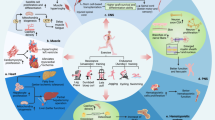
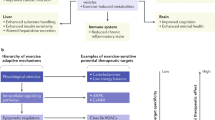
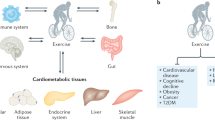
Abstract
Exercise mimetics are a proposed class of therapeutics that specifically mimic or enhance the therapeutic effects of exercise. Increased physical activity has demonstrated positive effects in preventing and ameliorating a wide range of diseases, including brain disorders such as Alzheimer disease and dementia, cancer, diabetes and cardiovascular disease. This article discusses the molecular mechanisms and signalling pathways associated with the beneficial effects of physical activity, focusing on effects on brain function and cognitive enhancement. Emerging therapeutic targets and strategies for the development of exercise mimetics, particularly in the field of central nervous system disorders, as well as the associated opportunities and challenges, are discussed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fiuza-Luces, C. et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 15, 731–743 (2018).
Febbraio, M. A. Health benefits of exercise — more than meets the eye! Nat. Rev. Endocrinol. 13, 72–74 (2017).
Gleeson, M. et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–615 (2011).
McTiernan, A. Mechanisms linking physical activity with cancer. Nat. Rev. Cancer 8, 205–211 (2008).
Hillman, C. H., Erickson, K. I. & Kramer, A. F. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 9, 58–65 (2008).
Wang, N., Liu, Y., Ma, Y. & Wen, D. High-intensity interval versus moderate-intensity continuous training: superior metabolic benefits in diet-induced obesity mice. Life Sci. 191, 122–131 (2017).
Bacurau, A. V. N. et al. Effect of a high-intensity exercise training on the metabolism and function of macrophages and lymphocytes of Walker 256 tumor–bearing rats. Exp. Biol. Med. 232, 1289–1299 (2007).
Hagar, A. et al. Endurance training slows breast tumor growth in mice by suppressing Treg cells recruitment to tumors. BMC Cancer 19, 536 (2019).
Horowitz, A. M. et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 369, 167–173 (2020).
Narkar, V. A. et al. AMPK and PPARδ agonists are exercise mimetics. Cell 134, 405–415 (2008).
Carey, A. L. & Kingwell, B. A. Novel pharmacological approaches to combat obesity and insulin resistance: targeting skeletal muscle with ‘exercise mimetics’. Diabetologia 52, 2015–2026 (2009).
Fan, W. & Evans, R. M. Exercise mimetics: impact on health and performance. Cell Metab. 25, 242–247 (2017).
Fan, W., Atkins, A. R., Yu, R. T., Downes, M. & Evans, R. M. Road to exercise mimetics: targeting nuclear receptors in skeletal muscle. J. Mol. Endocrinol. 51, T87–T100 (2013).
Wall, C. E., Yu, R. T., Atkins, A. R., Downes, M. & Evans, R. M. Nuclear receptors and AMPK: can exercise mimetics cure diabetes? J. Mol. Endocrinol. 57, R49–R58 (2016).
Alkadhi, K. A. Exercise as a positive modulator of brain function. Mol. Neurobiol. 55, 3112–3130 (2018).
McDonnell, M. N., Smith, A. E. & Mackintosh, S. F. Aerobic exercise to improve cognitive function in adults with neurological disorders: a systematic review. Arch. Phys. Med. Rehabil. 92, 1044–1052 (2011).
Ströhle, A. et al. Drug and exercise treatment of Alzheimer disease and mild cognitive impairment: a systematic review and meta-analysis of effects on cognition in randomized controlled trials. Am. J. Geriatr. Psychiatry 23, 1234–1249 (2015).
Cammisuli, I. A. & Fusi, J. Aerobic exercise effects upon cognition in Alzheimer’s disease: a systematic review of randomized controlled trials. Arch. Ital. Biol. 156, 54–63 (2018).
Farina, N., Rusted, J. & Tabet, N. The effect of exercise interventions on cognitive outcome in Alzheimer’s disease: a systematic review. Int. Psychogeriatr. 26, 9–18 (2014).
Li, X., Guo, R., Wei, Z., Jia, J. & Wei, C. Effectiveness of exercise programs on patients with dementia: a systematic review and meta-analysis of randomized controlled trials. BioMed. Res. Int. 2019, 1–16 (2019).
