of response to stimuli, and altered transcriptional and epigenetic states4,5. This interaction is exploited by tumour cells to maintain immune tolerance, although it is also used physiologically to limit the extent of autoimmune inflammation, maintain fetal tolerance during pregnancy and prevent the rejection of transplanted organs. Relative to PD-1 (refs6,7), CTLA4 has a more proximal role in immune activation, engaging with the dendritic cell ligand B7 (also known as CD80) and acting as a higher affinity competitor to CD28, thus limiting the extent of T cell activation at the priming stage. Expression of CTLA4 also probably enhances the function and promotes the expansion of regulatory T (Treg) cells in the tumour microenvironment8. A more detailed description of the mechanisms of these various immune checkpoints is provided elsewhere9,10.
Abrogation of immune-checkpoint signalling has several consequences. Data from preclinical studies demonstrate that genetic ablation of Ctla4 in mice results in death in early life (typically at 3–4 weeks of age) owing to lymphoproliferation and profound multiorgan autoimmunity11,12,13. By contrast, deletion of Pd1 or Pdl1 results in less-severe, background-dependent, model-specific effects ranging from arthritis to cardiomyopathy, which usually occur later in life (from 5 to 30 weeks of age)14,15. The clinical experience mimics this pattern, in that CTLA4 inhibition results in a high incidence of dose-dependent toxicities (high-grade toxicities in 38.6% and 57.9% of patients with metastatic melanoma receiving ipilimumab 3 mg/kg or 10 mg/kg, respectively)16. By contrast, PD-1 or PD-L1 blockade causes high-grade adverse events in only 10–15% of patients, with similar incidences seen with different agents and across the range of clinically used doses17,18. Patterns of response also vary substantially between classes: CTLA4 inhibition has limited activity as monotherapy outside of melanoma (and is associated with a response rate of only 20% in patients with metastatic melanoma)19. By contrast, antibodies targeting PD-1 or PD-L1 have clinical activity in nearly 20 different cancer types, with response rates ranging from 10–30% (in many carcinogen-induced solid tumours, including those of the liver, bladder and kidneys), to 40–50% (in melanoma, microsatellite unstable and/or mismatch repair-deficient cancers and strongly PD-L1+ non-small-cell lung cancers (NSCLCs)) and to as high as 65–75% (in Hodgkin lymphoma)20,21. Combination PD-1 and CTLA4 blockade are also being used in many different types of cancer, and are associated with additive or possibly synergistic responses in several clinical settings. For example, in metastatic melanoma, treatment with the anti-PD-1 antibody nivolumab plus the anti-CTLA4 antibody ipilimumab is associated with a 59% response rate (compared with 43% for nivolumab alone and 15–20% for ipilimumab alone)22, and in renal cell carcinoma (RCC) approximately 40% (versus ~25% with nivolumab alone and probably minimal activity for ipilimumab monotherapy)23,24. This combination is also approved for use in patients with NSCLC, microsatellite instability-high colorectal cancer or hepatocellular carcinoma, in whom CTLA4 inhibition has minimal (or poorly characterized) activity as a monotherapy25. Unsurprisingly, concurrent inhibition of these non-redundant immune checkpoints also augments the risk of autoimmune toxicities, resulting in increased incidences of high-grade immune-related adverse events (irAEs)23,26. Specifically, nivolumab monotherapy, ipilimumab monotherapy and the combination of ipilimumab plus nivolumab have been shown to lead to high-grade adverse events in 23%, 28% and 59% of patients with advanced-stage melanoma, respectively27.
A feature that is common to all ICIs (whether they inhibit PD-1, PD-L1 or CTLA4), and largely distinct from many other classes of cancer therapeutic agents, is the potential for long-lasting, possibly indefinite responses, even in patients with metastatic solid tumours (Table 1) — and particularly in patients treated in earlier stages (Table 2). Duration of response is heterogeneous among patients, although those with tumours associated with higher baseline response rates (such as melanoma) tend to have longer responses. Of note, treatment might be safely discontinued in some patients who have prolonged excellent responses, although the timing and how best to identify eligible patients remain unclear and the oncological safety of this approach will probably vary between tumour types. For example, patients with NSCLC who have either a response or stable disease after 1 year of nivolumab were randomized to continued therapy or observation: continuous treatment resulted in superior overall survival (OS) compared with therapy discontinuation (median OS not reached versus 32.5 months, HR 0.61)28. By contrast, several non-randomized studies have indicated excellent outcomes after treatment discontinuation in patients with metastatic melanoma who have ongoing responses after either 1 or 2 years of therapy, with disease progression rarely observed over the ensuing 2–5 years29,30,31.
In patients who ultimately have disease relapse despite an initial clinical response (acquired resistance), several mechanisms of treatment failure have been identified, including upregulation of alternative immune checkpoints, defective antigen presentation, a lack of IFNγ response and T cell exclusion32,33,34. By contrast, patients who have durable responses appear to have broadened peripheral T and B cell repertoires, and seem to develop immunological memory (which might also have implications for broader and longer-term T cell-mediated toxicity)35,36. The molecular underpinnings of these responses are not entirely clear; potential mechanisms could include complete immune-mediated cytological eradication, generation of immune memory that forestalls disease recurrence or induction of a protracted ‘stalemate’ between residual cancer and immune cells37. Irrespective of the mechanisms of action, the extended duration of the therapeutic effects of ICIs often far surpasses their pharmacokinetic half-life38,39. This persistent pharmacodynamic effect (manifesting clinically as durable responses) also has implications for toxicities. In this Review, we describe the available data and clinical experience to date from patients who have chronic and/or irreversible toxicities from ICIs. We also discuss the clinical implications of these effects, including their relevance to treatment decision making, and their long-term implications for other inflammatory processes (Table 2).
Immune activation and irAEs
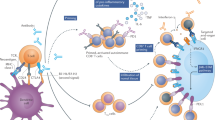
The immune activation that underlies most irAEs might be coupled with the activity required for antitumour immune responses (Fig. 1). This tumour-specific hypothesis is supported by the modest but reproducible positive correlation between therapeutic responses and incidence of irAEs40,41,42. Correlative studies provide additional circumstantial evidence for such a mechanistic association, demonstrating the existence of shared T cell receptor sequences and/or upregulated organ-specific transcripts between tumours and non-malignant tissues affected by toxicities43,44. Furthermore, the occurrence of vitiligo (an autoimmune response to melanocytes) provides a reliable indicator of antitumour activity of ICIs exclusively in patients with melanoma, also suggesting that irAEs and antitumour immunity might be mechanistically linked45. If these positive and negative outcomes do indeed arise from the same processes, long-term responders could have a higher risk of chronic toxicities than those who fail to benefit.
Schema depicting the interaction of T cells with malignant or non-malignant cells, and the molecular mechanisms of immune-checkpoint blockade. Tumour-specific (left) and non-tumour-specific (right) aspects associated with the development of irAEs are also included. ADCC, antibody-dependent cellular cytotoxicity; NK cell, natural killer cell; TCR, T cell receptor.
Evidence also exists suggesting that irAEs have mechanisms that are not related to antitumour activity, including those involving the microbiome, and viral or tissue-specific factors46,47,48,49,50,51. The divergence of irAEs is likely to be reflected in distinct and/or highly diverse mechanisms for each type of event. Notably, distinct cell types are implicated as dominant cellular populations and critical drivers in different preclinical models and biopsy samples obtained from affected tissues. For example, tissue-resident memory CD8+ T cells were the most abundant cell type in colon biopsy samples obtained from a cohort of patients with ICI-induced colitis, while cytotoxic activated memory CD4+ T cells were most prevalent in the brain of one patient with fatal encephalitis49,50,52,53. Targeted inhibition of particular cytokines, such as IL-6, could provide a method for uncoupling antitumour from antihost immune responses in preclinical models54,55. Ultimately, ‘one size fits all’ mechanistic explanations are unlikely to be forthcoming and might simply not exist: irAEs probably arise from both tumour-related and/or tumour-unrelated factors. Furthermore, the specific mechanisms of acute versus chronic irAEs remain poorly understood. A more detailed overview of the mechanisms of the various irAEs associated with ICIs is provided elsewhere56.