Uhrbrand, A., Stenager, E., Pedersen, M. S. & Dalgas, U. Parkinson’s disease and intensive exercise therapy – a systematic review and meta-analysis of randomized controlled trials. J. Neurol. Sci. 353, 9–19 (2015).
Shu, H.-F. et al. Aerobic exercise for Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE 9, e100503 (2014).
Cruickshank, T. M., Reyes, A. R. & Ziman, M. R. A systematic review and meta-analysis of strength training in individuals with multiple sclerosis or Parkinson disease. Medicine 94, e411 (2015).
Luo, L. et al. Effect of high-intensity exercise on cardiorespiratory fitness in stroke survivors: a systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 63, 59–68 (2020).
Vanderbeken, I. & Kerckhofs, E. A systematic review of the effect of physical exercise on cognition in stroke and traumatic brain injury patients. NeuroRehabilitation 40, 33–48 (2017).
Hung, S. H. et al. Pre-stroke physical activity and admission stroke severity: a systematic review. Int. J. Stroke https://doi.org/10.1177/1747493021995271 (2021).
Meng, L. et al. Effects of exercise in patients with amyotrophic lateral sclerosis: a systematic review and meta-analysis. Am. J. Phys. Med. Rehabil. 99, 801–810 (2020).
Álvarez-Bueno, C. et al. The effect of physical activity interventions on children’s cognition and metacognition: a systematic review and meta-analysis. J. Am. Acad. Child. Adolesc. Psychiatry 56, 729–738 (2017).
Northey, J. M., Cherbuin, N., Pumpa, K. L., Smee, D. J. & Rattray, B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br. J. Sports Med. 52, 154–160 (2018).
Barha, C. K., Davis, J. C., Falck, R. S., Nagamatsu, L. S. & Liu-Ambrose, T. Sex differences in exercise efficacy to improve cognition: a systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocrinol. 46, 71–85 (2017).
Firth, J. et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr. Bull. 43, 546–556 (2017).
Firth, J., Cotter, J., Elliott, R., French, P. & Yung, A. R. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol. Med. 45, 1343–1361 (2015).
Dauwan, M., Begemann, M. J. H., Heringa, S. M. & Sommer, I. E. Exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: a systematic review and meta-analysis. Schizophr. Bull. 42, 588–599 (2016).
Neufer, P. D. et al. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab. 22, 4–11 (2015).
Nithianantharajah, J. & Hannan, A. J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697–709 (2006).
De Vincenti, A. P., Ríos, A. S., Paratcha, G. & Ledda, F. Mechanisms that modulate and diversify BDNF functions: implications for hippocampal synaptic plasticity. Front. Cell. Neurosci. 13, 135 (2019).
Kim, S. et al. Roles of myokines in exercise-induced improvement of neuropsychiatric function. Pflügers Arch. Eur. J. Physiol. 471, 491–505 (2019).
McGee, S. L. & Walder, K. R. Exercise and the skeletal muscle epigenome. Cold Spring Harb. Perspect. Med. 7, a029876 (2017).
Pillon, N. J. et al. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat. Commun. 11, 470 (2020).
McOmish, C. E. & Hannan, A. J. Enviromimetics: exploring gene environment interactions to identify therapeutic targets for brain disorders. Expert Opin. Ther. Targets 11, 899–913 (2007).
Boa, B. C. S., Yudkin, J. S., van Hinsbergh, V. W. M., Bouskela, E. & Eringa, E. C. Exercise effects on perivascular adipose tissue: endocrine and paracrine determinants of vascular function: exercise, adipose tissue and vascular function. Br. J. Pharmacol. 174, 3466–3481 (2017).
Benatti, F. B. & Pedersen, B. K. Exercise as an anti-inflammatory therapy for rheumatic diseases — myokine regulation. Nat. Rev. Rheumatol. 11, 86–97 (2015).
Ruiz-Casado, A. et al. Exercise and the hallmarks of cancer. Trends Cancer 3, 423–441 (2017).
Dethlefsen, C., Pedersen, K. S. & Hojman, P. Every exercise bout matters: linking systemic exercise responses to breast cancer control. Breast Cancer Res. Treat. 162, 399–408 (2017).
American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription (Wolters Kluwer, 2017).