Timing and resolution
Distinct ICI regimens have disparate toxicity profiles. High-grade irAEs typically occur in a dose-dependent fashion with regimens containing anti-CTLA4 antibodies (30–55% for the combination of ipilimumab plus nivolumab), but are not dose-dependent with anti-PD-1–PD-L1 antibodies administered as monotherapies (10–15% incidence)26. The incidence, clinical presentation and management of the most common irAEs are discussed in more detail elsewhere56,57. To briefly summarize, these effects are more frequent in organs with extensive environmental interfaces (such as the skin, lungs, liver and gastrointestinal tract) or those harbouring presumed pre-existing, or smouldering, autoimmunity (such as the thyroid and joints). Nonetheless, irAEs can occur in essentially any organ system, including the heart, bone marrow, kidneys, bones, pituitary gland and others. IrAEs occur most often during the first three months of treatment but can arise at any time on therapy or even several months after treatment cessation58. Despite the lack of robust evidence from randomized clinical trials, acute severe irAEs are managed with reasonable effectiveness by providing symptom management, withholding ICIs and administering high-dose glucocorticoids (or potentially other immunosuppressive medications for steroid-refractory irAEs)59,60,61. In general, high-dose glucocorticoids do not appear to interfere with antitumour responses, although data from several studies suggest that administration of steroids within a few weeks of starting treatment might result in inferior outcomes42,62,63.
The majority of irAEs arise early in the course of treatment, although delayed events, defined as irAEs arising after 1 year of therapy, are also possible. In one series of 118 patients, the estimated incidence of high-grade, delayed irAEs was 5.3%64. Many patients with delayed events had already reported an acute irAE (58%), although later events generally occurred at a distinct organ site (86%). The most frequent delayed irAEs were colitis, rash and pneumonitis, and two patients died from toxicities. Of note, most patients (74%) were receiving anti-PD-1 antibodies at irAE onset while 12% had discontinued within the previous 3 months and 14% had discontinued >3 months previously. These observations reinforce the idea that irAEs (either acute or delayed) can arise sporadically after discontinuation, but usually occur during active treatment. Thus, the possibility of developing delayed irAEs with extended durations of treatment should be considered when deciding whether to continue ICIs in patients with prolonged responses. Current data are limited, although possible approaches to guide this decision could include PET–CT or circulating tumour DNA (ctDNA)-based monitoring (with therapy cessation in patients with a metabolic complete response29 and/or consistently undetectable ctDNA)65,66,67.
The timing of irAE resolution is also not entirely understood. In theory, discrete immune perturbations, which include treatment with an ICI, resulting in autoimmune inflammation either could result in a temporary state that is reversible by withholding therapy followed by immune suppression and/or modulation if needed, or could incite an irreversible autoimmune disorder, particularly if the causative factor (ICI administration) is not withdrawn. The early clinical-trial experience largely suggests the former scenario, as most irAE symptoms improved or resolved upon treatment with high-dose glucocorticoids. Most syndromes and histopathological features seen in the involved organs closely mimicked autoimmune disorders, although genuine new-onset, chronic autoimmune diseases (such as lupus erythematosus or inflammatory bowel disease) have seemed extremely rare (with some exceptions, as discussed below). Despite this low incidence, chronic irAEs have emerged as a major clinical concern.
Chronic irAEs
Acute irAEs have thus far received the bulk of the attention owing to their more dramatic clinical presentation and need for urgent treatment. However, retrospective data published in May 2021 suggest that chronic irAEs (defined as those persisting for >12 weeks after discontinuation of an anti-PD-1–PD-L1 antibody) are more prevalent than previously recognized, occurring in 43.2% of patients68. This lack of recognition is likely to have occurred and persisted for several reasons. First, as noted, most acute irAEs will at least improve with steroids and often resolve altogether69. Second, adverse event reporting in clinical trials tends to focus on the most frequent treatment-related toxicities (those that occur in ≥10% of participants). Thus, low-frequency events are usually under-reported and under-recognized regardless of their aggregate prevalence. Third, most initial clinical trials have enrolled patients with metastatic cancer. Characterizing chronic and long-term events in patients with metastatic disease is challenging because these patients often have a limited life expectancy, thus constraining long-term follow-up. Such patients might also receive subsequent systemic therapies, surgery and/or radiotherapy, rendering toxicity attribution even more difficult. Finally, the presence of multiple co-morbidities, which is common in patients with cancer, might further impair the identification of chronic irAEs. Despite these challenges, the durable clinical responses seen in a subset of patients receiving ICIs has enabled several follow-up studies to generate preliminary insights. In these studies, endocrinopathies (such as hypothyroidism and newly emergent type 1 diabetes) and rheumatological toxicities (such as arthritis) emerged as the most common chronic irAEs. Various other low-prevalence events also appeared, including neuropathy, dermatitis and pneumonitis70,71, revealing a more nuanced picture of the possibility of chronic irAEs.
These observations raise the question as to why some irAEs fail to resolve. We hypothesize two distinct scenarios: burnout or smouldering inflammation. Burnout refers to irreversible damage of the relevant cells, thus precluding physiological recovery (Fig. 2). Endocrinopathies provide the archetypal example of this effect. In patients with ICI-induced forms of hypothyroidism (which is generally preceded by thyroiditis), hypophysitis or type 1 diabetes, the hormone-secreting cells are irretrievably damaged or ablated by the inflammatory process. Support for this notion includes the lack of recovery even with early utilization of high-dose steroids72,73. These syndromes are rarely reversible (in contrast to most other irAEs) and typically necessitate the use of exogenous hormone replacement therapy, suggesting that the relevant cell types are truly ‘burned out’. Other irAEs might also fit into this category, including neuropathies (with persistently injured peripheral nerves) and xerostomia (with chronic salivary gland scarring leading to outflow obstruction and/or decreased saliva production). By contrast, a smouldering inflammation phenotype might more closely resemble classic autoimmunity, in which ICIs trigger persistent subacute or chronic inflammation. ICI-associated inflammatory arthritis provides the classic example of this phenotype, in which the polyarticular involvement closely mimics that of rheumatoid arthritis (RA) in many patients and evolves into a chronic condition in approximately half of all affected patients74. The estimated risk of acute irAEs evolving into chronic events varies substantially between events (Fig. 3).
a | Smouldering toxicities characterized by off-target T cell activation that may wax and wane over time. Examples include rheumatoid arthritis-like inflammation of the joints. Such effects often resolve on treatment withdrawal and/or steroids b, c | Burnout toxicities characterized by irreversible damage to the relevant cells, typified by immune-checkpoint inhibitor-mediated endocrinopathies. Examples include destruction of the hormone-secreting cells of the pancreas (b) or thyroid (c). Such toxicities are usually irreversible and require permanent hormone-replacement therapy.
The exact risks of acute toxicities becoming chronic (defined as persisting for at least 12 weeks beyond treatment cessation) are currently unknown, although endocrinopathies, arthritis, xerostomia, neurotoxicities and ocular events are generally more likely to become chronic toxicities. Immune-related adverse events affecting the visceral organs seem to have a lower risk of becoming chronic. Percentages expressed are the percentages of acute toxicities that become chronic (defined as those that persist for at least 12 weeks following immune-checkpoint inhibitor discontinuation) from ref.68.
The definition of a ‘chronic irAE’ lacks a general consensus. In our retrospective study assessing patients with melanoma who received an adjuvant anti-PD-1 antibody, we defined these as irAEs lasting ≥12 weeks following treatment discontinuation68. By this definition, approximately 43% of patients had at least one chronic irAE. However, additional definitions could be proposed (including for those with longer-term manifestations), and multidisciplinary input will be needed to establish a consensus definition.
Endocrine irAEs
The earliest recognized chronic irAEs were those affecting the endocrine organs, as seen in 15–40% of patients receiving ICIs. Endocrine toxicities, unlike other irAEs, are usually not managed using high-dose steroids, as data from several small-cohort studies suggest that steroids have no effect on either initial severity or ultimate resolution73,75. Furthermore, these events do not necessitate ICI discontinuation; the cornerstone of their management is replacement of the relevant hormone76.
Hypothyroidism is the most common endocrine irAE, occurring in around 10% of patients receiving anti-PD-1–PD-L1 antibodies as monotherapy and in up to 20% receiving ipilimumab plus nivolumab. Prior to hypothyroidism, approximately half of all patients have destructive thyroiditis resulting in excessive thyroid hormone secretions (thyrotoxicosis), which is often asymptomatic77. The median time to onset is approximately 6 weeks after commencing therapy, although hypothyroidism can arise at any time on therapy78. The preceding thyrotoxicosis is generally transient, although the subsequent thyroid hormone deficiency tends to be a lifelong condition75,79,80,81. Systematic efforts to wean patients off thyroid replacement (to confirm persistence) have, to our knowledge, not been made, although anecdotally we have not been successful in our attempts to discontinue hormone replacement therapy in such circumstances. Of note, thyroid dysfunction seems to be associated with a superior prognosis, although as with other toxicity–survival associations, time-dependent variables might be confounding82,83.