Fletcher, G. F. et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation 104, 1694–1740 (2001).
Piercy, K. L. et al. The physical activity guidelines for Americans. JAMA 320, 2020 (2018).
Sarzynski, M. A., Ghosh, S. & Bouchard, C. Genomic and transcriptomic predictors of response levels to endurance exercise training. J. Physiol. 595, 2931–2939 (2017).
Hoffman, N. J. Omics and exercise: global approaches for mapping exercise biological networks. Cold Spring Harb. Perspect. Med. 7, a029884 (2017).
Al-Khelaifi, F. et al. Metabolic GWAS of elite athletes reveals novel genetically-influenced metabolites associated with athletic performance. Sci. Rep. 9, 19889 (2019).
Bouchard, C., Rankinen, T. & Timmons, J. A. Genomics and genetics in the biology of adaptation to exercise. Compr. Physiol. 1, 1603–1648 (2011).
Patel, H. et al. Aerobic vs anaerobic exercise training effects on the cardiovascular system. World J. Cardiol. 9, 134–138 (2017).
Hawley, J. A., Hargreaves, M., Joyner, M. J. & Zierath, J. R. Integrative biology of exercise. Cell 159, 738–749 (2014).
Guerrieri, D., Moon, H. Y. & van Praag, H. Exercise in a pill: the latest on exercise-mimetics. Brain Plasticity 2, 153–169 (2017).
Hunter, P. Exercise in a bottle: elucidating how exercise conveys health benefits might lead to new therapeutic options for a range of diseases from cancer to metabolic syndrome. EMBO Rep. 17, 136–138 (2016).
Catoire, M. & Kersten, S. The search for exercise factors in humans. FASEB J. 29, 1615–1628 (2015).
Choi, S. H. et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361, eaan8821 (2018).
Tari, A. R. et al. Are the neuroprotective effects of exercise training systemically mediated? Prog. Cardiovascular Dis. 62, 94–101 (2019).
Delezie, J. & Handschin, C. Endocrine crosstalk between skeletal muscle and the brain. Front. Neurol. 9, 698 (2018).
Lucassen, P. J., Fitzsimons, C. P., Salta, E. & Maletic-Savatic, M. Adult neurogenesis, human after all (again): classic, optimized, and future approaches. Behav. Brain Res. 381, 112458 (2020).
Rendeiro, C. & Rhodes, J. S. A new perspective of the hippocampus in the origin of exercise–brain interactions. Brain Struct. Funct. 223, 2527–2545 (2018).
Eriksson, P. S. et al. Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317 (1998).
Malvaut, S. & Saghatelyan, A. The role of adult-born neurons in the constantly changing olfactory bulb network. Neural Plasticity 2016, 1–8 (2016).
Sakamoto, M., Kageyama, R. & Imayoshi, I. The functional significance of newly born neurons integrated into olfactory bulb circuits. Front. Neurosci. 8, 121 (2014).
Bicker, F. et al. Neurovascular EGFL7 regulates adult neurogenesis in the subventricular zone and thereby affects olfactory perception. Nat. Commun. 8, 15922 (2017).
Bédard, A. & Parent, A. Evidence of newly generated neurons in the human olfactory bulb. Dev. Brain Res. 151, 159–168 (2004).
Oomen, C. A., Bekinschtein, P., Kent, B. A., Saksida, L. M. & Bussey, T. J. Adult hippocampal neurogenesis and its role in cognition. Wiley Interdiscip. Rev. Cogn. Sci. 5, 573–587 (2014).
Bonzano, S., Bovetti, S., Gendusa, C., Peretto, P. & De Marchis, S. Adult born olfactory bulb dopaminergic interneurons: molecular determinants and experience-dependent plasticity. Front. Neurosci. 10, 189 (2016).
Sun, W., Kim, H. & Moon, Y. Control of neuronal migration through rostral migration stream in mice. Anat. Cell Biol. 43, 269 (2010).
Toda, T., Parylak, S. L., Linker, S. B. & Gage, F. H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 24, 67–87 (2019).
Xiong, Y., Mahmood, A. & Chopp, M. Emerging treatments for traumatic brain injury. Expert Opin. Emerg. Drugs 14, 67–84 (2009).
Vivar, C., Potter, M. C. & van Praag, H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr. Top. Behav. Neurosci. 15, 189–210 (2013).