Hypophysitis is nearly unique to patients receiving ICIs. This condition occurs more often (5–10%) and with an earlier onset (median 9–12 weeks) in patients receiving ipilimumab-based regimens than in those receiving anti-PD-1–PD-L1 antibodies (<1%, median onset ~26 weeks)72. The predilection for those receiving ipilimumab seems to relate to expression of CTLA4 on the hormone-secreting cells of the pituitary gland, leading to antibody and complement binding48. Acutely, patients presenting with hypophysitis usually have symptoms of pituitary inflammation (headache, nausea and diplopia) and/or symptoms related to secondary adrenal insufficiency (fatigue and nausea). The inflammatory symptoms are generally transient, although hypopituitarism is usually permanent, and requires the replacement of glucocorticoids and often also thyroid and gonadal hormones84. Patients should receive the lowest physiological dose required to maintain quality of life (generally prednisone 5–7.5 mg daily or hydrocortisone 20–30 mg administered as two divided daily doses). Similar to hypothyroidism, hypopituitarism rarely resolves.
Other endocrine irAEs, although less common, are similarly persistent. ICI-induced diabetes mellitus (ICI–DM) occurs in <1% of treated patients but might present as diabetic ketoacidosis and generally requires lifelong insulin supplementation85,86. In marked contrast to hypophysitis, ICI–DM is almost exclusively seen in patients receiving anti-PD-1–PD-L1 antibodies, and only rarely with anti-CTLA4 monotherapy87. Serum amylase and lipase levels are often elevated at the time of ICI–DM diagnosis, suggesting that exocrine pancreatic inflammation might also have a role in pathogenesis, although whether persistent pancreatic insufficiency or chronic pancreatitis can frequently co-occur with ICI–DM is currently unclear88. Primary adrenal insufficiency is less common than hypopituitarism, albeit with a similar presentation, although this endocrinopathy might also involve mineralocorticoid deficiency and thus present with hypotension89.
The long-term effects of endocrine toxicities largely relate to the need to provide and maintain ongoing hormone replacement therapy rather than the presence of any chronic symptoms. Co-management with input from an endocrinologist might be helpful in optimizing the doses of replacement hormones and assessing for the uncommon cases where discontinuation might be possible. Assessment for hypogonadism is also warranted in patients with pituitary insufficiency. Studies involving fertility specifically in patients with hypopituitarism from ICIs, and studies on fertility in female patients treated with ICIs have, to our knowledge, not thus far been conducted. Data from several small cohorts suggest that male patients generally have preserved fertility following ICIs, although an inflammatory impairment of spermatogenesis remains possible in rare cases90,91.
Rheumatological irAEs
Given the spectrum of rheumatological autoimmune disorders, the occurrence of a wide variety of rheumatological irAEs seems unsurprising. Interestingly, systemic lupus erythematosus and mixed connective tissue disorder are thus far not associated with ICIs, although syndromes resembling RA, polymyalgia rheumatica, polymyositis and Sjögren syndrome all occur, potentially providing clues to the pathobiology of these syndromes and the role of immune checkpoints92. These syndromes closely resemble established rheumatological conditions, although certain key differences also exist, including a lack of a genetic association with HLA-B*27, and certain key clinical distinctions. Specifically, inflammatory arthritis tends to arise in the large or medium joints with possible migration to small joints over time. While this syndrome most closely resembles RA, it also has features reminiscent of spondyloarthropathies, including enthesitis93. Most studies indicate that the majority of patients are seronegative (for rheumatoid factor or cyclic citrullinated peptide), although in one series all six patients were seropositive; thus, the true incidence is not clearly defined93,94,95. Approximately half of all patients have lingering symptoms of arthritis lasting at least 6–12 months after discontinuation of an ICI that, in our experience, will evolve into a truly chronic condition in a subset96. Furthermore, the response to steroids is often suboptimal in both the acute and chronic setting, as data from one series indicate that two thirds of patients require disease-modifying antirheumatic drugs (DMARDs)97. Notably, these data also suggest that the use of steroids and DMARDs is not associated with a worsening of oncological outcomes97. When patients develop a more chronic phenotype, non-steroidal anti-inflammatory drugs and other conservative measures suffice in certain patients, although chronic low-dose steroids or DMARDs are required in others. Data from small series suggest that ICI-induced chronic arthritis has major negative effects on quality of life, at least comparable to those of other irAEs98. Additional data are needed to quantify the long-term course in these patients. Among other rheumatological irAEs, ICI-induced sicca syndrome has similarities to and also differences from Sjögren syndrome, in that xerostomia predominates rather than eye involvement99. Most patients report subjective improvements with sialagogues and/or steroids, although salivary flow frequently remains suppressed100.
A major challenging question, which is applicable to several organ systems but is most apparent in the context of rheumatological irAEs, is the management of chronic low-grade toxicities. Rheumatological irAEs might impair quality of life but are usually not sufficiently severe to justify ongoing high-dose steroids or ICI discontinuation (at least based on patient and/or physician preference). Thus, patients often continue to receive low-dose steroids and/or DMARDs in the presence of ongoing ICI therapy. The optimal strategy, in terms of optimal immunosuppressant choice or dose, timing of ICI discontinuation and antitumour outcomes remains poorly defined.
Gastrointestinal irAEs
Colitis occurs in up to 5% of patients receiving anti-PD-1 antibodies, and generally presents as diarrhoea and less often as abdominal pain and haematochezia. However, any grade diarrhoea (44%) or severe diarrhoea/colitis (15%) occur more often in patients receiving ipilimumab-containing regimens27,101. Hepatitis arises in 3–10% of patients (more often with ipilimumab-containing regimens) and is asymptomatic or might present as non-specific symptoms including malaise and myalgias, or less often as jaundice and acute liver failure. These high-profile events are major considerations in the acute setting but generally resolve with acute management and rarely evolve into a permanent phenotype (although symptoms can take multiple months to resolve, and thus meet the definition of chronic)68,102,103. Diarrhoea that fails to improve with steroids and/or other conventional immunomodulators (such as the anti-TNF antibody infliximab) should be evaluated carefully to rule out other causes, including ICI-induced coeliac disease, which has been reported rarely and necessitates a gluten-free diet (however, treatment-refractory colitis remains a more likely diagnosis than coeliac disease)104. Late-onset diarrhoea might also reflect pancreatic insufficiency. A 1% rate of steatorrhoea was reported in patients receiving anti-PD-1 antibodies in one cohort, with a median onset of 9 months105. Of note, 10% of this cohort developed radiographic signs of pancreatic atrophy, although the mechanisms and the clinical significance of this finding in asymptomatic patients remain to be seen. We are not aware of a chronic hepatitis phenotype that arises as a result of treatment with ICIs, although the possibility of subclinical liver injury, including following pre-existing liver inflammation (such as steatohepatitis), cannot entirely be ruled out.
Pulmonary irAEs
Pneumonitis is one of the major sources of both morbidity and mortality from anti-PD-1 antibodies, and most commonly presents as a dry cough, reduced oxygen saturation and bilateral ground-glass opacities (although a variety of distinct radiographic appearances can occur, including interstitial and organizing pneumonia). This presentation can overlap with that of COVID-19 or other viral infections, which should be ruled out106. Pneumonitis symptoms are usually responsive to steroids and/or second-line immunomodulation, although most patients have persistent imaging findings at least 1–2 years after symptom onset107,108. Most of these patients, however, have resolution of their symptoms, and detailed studies designed to assess for mild and/or subclinical decrements in lung function have not yet been performed. Paradoxically, data from preclinical models in at least one study suggest that anti-PD-1 antibodies could ameliorate ICI-induced pulmonary fibrosis109. We have also observed uncommon cases of patients with chronic wheezing or cough without radiographic evidence of pneumonitis with anti-PD-1 antibodies. Symptoms in these patients have generally improved over 6–12 months with inhalers and drug withdrawal, although we are not aware of any published reports in this area (and cannot completely rule out an atypical infectious trigger). Sarcoidosis might also complicate therapy but this is usually highly steroid-responsive (or will self-resolve) and rarely evolves into a chronic phenotype110. Reactivation of pulmonary tuberculosis can also occur111,112, although data from a retrospective study suggest a similar incidence in patients with cancer who did not receive ICIs, thus making it unclear whether ICIs have a role in this effect113.