El-Sayes, J., Harasym, D., Turco, C. V., Locke, M. B. & Nelson, A. J. Exercise-induced neuroplasticity: a mechanistic model and prospects for promoting plasticity. Neuroscientist 25, 65–85 (2019).
Parrini, M. et al. Aerobic exercise and a BDNF-mimetic therapy rescue learning and memory in a mouse model of Down syndrome. Sci. Rep. 7, 16825 (2017).
Anacker, C. & Hen, R. Adult hippocampal neurogenesis and cognitive flexibility — linking memory and mood. Nat. Rev. Neurosci. 18, 335–346 (2017).
Fernandes, J., Arida, R. M. & Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 80, 443–456 (2017).
Reichmann, F. et al. Environmental enrichment induces behavioural disturbances in neuropeptide Y knockout mice. Sci. Rep. 6, 28182 (2016).
Dremencov, E. et al. Effect of physical exercise and acute escitalopram on the excitability of brain monoamine neurons: in vivo electrophysiological study in rats. Int. J. Neuropsychopharmacol. 20, 585–592 (2017).
Graff, R. M. et al. β2-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav. Immun. 74, 143–153 (2018).
Garcia, P. C., Real, C. C. & Britto, L. R. The impact of short and long-term exercise on the expression of Arc and AMPARs during evolution of the 6-hydroxy-dopamine animal model of Parkinson’s disease. J. Mol. Neurosci. 61, 542–552 (2017).
Nauer, R. K., Dunne, M. F., Stern, C. E., Storer, T. W. & Schon, K. Improving fitness increases dentate gyrus/CA3 volume in the hippocampal head and enhances memory in young adults. Hippocampus 30, 488–504 (2020).
van Praag, H., Kempermann, G. & Gage, F. H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270 (1999).
Farmer, J. et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 124, 71–79 (2004).
Vaynman, S., Ying, Z. & Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 20, 2580–2590 (2004).
Saraulli, D., Costanzi, M., Mastrorilli, V. & Farioli-Vecchioli, S. The long run: neuroprotective effects of physical exercise on adult neurogenesis from youth to old age. Curr. Neuropharmacol. 15, 519–533 (2017).
Islam, M. R. et al. Diffusion tensor-MRI detects exercise-induced neuroplasticity in the hippocampal microstructure in mice. Brain Plasticity 5, 147–159 (2020).
Codd, L. N., Blackmore, D. G., Vukovic, J. & Bartlett, P. F. Exercise reverses learning deficits induced by hippocampal injury by promoting neurogenesis. Sci. Rep. 10, 19269 (2020).
Contrepois, K. et al. Molecular choreography of acute exercise. Cell 181, 1112–1130.e16 (2020).
Tapia-Arancibia, L., Aliaga, E., Silhol, M. & Arancibia, S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res. Rev. 59, 201–220 (2008).
Neeper, S. A., Gómez-Pinilla, F., Choi, J. & Cotman, C. W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 726, 49–56 (1996).
Bekinschtein, P., Oomen, C. A., Saksida, L. M. & Bussey, T. J. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin. Cell Dev. Biol. 22, 536–542 (2011).
Wang, R. & Holsinger, R. M. D. Exercise-induced brain-derived neurotrophic factor expression: therapeutic implications for Alzheimer’s dementia. Ageing Res. Rev. 48, 109–121 (2018).
Pang, T. Y. C., Stam, N. C., Nithianantharajah, J., Howard, M. L. & Hannan, A. J. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington’s disease transgenic mice. Neuroscience 141, 569–584 (2006).
Phillips, C., Baktir, M. A., Srivatsan, M. & Salehi, A. Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Front. Cell. Neurosci. 8, 170 (2014).
Squinto, S. P. et al. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell 65, 885–893 (1991).
Huang, E. J. & Reichardt, L. F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 (2001).
Kowiański, P. et al. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol. Neurobiol. 38, 579–593 (2018).
Egan, M. F. et al. The BDNF Val66Met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 (2003).
Ieraci, A., Madaio, A. I., Mallei, A., Lee, F. S. & Popoli, M. Brain-derived neurotrophic factor Val66Met human polymorphism impairs the beneficial exercise-induced neurobiological changes in mice. Neuropsychopharmacology 41, 3070–3079 (2016).
Seo, J.-H. et al. Physical exercise ameliorates psychiatric disorders and cognitive dysfunctions by hippocampal mitochondrial function and neuroplasticity in post-traumatic stress disorder. Exp. Neurol. 322, 113043 (2019).
Park, S.-S., Park, H.-S., Kim, C.-J., Baek, S.-S. & Kim, T.-W. Exercise attenuates maternal separation-induced mood disorder-like behaviors by enhancing mitochondrial functions and neuroplasticity in the dorsal raphe. Behav. Brain Res. 372, 112049 (2019).
Venezia, A. C., Guth, L. M., Sapp, R. M., Spangenburg, E. E. & Roth, S. M. Sex-dependent and independent effects of long-term voluntary wheel running on Bdnf mRNA and protein expression. Physiol. Behav. 156, 8–15 (2016).
Walsh, J. J. & Tschakovsky, M. E. Exercise and circulating BDNF: mechanisms of release and implications for the design of exercise interventions. Appl. Physiol. Nutr. Metab. 43, 1095–1104 (2018).
Mattson, M. P. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann. N. Y. Acad. Sci. 1144, 97–112 (2008).
Zheng, F., Zhou, X., Moon, C. & Wang, H. Regulation of brain-derived neurotrophic factor expression in neurons. Int. J. Physiol. Pathophysiol. Pharmacol. 4, 188–200 (2012).
Shi, K., Liu, X., Hou, L., Qiao, D. & Lin, X. Effects of exercise on mGluR-mediated glutamatergic transmission in the striatum of hemiparkinsonian rats. Neurosci. Lett. 705, 143–150 (2019).
Alenina, N. & Klempin, F. The role of serotonin in adult hippocampal neurogenesis. Behav. Brain Res. 277, 49–57 (2015).
Klempin, F. et al. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J. Neurosci. 33, 8270–8275 (2013).
Gutknecht, L. et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J. Neural Transm. 115, 1127–1132 (2008).
Kim, D. D., Barr, A. M., Honer, W. G. & Procyshyn, R. M. Exercise-induced hippocampal neurogenesis: 5-HT3 receptor antagonism by antipsychotics as a potential limiting factor in schizophrenia. Mol. Psychiatry 23, 2252–2253 (2018).
Kondo, M., Nakamura, Y., Ishida, Y. & Shimada, S. The 5-HT3 receptor is essential for exercise-induced hippocampal neurogenesis and antidepressant effects. Mol. Psychiatry 10, 1428–1437 (2014).
Rogers, J. et al. Paradoxical effects of exercise on hippocampal plasticity and cognition in mice with a heterozygous null mutation in the serotonin transporter gene. Br. J. Pharmacol. 176, 3279–3296 (2019).
Jensen, E. W., Espersen, K., Kanstrup, I. L. & Christensen, N. J. Exercise-induced changes in plasma catecholamines and neuropeptide Y: relation to age and sampling times. J. Appl. Physiol. 76, 1269–1273 (1994).
Rämson, R., Jürimäe, J., Jürimäe, T. & Mäestu, J. The effect of 4-week training period on plasma neuropeptide Y, leptin and ghrelin responses in male rowers. Eur. J. Appl. Physiol. 112, 1873–1880 (2012).
Chen, J.-X., Zhao, X., Yue, G.-X. & Wang, Z.-F. Influence of acute and chronic treadmill exercise on rat plasma lactate and brain NPY, L-ENK, DYN A1–13. Cell. Mol. Neurobiol. 27, 1–10 (2007).
Joksimovic, J. et al. The role of neuropeptide-Y in nandrolone decanoate-induced attenuation of antidepressant effect of exercise. PLoS ONE 12, e0178922 (2017).
Watson, C. N., Belli, A. & Di Pietro, V. Small non-coding RNAs: new class of biomarkers and potential therapeutic targets in neurodegenerative disease. Front. Genet. 10, 364 (2019).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Lennox, K. A. & Behlke, M. A. Chemical modification and design of anti-miRNA oligonucleotides. Gene Ther. 18, 1111–1120 (2011).
Mattes, J., Yang, M. & Foster, P. S. Regulation of microrna by antagomirs: a new class of pharmacological antagonists for the specific regulation of gene function? Am. J. Respir. Cell Mol. Biol. 36, 8–12 (2007).
Ling, H., Fabbri, M. & Calin, G. A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 12, 847–865 (2013).