Cardiovascular irAEs
Acute fulminant myocarditis was the first recognized cardiovascular irAE to be associated with an ICI, often presenting with electrocardiographic disturbances, including arrythmias and concurrent myositis114,115. However, increased recognition of this irAE and the resulting surveillance strategies led to the detection of more subtle forms of cardiac inflammation, ranging from smouldering myocarditis to asymptomatic elevations in serum troponin I116,117,118. An important unanswered question in the field relates to the long-term cardiac sequelae of these more subacute presentations of myocarditis. Extrapolating from non-ICI-associated myocarditis, one might expect that patients who recover from ICI-associated myocarditis would also have chronic consequences related to residual cardiomyopathy119. For example, skeletal muscle involvement can occur and might lead to paralysis or respiratory compromise (owing to diaphragmatic involvement) with the resultant complications of critical illness92,120. Other recognized cardiac sequelae include pericarditis, which is more common among patients with lung cancer receiving anti-PD-1–PD-L1 antibodies and is generally less fulminant and more responsive to corticosteroids121,122. Vascular irAEs have also been described and these include acute vasculitis, especially temporal arteritis and polymyalgia rheumatica122. In aggregate, these events are likely to occur in up to 1–2% of treated patients.
The more chronic sequelae of combination regimens involving an ICI and other traditional or targeted therapies is another important area of investigation, both of which might have their own inherent chronic cardiac toxicities123. An example is provided by the increasing use of ICIs in patients with breast cancer who previously received anthracyclines and radiotherapy124. Similarly, in patients with RCC and those with several other cancers, ICIs are often combined with VEGF inhibitors such as axitinib or lenvatinib, which have been associated with a number of cardiovascular sequelae including hypertension, vascular disease and cardiomyopathy125.
Neurological irAEs
Neurological irAEs occur in up to 5% of patients and are more common with ipilimumab-containing regimens. These events can affect the neuromuscular junction (myasthenia gravis and Lambert–Eaton myasthenic syndrome), the central nervous system (meningoencephalitis) and peripheral nerves (both sensory and motor neuropathy, including Guillain–Barré syndrome)126,127. Meningoencephalitis usually resolves with acute management (although rarely can be fatal), and whether chronic deficits occur in some patients remains unclear. Myasthenia gravis might present with acetylcholinesterase receptor (AchR) positivity and evolve into a stereotypical chronic syndrome128, but can also co-occur with myositis and myocarditis, and is often AchR-negative in this scenario. The long-term outcomes of ICI-associated myasthenia gravis are not well-characterized, although in most patients it appears to either completely resolve or remain controlled with disease-specific therapy129,130. ICI-associated Guillain–Barré syndrome has a high fatality rate (six of 31 patients (19%) in one series died of this irAE), and even patients whose symptoms do improve with immunomodulation often have residual weakness and/or sensory loss (in one series, 68% of patients had residual symptoms)126,131. Peripheral neuropathy seems to be the neurological irAE that is most likely to evolve into a chronic phenotype132. Peripheral sensory neuropathy has been reported in approximately 2% of patients who received adjuvant anti-PD-1 antibodies for resected melanoma and nearly half of these patients developed chronic peripheral neuropathy68.
Cutaneous irAEs
Skin toxicities are among the most common complications seen among patients receiving ICIs, and include various inflammatory dermatitis syndromes, pruritus and vitiligo. Dermatitis is usually manageable with topical or occasionally systemic steroids. Pruritus without rash might be the result of a neurogenic itch, and respond more effectively to GABAergic agonists (such as pregabalin) than to steroids133. These events often present a challenging clinical dilemma as bothersome but not life-threatening irAEs that do not seem sufficiently symptomatic to discontinue treatment or require high-dose steroids that nonetheless can negatively affect quality of life. Ongoing use of antihistamines, topical steroids and/or GABAergic agonists might all be required in patients with ICI-induced forms of dermatitis or pruritus. Involvement of a dermatologist in the co-management of cutaneous irAEs leads to higher treatment rates and even improved survival (potentially owing to the facilitation of ICI continuation)134. In our experience, even though dermatitis and pruritus frequently linger for weeks or months beyond treatment discontinuation, these irAEs tend to ultimately resolve (although this situation has not been well-defined in the literature, to our knowledge). Vitiligo, which is far more common in patients with melanoma than in those with other cancers, often fails to resolve and might become a lifelong complication. Severe cutaneous reactions, such as Stephens–Johnson syndrome and bullous pemphigoid, might also have life-threatening and/or long-term consequences135.
Other irAEs
Similar to acute irAEs, a wide constellation of low-frequency events might ultimately become chronic. Nephritis, while usually steroid-responsive136, required haemodialysis in approximately 10% of patients in one series; half of these patients failed to recover adequate renal function and required ongoing renal replacement therapy137. Haematological toxicities, including idiopathic thrombocytopenic purpura, aplastic anaemia, haemophagocytic lymphangiohistiocytosis and pure red cell aplasia can also all occur, albeit rarely, and might be fatal138, although most of these irAEs resolve and do not evolve into a chronic condition139. Ocular symptoms might include uveitis and conjunctivitis, and can be managed using ophthalmic steroids140. We found that the few affected patients in our study often had chronic symptoms68, although data from larger series of patients with ICI-related uveitis suggest that most patients have symptom improvement with treatment141. Other constitutional symptoms such as fatigue might also remain after ICI discontinuation, although, in our experience, this manifestation will usually ultimately improve and/or resolve. The pathophysiology, incidence and time course of these non-classic irAE sequelae have not been studied systematically.
Patients with pre-existing autoimmune disorders are largely excluded from clinical trials, and their long-term outcomes are not well described. Data from a number of studies suggest that these patients have similar or slightly higher rates of classic irAEs, and similar antitumour responses compared with those of individuals without autoimmune disease, although these patients also have high incidences of autoimmune flares (20–25%)142,143,144,145. In our anecdotal experience, any autoimmune disease flares will usually improve following treatment discontinuation, although the long-term outcomes in these patients need to be studied in more detail. Patients with a history of solid organ transplantation are another challenging population. These patients have a high risk of allograft rejection on receiving an ICI (approximately 50%)146, which can lead to chronic complications (such as a need for long-term dialysis following rejection of a donor kidney) or have fatal consequences.
Fatal irAEs
Fatal irAEs are rare but can arise owing to overwhelming autoinflammation that is refractory to steroids and/or other immunosuppressants. Although these events clearly belong in a different category than chronic toxicities, the risks of fatal events need to be particularly considered in the context of the potentially durable responses generated by ICIs (such as early-onset fatal toxicity in a patient who might have had a response lasting years or even decades). These events highlight the need for effective rescue agents and rigorous studies to provide additional evidence-based approaches to the treatment of refractory irAEs147,148,149. Data from meta-analyses suggest that fatal irAEs occur at low rates, ranging from 0.4% with anti-PD-1–PD-L1 antibodies as monotherapy to approximately 1.2% with combination anti-CTLA4–anti-PD-1 regimens150. Although non-trivial, these fatality rates compare relatively favourably with those associated with cytotoxic chemotherapy, molecularly targeted therapy and even high-risk surgical procedures for cancer151,152,153,154. The incidence of death from irAEs is also many-fold lower than that associated with cancer, even in indications with regimens that provide high durable response rates where toxicities are also a common occurrence. For example, in CheckMate-067, a phase III clinical trial in which patients with metastatic melanoma received ipilimumab 3 mg/kg and nivolumab 1 mg/kg versus either agent as monotherapy, two patients receiving this combination died of fatal toxicities compared with 130 cancer-related deaths at the last follow-up cut-off point69.
Fatalities can arise from various organ-specific irAEs. Myocarditis is the irAE with the highest fatality rate (25–50% of patients)114,115, largely owing to the refractory arrhythmias seen in severe cases. Myositis can also accompany myocarditis, resulting in diaphragmatic paralysis, respiratory failure and failure to gain independence from ventilatory support92. Pneumonitis is fatal in 10–15% of patients, generally owing to respiratory failure155. Hepatitis (from fulminant hepatic failure) and colitis (from colon perforation or less frequently from voluminous diarrhoea) can also cause death, although typically with a much lower incidence102,103. Neurological irAEs, while uncommon, can also be fatal in 10–15% of patients, often owing to refractory or prolonged Guillain–Barré syndrome, encephalitis or myasthenia gravis-like syndrome126. Of note, fatal irAEs often arise early in the course of therapy (earlier than most non-fatal events), at a median of 15 days (combination therapy) and 40 days (anti-PD-1 antibodies as monotherapy)150, suggesting that pre-existing, organ-specific inflammation that is rapidly unleashed by ICI initiation might be responsible for these events. A trend towards older age of patients with fatal irAEs (compared with non-fatal events) has also been observed, suggesting that older age and the associated decreased functional reserve might predispose patients to a higher risk of death150. Prolonged immunosuppression might be the proximate cause of death in patients with protracted, refractory toxicities because the presence of opportunistic infections might also complicate the clinical course156.
Clinicians should incorporate these non-negligible but low risks of fatal irAEs into treatment decision making in several ways. These risks of uncommon fatal irAEs are appropriately overshadowed by the near universal lethality of untreated metastatic cancer, particularly when considering potentially more toxic (and often less effective) alternative therapies. Nonetheless, for a subset of patients with low-risk disease, oncologists should consider the risks of fatal irAEs when selecting therapy. For example, patients with low-volume metastatic melanoma and/or severe co-morbidities could consider an anti-PD-1 antibody only, with combination therapy reserved for the onset of disease progression157. Analogously, in patients with very low-risk AJCC stage IIIA melanoma, withholding adjuvant therapy and treatment only on disease progression could be considered. Similarly, in patients with stage III BRAFV600E-mutant melanoma, BRAF/MEK inhibition might be considered in this setting158, although the risks of cancer recurrence (and the often unclear risks of fatal toxicities with alternative treatments) must be taken into account. These remain individualized decisions, however, and universal recommendations surrounding the risk of uncommon chronic or fatal irAEs remain challenging. Another point of contention surrounds monitoring and surveillance. Given the risk of fatal irAEs in patients receiving combination anti-PD-1 and anti-CTLA-4 antibodies (approximately 1.2%) and a typically early onset (median onset prior to the second dose), we recommend weekly laboratory and clinical monitoring for the first 3–4 weeks in patients receiving combination therapy, including testing for serum troponin I. One could reasonably argue that without clear data to support close monitoring, a 0.4% risk of fatal irAEs in patients receiving anti-PD-1 antibodies as monotherapy does not justify an analogous surveillance approach.
Implications for rechallenge
Another long-term implication of irAEs is whether patients who derived some benefit from treatment but also had severe toxicities should be rechallenged upon resolution of the irAE. In many patients this is an unnecessary consideration, particularly in those who have delayed irAEs after a complete response, or in those with clear disease progression on therapy. Nonetheless, clinicians might seek to rechallenge a subset of patients, including those with early-onset toxicities who might benefit from further therapy, or patients in whom an ICI was stopped while still responding and then later had disease progression.
Prospective data on the safety of rechallenge are currently lacking, although understanding certain key trends can assist in treatment decision-making. Data from retrospective studies suggest that recurrence of irAEs occurs in approximately 25–50% of patients rechallenged with anti-PD-1–PD-L1 antibodies159,160,161. De-escalation of therapy (such as de-escalation from combination anti-PD-1–anti-CTLA4 antibodies to anti-PD-1 monotherapy) seems to be associated with a lower risk of irAE recurrence (18% in one series), particularly for colitis (<5%)162. Determining which patients have the highest risk of recurrent irAEs is currently challenging; data from one series suggest that colitis, pneumonitis and hepatitis recur more frequently on rechallenge than do other irAEs; older age is also associated with irAE recurrence159. Data from other series suggest low rates of hepatitis recurrence, thus adding a further level of uncertainty163. A longer delay between discontinuation and rechallenge would also presumably decrease the risk of recurrent toxicities (for example, in a patient who is re-treated >12 months after discontinuing therapy owing to an irAE), although the data for such associations are currently not clear. Clinicians should consider both the type and severity of the irAE, as well as the clinical need for rechallenge in making decisions regarding the reintroduction of therapy. If rechallenge is undertaken, close clinical and/or laboratory monitoring should be used to assess for possible irAE recurrence.
A theoretical approach that has yet to undergo widespread clinical testing is to resume ICIs in conjunction with selective immunomodulatory therapy. Data from a series of five patients suggest that ongoing administration of infliximab enables the resumption of ICIs in patients with colitis164. Data from preclinical studies suggest that this approach might even improve the antitumour activity of anti-PD-1 antibodies165. Similarly, targeted inhibition of cytokines such as IL-6 could theoretically uncouple antitumour immune responses from irAEs and permit safe rechallenge. Several studies testing these approaches clinically are currently ongoing (for example NCT03293784, NCT03999749 and NCT04940299).
Effects on other immune processes
Immune dysfunction has a role in many chronic diseases outside classic autoimmunity. Thus, perturbations involving prolonged treatment with ICIs could theoretically influence several diverse pathobiological processes including atherosclerosis, obesity and neuroinflammation, potentially over very prolonged timelines (Fig. 4). Data from preclinical models suggest that genetic inactivation of PD-1–PD-L1 potentiates the burden of atherosclerosis and promotes the infiltration of macrophages, CD4+ T cells and CD8+ T cells into atherosclerotic plaques166,167. Other high-dimensional sequencing studies of atherosclerotic plaques have shown high levels of CD8+ tissue-resident memory cells with both activation and exhaustion markers, identifying a cellular population with functions that could be activated or promoted using ICIs, potentially leading to atherosclerosis and/or plaque rupture168. Early clinical data support this concern: data from a matched cohort study indicate a threefold higher incidence of atherosclerotic cardiovascular events in the 2 years following ICI therapy compared with a similar pretreatment time frame169. However, conclusively linking this association epidemiologically can be difficult owing to the presence of several confounding variables, including ascertainment bias in patients with advanced-stage cancer, who are typically subject to more intensive clinical monitoring following diagnosis. Perhaps more convincingly, data from several small-cohort imaging studies suggest an increased incidence of progression of aortic plaques and inflammatory activity following treatment with ICIs169,170. The growing appreciation of inflammation as a major contributor to atherosclerosis provides evidence supporting the biological plausibility of this suggestion171. Intriguingly, data from a small-cohort study suggest superior oncological outcomes in patients receiving β-blockers, although several other studies suggest either no benefit or even potentially worse survival outcomes172,173,174.
The relevant time frame of important patient-specific/toxicity-related factors (top), includes acute toxicities, which are largely relevant while patients are on therapy, chronic toxicities, which probably slowly decline over time, and the potential (theoretical) effects of such toxicities on other immune processes such as atherosclerosis, which could increase over time. Tumour-specific factors, including the risks of recurrence and the need for rechallenge, both of which appear to decrease over time are depicted below the timeline. ICI, immune-checkpoint inhibitor.
Regarding the effects of ICIs in patients with obesity, and the related obesity-mediated inflammation, preclinical studies suggest that adipose tissue T cells express markers of T cell exhaustion and that T cells have a key role in obesity-related complications (such as steatohepatitis). These observations raise the question as to whether ICIs might reactivate these T cells and exacerbate the severity of obesity and its related complications175,176. ICIs cause increased obesity-associated inflammation in the mammary fat pads of mice with obesity-associated breast cancer177. Interestingly, data from another model suggest that adipocyte-dependent Pdl1 knockout results in decreased tumour growth but increased obesity-associated inflammation178. As an aside, several studies notably suggest that obesity correlates with improved responses to ICIs179,180, although others have not found such an association181,182. Similarly, some but not all studies suggest that obesity might increase the risk of irAEs181,183,184,185.
Data from a variety of studies demonstrate that post-injury inflammation (such as that seen following ischaemia and/or trauma) is associated with high levels of activated T cells and high levels of immune-checkpoint expression186. However, functional studies differ regarding whether PD-1–PD-L1 inhibition or gene knockout is either protective187 or detrimental in this setting188. Similarly, inhibition of PD-1–PD-L1 has been reported to improve the pathology of both Alzheimer disease and tauopathy in mouse models, although this has yet to be clinically confirmed189,190. Clinical trials assessing the effects of modulation of immune-checkpoint signalling pathways on neurological processes might be an intriguing research direction in this challenging set of diseases.
Conclusions
The potential for durable responses, activity in a broad range of cancers and generally manageable toxicities has rendered ICIs an attractive and widely used treatment option for patients with cancer. This widespread use has broadened treatment-related and survivorship considerations beyond generating an antitumour immune response to lifelong effects on quality of life. In addition to chronic, classic irAEs, the long-term effects on global immune function triggered by blocking immune-checkpoint molecules remain unclear and need further study.
References
Haslam, A. & Prasad, V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw. Open. 2, e192535 (2019).
Vilgelm, A. E., Johnson, D. B. & Richmond, A. Combinatorial approach to cancer immunotherapy: strength in numbers. J. Leukoc. Biol. 100, 275–290 (2016).
Topalian, S. L. et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 5, 1411–1420 (2019).
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Philip, M. et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456 (2017).
Barton, B. M., Xu, R., Wherry, E. J. & Porrett, P. M. Pregnancy promotes tolerance to future offspring by programming selective dysfunction in long-lived maternal T cells. J. Leukoc. Biol. 101, 975–987 (2017).
Shim, Y. J. et al. Early T cell infiltration is modulated by programed cell death-1 protein and its ligand (PD-1/PD-L1) interactions in murine kidney transplants. Kidney Int. 98, 897–905 (2020).
Selby, M. J. et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 1, 32–42 (2013).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Saha, B. et al. Toxic shock syndrome toxin-1-induced death is prevented by CTLA4Ig. J. Immunol. 157, 3869–3875 (1996).
Waterhouse, P. et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 (1995).
Tivol, E. A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995).
Nishimura, H. et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 (1999).
Nishimura, H. et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 (2001).
Tarhini, A. A. et al. Phase III study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma: North American Intergroup E1609. J. Clin. Oncol. 38, 567–575 (2020).
Eggermont, A. M. M. et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III melanoma: updated results from the EORTC 1325-MG/KEYNOTE-054 trial. J. Clin. Oncol. 38, 3925–3936 (2020).
Weber, J. et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 377, 1824–1835 (2017).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Yarchoan, M., Hopkins, A. & Jaffee, E. M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 377, 2500–2501 (2017).
Zhao, B., Zhao, H. & Zhao, J. Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials. Ther. Adv. Med. Oncol. 12, 1758835920937612 (2020).
Larkin, J. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381, 1535–1546 (2019).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Wong, S. K., Beckermann, K. E., Johnson, D. B. & Das, S. Combining anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) and -programmed cell death protein 1 (PD-1) agents for cancer immunotherapy. Expert Opin. Biol. Ther. 21, 1623–1634 (2021).
Wolchok, J. D. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377, 1345–1356 (2017).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Waterhouse, D. M. et al. Continuous versus 1-year fixed-duration nivolumab in previously treated advanced non-small-cell lung cancer: CheckMate 153. J. Clin. Oncol. 38, 3863–3873 (2020).
Jansen, Y. J. L. et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann. Oncol. 30, 1154–1161 (2019).
Robert, C. et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J. Clin. Oncol. 36, 1668–1674 (2018).
Betof Warner, A. et al. Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J. Clin. Oncol. 38, 1655–1663 (2020).
Johnson, D. B. et al. Tumor-specific MHC-II expression drives a unique pattern of resistance to immunotherapy via LAG-3/FCRL6 engagement. JCI Insight 3, e120360 (2018).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Sade-Feldman, M. et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 8, 1136 (2017).
Kato, T. et al. Peripheral T cell receptor repertoire features predict durable responses to anti-PD-1 inhibitor monotherapy in advanced renal cell carcinoma. Oncoimmunology 10, 1862948 (2021).
Spassova, I. et al. Predominance of central memory T cells with high T-cell receptor repertoire diversity is associated with response to PD-1/PD-L1 inhibition in Merkel cell carcinoma. Clin. Cancer Res. 26, 2257–2267 (2020).
Han, J. et al. Resident and circulating memory T cells persist for years in melanoma patients with durable responses to immunotherapy. Nat. Cancer 2, 300–311 (2021).
Brahmer, J. R. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010).
Patnaik, A. et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin. Cancer Res. 21, 4286–4293 (2015).
Das, S. & Johnson, D. B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 7, 306 (2019).
Quach, H. T. et al. Association of anti-programmed cell death 1 cutaneous toxic effects with outcomes in patients with advanced melanoma. JAMA Oncol. 5, 906–908 (2019).
Eggermont, A. M. M. et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 6, 519–527 (2020).
Berner, F. et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 5, 1043–1047 (2019).
Johnson, D. B. et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 375, 1749–1755 (2016).
Guida, M. et al. Immune checkpoint inhibitor associated vitiligo and its impact on survival in patients with metastatic melanoma: an Italian Melanoma Intergroup study. ESMO Open 6, 100064 (2021).
Johnson, D. B. et al. A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat. Med. 25, 1243–1250 (2019).
Dubin, K. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391 (2016).
Iwama, S. et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 6, 230ra45 (2014).
Wei, S. C. et al. A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov. 11, 614–625 (2021).
Luoma, A. M. et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell 182, 655–671.e22 (2020).
Andrews, M. C. et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 27, 1432–1441 (2021).
Das, R. et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Invest. 128, 715–720 (2018).
Yasuda, Y. et al. CD4+ T cells are essential for the development of destructive thyroiditis induced by anti-PD-1 antibody in thyroglobulin-immunized mice. Sci. Transl. Med. 13, eabb7495 (2021).
Johnson, D. H. et al. Interleukin-6 is potential target to de-couple checkpoint inhibitor-induced colitis from antitumor immunity. J. Clin. Oncol. 37, 2616 (2019).
Stroud, C. R. et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J. Oncol. Pharm. Pract. 25, 551–557 (2019).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Johnson, D. B., Chandra, S. & Sosman, J. A. Immune checkpoint inhibitor toxicity in 2018. JAMA 320, 1702–1703 (2018).
Weber, J. S. et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35, 785–792 (2017).
Thompson, J. A. et al. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1.2020. J. Natl Compr. Canc Netw. 18, 230–241 (2020).
Brahmer, J. R. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 9, 002435e (2021).
Haanen, J. et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29, iv264–iv266 (2018).
Bai, X. et al. Early use of high-dose glucocorticoid for the management of irAE is associated with poorer survival in patients with advanced melanoma treated with anti-PD-1 monotherapy. Clin. Cancer Res. 27, 5993–6000 (2021).
Dearden, H. et al. Hyperacute toxicity with combination ipilimumab and anti-PD1 immunotherapy. Eur. J. Cancer 153, 168–178 (2021).
Owen, C. N. et al. Delayed immune-related adverse events with anti-PD-1-based immunotherapy in melanoma. Ann. Oncol. 32, 917–925 (2021).
Dimitriou, F. et al. FDG-PET to predict long-term outcome from anti-PD1 therapy in metastatic melanoma. Ann. Oncol. 33, 99–106 (2021).
Lee, R. J. et al. Circulating tumor DNA predicts survival in patients with resected high-risk stage II/III melanoma. Ann. Oncol. 29, 490–496 (2018).
Ott, P. A. et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol. 37, 318–327 (2019).
Patrinely, J. R. Jr et al. Chronic immune-related adverse events following adjuvant anti-PD-1 therapy for high-risk resected melanoma. JAMA Oncol. 7, 744–748 (2021).
Wang, L. X. et al. Health care utilization and steroid-refractory toxicities from immune checkpoint inhibitors. Cancer 126, 322–328 (2020).
Johnson, D. B. et al. Survivorship in immune therapy: assessing chronic immune toxicities, health outcomes, and functional status among long-term ipilimumab survivors at a single referral center. Cancer Immunol. Res. 3, 464–469 (2015).
Patrinely, J. R. Jr et al. Survivorship in immune therapy: assessing toxicities, body composition and health-related quality of life among long-term survivors treated with antibodies to programmed death-1 receptor and its ligand. Eur. J. Cancer 135, 211–220 (2020).
Faje, A. et al. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur. J. Endocrinol. 181, 211–219 (2019).
Faje, A. T. et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 124, 3706–3714 (2018).
Cappelli, L. C. & Bingham, C. O. 3rd Spectrum and impact of checkpoint inhibitor-induced irAEs. Nat. Rev. Rheumatol. 17, 69–70 (2021).
Ma, C. et al. The impact of high-dose glucocorticoids on the outcome of immune-checkpoint inhibitor-related thyroid disorders. Cancer Immunol. Res. 7, 1214–1220 (2019).
Wright, J. J., Powers, A. C. & Johnson, D. B. Endocrine toxicities of immune checkpoint inhibitors. Nat. Rev. Endocrinol. 40, 17–65 (2021).
Muir, C. A. et al. Thyroid immune-related adverse events following immune checkpoint inhibitor treatment. J. Clin. Endocrinol. Metab. 106, e3704–e3713 (2021).
Iyer, P. C. et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid 28, 1243–1251 (2018).
Lee, H. et al. Characterization of thyroid disorders in patients receiving immune checkpoint inhibition therapy. Cancer Immunol. Res. 5, 1133–1140 (2017).
Tan, M. H. et al. Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: a scoping review of case reports. Clin. Diabetes Endocrinol. 5, 1 (2019).
Al Mushref, M. et al. Thyroid dysfunction, recovery, and prognosis in melanoma patients treated with immune checkpoint inhibitors: a retrospective review. Endocr. Pract. 26, 36–42 (2020).
Sakakida, T. et al. Clinical features of immune-related thyroid dysfunction and its association with outcomes in patients with advanced malignancies treated by PD-1 blockade. Oncol. Lett. 18, 2140–2147 (2019).
Street, S. et al. The positive effect of immune checkpoint inhibitor-induced thyroiditis on overall survival accounting for immortal time bias: a retrospective cohort study of 6,596 patients. Ann. Oncol. 32, 1050–1051 (2021).
Garon-Czmil, J. et al. Immune check point inhibitors-induced hypophysitis: a retrospective analysis of the French Pharmacovigilance database. Sci. Rep. 9, 19419 (2019).
de Filette, J. et al. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm. Metab. Res. 51, 145–156 (2019).
Tsang, V. H. M. et al. Checkpoint inhibitor-associated autoimmune diabetes is distinct from type 1 diabetes. J. Clin. Endocrinol. Metab. 104, 5499–5506 (2019).
Wright, J. J. et al. Increased reporting of immune checkpoint inhibitor-associated diabetes. Diabetes Care 41, e150–e151 (2018).
Stamatouli, A. M. et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 67, 1471–1480 (2018).
Grouthier, V. et al. Immune checkpoint inhibitor-associated primary adrenal insufficiency: WHO VigiBase Report Analysis. Oncologist 25, 696–701 (2020).
Salzmann, M. et al. Male fertility during and after immune checkpoint inhibitor therapy: a cross-sectional pilot study. Eur. J. Cancer 152, 41–48 (2021).
Scovell, J. M. et al. Association of impaired spermatogenesis with the use of immune checkpoint inhibitors in patients with metastatic melanoma. JAMA Oncol. 6, 1297–1299 (2020).
Allenbach, Y. et al. Immune checkpoint inhibitor-induced myositis, the earliest and most lethal complication among rheumatic and musculoskeletal toxicities. Autoimmun. Rev. 19, 102586 (2020).
Cappelli, L. C. et al. Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Semin. Arthritis Rheum. 48, 553–557 (2018).
Belkhir, R. et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann. Rheum. Dis. 76, 1747–1750 (2017).
Naidoo, J. et al. Inflammatory arthritis: a newly recognized adverse event of immune checkpoint blockade. Oncologist 22, 627–630 (2017).
Braaten, T. J. et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann. Rheum. Dis. 25, 696–701 (2019).
Roberts, J. et al. Rheumatic immune-related adverse events associated with cancer immunotherapy: a nationwide multi-center cohort. Autoimmun. Rev. 19, 102595 (2020).
Cappelli, L. C. et al. Immune checkpoint inhibitor-induced inflammatory arthritis: a qualitative study identifying unmet patient needs and care gaps. BMC Rheumatol. 4, 32 (2020).
Cappelli, L. C. et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann. Rheum. Dis. 76, 43–50 (2017).
Warner, B. M. et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist 24, 1259–1269 (2019).
Wang, D. Y., Ye, F., Zhao, S. & Johnson, D. B. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: a systematic review and meta-analysis. Oncoimmunology 6, e1344805 (2017).
Patrinely, J. R. Jr et al. A multicenter characterization of hepatitis associated with immune checkpoint inhibitors. Oncoimmunology 10, 1875639 (2021).
Wang, D. Y. et al. Clinical characterization of colitis arising from anti-PD-1 based therapy. Oncoimmunology 8, e1524695 (2019).
Badran, Y. R. et al. Immune checkpoint inhibitor-associated celiac disease. J. Immunother. Cancer 8, e000958 (2020).
Eshet, Y. et al. Clinical significance of pancreatic atrophy induced by immune-checkpoint inhibitors: a case-control study. Cancer Immunol. Res. 6, 1453–1458 (2018).
Sullivan, R. J. et al. COVID-19 and immune checkpoint inhibitors: initial considerations. J. Immunother. Cancer 8, e000933 (2020).
Johnson, D. B. et al. Anti-PD-1-induced pneumonitis is associated with persistent imaging abnormalities in melanoma patients. Cancer Immunol. Res. 7, 1755–1759 (2019).
Dobre, I. A. et al. Outcomes of patients with interstitial lung disease receiving programmed cell death 1 inhibitors: a retrospective case series. Clin. Lung Cancer 22, e738–e744 (2021).
Celada, L. J. et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci. Transl. Med. 10, eaar8356 (2018).
Chorti, E. et al. Drug-induced sarcoidosis-like reaction in adjuvant immunotherapy: increased rate and mimicker of metastasis. Eur. J. Cancer 131, 18–26 (2020).
Tezera, L. B. et al. Anti-PD-1 immunotherapy leads to tuberculosis reactivation via dysregulation of TNF-α. eLife 9, e52668 (2020).
Anastasopoulou, A. et al. Reactivation of tuberculosis in cancer patients following administration of immune checkpoint inhibitors: current evidence and clinical practice recommendations. J. Immunother. Cancer 7, 239 (2019).
Bae, S. et al. Risk of tuberculosis in patients with cancer treated with immune checkpoint inhibitors: a nationwide observational study. J. Immunother. Cancer 9, e002960 (2021).
Moslehi, J. J. et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 391, 933 (2018).
Mahmood, S. S. et al. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 71, 1755–1764 (2018).
Bonaca, M. P. et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation 140, 80–91 (2019).
Norwood, T. G. et al. Smoldering myocarditis following immune checkpoint blockade. J. Immunother. Cancer 5, 91 (2017).
Waliany, S. et al. Myocarditis surveillance with high-sensitivity troponin i during cancer treatment with immune checkpoint inhibitors. JACC Cardio. Oncol. 3, 137–139 (2021).
Ammirati, E. et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ. Heart Fail. 13, e007405 (2020).
Kadota, H. et al. Immune checkpoint inhibitor-induced myositis: a case report and literature review. Curr. Rheumatol. Rep. 21, 10 (2019).
Ala, C. K., Klein, A. L. & Moslehi, J. J. Cancer treatment-associated pericardial disease: epidemiology, clinical presentation, diagnosis, and management. Curr. Cardiol. Rep. 21, 156 (2019).
Salem, J. E. et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 19, 1579–1589 (2018).
Moslehi, J. J. Cardiovascular toxic effects of targeted cancer therapies. N. Engl. J. Med. 375, 1457–1467 (2016).
Moslehi, J. The cardiovascular perils of cancer survivorship. N. Engl. J. Med. 368, 1055–1056 (2013).
Bair, S. M., Choueiri, T. K. & Moslehi, J. Cardiovascular complications associated with novel angiogenesis inhibitors: emerging evidence and evolving perspectives. Trends Cardiovasc. Med. 23, 104–113 (2013).
Johnson, D. B. et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J. Immunother. Cancer 7, 134 (2019).
Guidon, A. C. et al. Consensus disease definitions for neurologic immune-related adverse events of immune checkpoint inhibitors. J. Immunother. Cancer 9, e002890 (2021).
Johnson, D. B. et al. Myasthenia gravis induced by ipilimumab in patients with metastatic melanoma. J. Clin. Oncol. 33, e122–e124 (2015).
Reynolds, K. L. & Guidon, A. C. Diagnosis and management of immune checkpoint inhibitor-associated neurologic toxicity: illustrative case and review of the literature. Oncologist 24, 435–443 (2019).
Haugh, A. M., Probasco, J. C. & Johnson, D. B. Neurologic complications of immune checkpoint inhibitors. Expert Opin. Drug Saf. 19, 479–488 (2020).
Janssen, J. B. E. et al. Immune checkpoint inhibitor-related Guillain-Barré syndrome: a case series and review of the literature. J. Immunother. 44, 276–282 (2021).
Dubey, D. et al. Varied phenotypes and management of immune checkpoint inhibitor-associated neuropathies. Neurology 93, e1093–e1103 (2019).
Phillips, G. S. et al. Treatment outcomes of immune-related cutaneous adverse events. J. Clin. Oncol. 37, 2746–2758 (2019).
Thompson, L. L. et al. Effect of dermatological consultation on survival in patients with checkpoint inhibitor-associated cutaneous toxicity. Br. J. Dermatol. 185, 627–635 (2021).
Molina, G. E., Reynolds, K. L. & Chen, S. T. Diagnostic and therapeutic differences between immune checkpoint inhibitor-induced and idiopathic bullous pemphigoid: a cross-sectional study. Br. J. Dermatol. 183, 1126–1128 (2020).
Lee, M. D. et al. Rapid corticosteroid taper versus standard of care for immune checkpoint inhibitor induced nephritis: a single-center retrospective cohort study. J. Immunother. Cancer 9, e002292 (2021).
Cortazar, F. B. et al. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J. Am. Soc. Nephrol. 31, 435–446 (2020).
Davis, E. J. et al. Hematologic complications of immune checkpoint inhibitors. Oncologist 24, 584–588 (2019).
Shiuan, E. et al. Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J. Immunother. Cancer 5, 8 (2017).
Sun, M. M. et al. Ophthalmic immune-related adverse events after anti-CTLA-4 or PD-1 therapy recorded in the American Academy of Ophthalmology Intelligent Research in Sight Registry. Ophthalmology 128, 910–919 (2021).
Dow, E. R., Yung, M. & Tsui, E. Immune checkpoint inhibitor-associated uveitis: review of treatments and outcomes. Ocul. Immunol. Inflamm. 29, 203–211 (2021).
Halle, B. R. et al. Immune checkpoint inhibitors in patients with pre-existing psoriasis: safety and efficacy. J. Immunother. Cancer 9, e003066 (2021).
Abu-Sbeih, H. et al. Immune checkpoint inhibitor therapy in patients with preexisting inflammatory bowel disease. J. Clin. Oncol. 38, 576–583 (2020).
Menzies, A. M. et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 28, 368–76 (2017).
Johnson, D. B. et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2, 234–240 (2016).
Kumar, V. et al. The safety and efficacy of checkpoint inhibitors in transplant recipients: a case series and systematic review of literature. Oncologist 25, 505–514 (2020).
Esfahani, K. et al. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N. Engl. J. Med. 380, 2375–2376 (2019).
Salem, J. E. et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N. Engl. J. Med. 380, 2377–2379 (2019).
Haanen, J. et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann. Oncol. 31, 724–744 (2020).
Wang, D. Y. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4, 1721–1728 (2018).
Pignon, J. P. et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 26, 3552–3559 (2008).
Long, G. V. et al. Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J. Clin. Oncol. 34, 871–878 (2016).
Cameron, J. L., Riall, T. S., Coleman, J. & Belcher, K. A. One thousand consecutive pancreaticoduodenectomies. Ann. Surg. 244, 10–15 (2006).
Gooley, T. A. et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 363, 2091–2101 (2010).
Naidoo, J. et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J. Clin. Oncol. 35, 709–717 (2017).
Kyi, C. et al. Opportunistic infections in patients treated with immunotherapy for cancer. J. Immunother. Cancer 2, 19 (2014).
Olson, D. J. et al. Pembrolizumab plus ipilimumab following anti-PD-1/L1 failure in melanoma. J. Clin. Oncol. 39, 2647–2655 (2021).
Dummer, R. et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. N. Engl. J. Med. 383, 1139–1148 (2020).
Dolladille, C. et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 6, 865–871 (2020).
Santini, F. C. et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol. Res. 6, 1093–1099 (2018).
Abu-Sbeih, H. et al. Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J. Clin. Oncol. 37, 2738–2745 (2019).
Pollack, M. H. et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann. Oncol. 29, 250–255 (2018).
Li, M. et al. Outcomes after resumption of immune checkpoint inhibitor therapy after high-grade immune-mediated hepatitis. Cancer 126, 5088–5097 (2020).
Badran, Y. R. et al. Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse events. J. Immunother. Cancer 7, 226 (2019).
Bertrand, F. et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat. Commun. 8, 2256 (2017).
Grabie, N. et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation 116, 2062–2071 (2007).
Gotsman, I. et al. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J. Clin. Invest. 117, 2974–2982 (2007).
Fernandez, D. M. et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 25, 1576–1588 (2019).
Drobni, Z. D. et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 142, 2299–2311 (2020).
Calabretta, R. et al. Immune checkpoint inhibitor therapy induces inflammatory activity in large arteries. Circulation 142, 2396–2398 (2020).
Libby, P. The changing landscape of atherosclerosis. Nature 592, 524–533 (2021).
Oh, M. S. et al. The impact of beta blockers on survival outcomes in patients with non-small-cell lung cancer treated with immune checkpoint inhibitors. Clin. Lung Cancer 22, e57–e62 (2021).
Oren, O. et al. Cardiovascular health and outcomes in cancer patients receiving immune checkpoint inhibitors. Am. J. Cardiol. 125, 1920–1926 (2020).
Wang, D. Y. et al. The impact of nonsteroidal anti-inflammatory drugs, beta blockers, and metformin on the efficacy of anti-PD-1 therapy in advanced melanoma. Oncologist 25, e602–e605 (2020).
Porsche, C. E. et al. Obesity results in adipose tissue T cell exhaustion. JCI Insight 6, e139793 (2021).
Breuer, D. A. et al. CD8(+) T cells regulate liver injury in obesity-related nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 318, G211–G224 (2020).
Pingili, A. K. et al. Immune checkpoint blockade reprograms systemic immune landscape and tumor microenvironment in obesity-associated breast cancer. Cell Rep. 35, 109285 (2021).
Wu, B. et al. Genetic ablation of adipocyte PD-L1 reduces tumor growth but accentuates obesity-associated inflammation. J. Immunother. Cancer 8, e000964 (2020).
McQuade, J. L. et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 19, 310–322 (2018).
Cortellini, A. et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J. Immunother. Cancer 7, 57 (2019).
Young, A. C. et al. Impact of body composition on outcomes from anti-PD1 +/− anti-CTLA-4 treatment in melanoma. J. Immunother. Cancer 8, e000821 (2020).
Boi, S. K. et al. Obesity diminishes response to PD-1-based immunotherapies in renal cancer. J. Immunother. Cancer 8, e000725 (2020).
Cortellini, A. et al. Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: a multicentre analysis of immune-related adverse events. Eur. J. Cancer 128, 17–26 (2020).
Leiter, A. et al. Metabolic disease and adverse events from immune checkpoint inhibitors. Eur. J. Endocrinol. 184, 857–865 (2021).
Heidelberger, V. et al. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Invest. N. Drugs 35, 436–441 (2017).
Kim, J. E., Patel, K. & Jackson, C. M. The potential for immune checkpoint modulators in cerebrovascular injury and inflammation. Expert Opin. Ther. Targets 25, 101–113 (2021).
Bodhankar, S. et al. PD-L1 monoclonal antibody treats ischemic stroke by controlling central nervous system inflammation. Stroke 46, 2926–2934 (2015).
Ren, X. et al. Programmed death-1 pathway limits central nervous system inflammation and neurologic deficits in murine experimental stroke. Stroke 42, 2578–2583 (2011).
Baruch, K. et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 22, 135–137 (2016).
Rosenzweig, N. et al. PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat. Commun. 10, 465 (2019).
Garon, E. B. et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J. Clin. Oncol. 37, 2518–2527 (2019).
Reck, M. et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥50%. J. Clin. Oncol. 39, 2339–2349 (2021).
Albiges, L. et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 5, e001079 (2020).
Ascierto, P. A. et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB–C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 21, 1465–1477 (2020).
Eggermont, A. M. M. et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 22, 643–654 (2021).
Spigel, D. R. et al. Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: an update from the PACIFIC trial [abstract]. J. Clin. Oncol. 39 (15 Suppl.), 8511 (2021).
Acknowledgements
The work of D.B.J. is funded by the Susan and Luke Stephens Directorship, the James C. Bradford Melanoma Fund, and the Van Stephenson Cancer Memorial Fund.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
D.B.J. has acted as a consultant and/or advisor for BMS, Catalyst, Iovance, Jansen, Mallinckrodt, Merck, Mosaic ImmunoEngineering, Novartis, Oncosec, Pfizer and Targovax, and receives research funding from BMS and Incyte. D.B.J. and J.J.M. have a patent pending for use of abatacept to reverse ICI toxicities. D.B.J. and J.M.B. have a patent pending for use of MHC II as a biomarker for ICI response. J.M.B. receives research funding from Genentech and Incyte. C.A.N. and J.J.M. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks K. Esfahani, S. Grover, S. Gupta and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Johnson, D.B., Nebhan, C.A., Moslehi, J.J. et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol 19, 254–267 (2022). https://doi.org/10.1038/s41571-022-00600-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-022-00600-w
This article is cited by
-
Augmenting MEK inhibitor efficacy in BRAF wild-type melanoma: synergistic effects of disulfiram combination therapy
Journal of Experimental & Clinical Cancer Research (2024)
-
Gut microbiome for predicting immune checkpoint blockade-associated adverse events
Genome Medicine (2024)
-
MicroRNAs as regulators of immune checkpoints in cancer immunotherapy: targeting PD-1/PD-L1 and CTLA-4 pathways
Cancer Cell International (2024)
-
Rare, late onset of immune checkpoint inhibitor-induced type 1 diabetes mellitus in a patient with small-cell lung cancer treated with serplulimab: a case report and review of the literature
Journal of Medical Case Reports (2024)
-
Targeting natural killer cells: from basic biology to clinical application in hematologic malignancies
Experimental Hematology & Oncology (2024)