Gomes, C. P. C. et al. Non-coding RNAs and exercise: pathophysiological role and clinical application in the cardiovascular system. Clin. Sci. 132, 925–942 (2018).
Silva, F. Cda et al. Effects of physical exercise on the expression of microRNAs: a systematic review. J. Strength. Cond. Res. 34, 270–280 (2020).
Bonilauri, B. & Dallagiovanna, B. Long non-coding RNAs are differentially expressed after different exercise training programs. Front. Physiol. 11, 567614 (2020).
Statello, L., Guo, C.-J., Chen, L.-L. & Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22, 96–118 (2021).
Ling, H. in Non-Coding RNAs in Colorectal Cancer (eds Slaby O. & Calin, G. A.) 229–237 (Springer, 2016).
Warner, K. D., Hajdin, C. E. & Weeks, K. M. Principles for targeting RNA with drug-like small molecules. Nat. Rev. Drug Discov. 17, 547–558 (2018).
Zhou, T., Kim, Y. & MacLeod, A. R. in Long Non-Coding RNAs (eds Feng, Y. & Zhang, L.) 199–213 (Springer, 2016).
Fernandes, J. et al. Hippocampal microRNA-mRNA regulatory network is affected by physical exercise. Biochim. Biophys. Acta Gen. Subj. 1862, 1711–1720 (2018).
Pons-Espinal, M. et al. MiR-135a-5p is critical for exercise-induced adult neurogenesis. Stem Cell Rep. 12, 1298–1312 (2019).
Improta-Caria, A. C. et al. Modulation of microRNAs as a potential molecular mechanism involved in the beneficial actions of physical exercise in Alzheimer disease. Int. J. Mol. Sci. 21, 4977 (2020).
Bao, T. et al. Spontaneous running wheel improves cognitive functions of mouse associated with miRNA expressional alteration in hippocampus following traumatic brain injury. J. Mol. Neurosci. 54, 622–629 (2014).
Miao, W. et al. Voluntary exercise prior to traumatic brain injury alters miRNA expression in the injured mouse cerebral cortex. Braz. J. Med. Biol. Res. 48, 433–439 (2015).
Hu, T. et al. miR21 is associated with the cognitive improvement following voluntary running wheel exercise in TBI mice. J. Mol. Neurosci. 57, 114–122 (2015).
Dharap, A., Pokrzywa, C. & Vemuganti, R. Increased binding of stroke-induced long non-coding RNAs to the transcriptional corepressors Sin3A and coREST. ASN Neuro 5, AN20130029 (2013).
Shang, J., Cheng, Q., Duan, S., Li, L. & Jia, L. Cognitive improvement following ischemia/reperfusion injury induced by voluntary running‑wheel exercise is associated with LncMALAT1‑mediated apoptosis inhibition. Int. J. Mol. Med. 41, 2715–2723 (2018).
Widmann, M., Nieß, A. M. & Munz, B. Physical exercise and epigenetic modifications in skeletal muscle. Sports Med. 49, 509–523 (2019).
McGowan, P. O. & Roth, T. L. Epigenetic pathways through which experiences become linked with biology. Dev. Psychopathol. 27, 637–648 (2015).
Urbano, A., Smith, J., Weeks, R. J. & Chatterjee, A. Gene-specific targeting of DNA methylation in the mammalian genome. Cancers 11, 1515 (2019).
Park, S.-Y. & Kim, J.-S. A short guide to histone deacetylases including recent progress on class II enzymes. Exp. Mol. Med. 52, 204–212 (2020).
Ganesan, A., Arimondo, P. B., Rots, M. G., Jeronimo, C. & Berdasco, M. The timeline of epigenetic drug discovery: from reality to dreams. Clin. Epigenet. 11, 174 (2019).
Ieraci, A., Mallei, A., Musazzi, L. & Popoli, M. Physical exercise and acute restraint stress differentially modulate hippocampal brain-derived neurotrophic factor transcripts and epigenetic mechanisms in mice: epigenetic modulation of Bdnf exons by exercise and stress. Hippocampus 25, 1380–1392 (2015).
Chen, M. J. & Russo-Neustadt, A. A. Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent. Hippocampus 19, 962–972 (2009).
Chen, M. J. & Russo-Neustadt, A. A. Running exercise- and antidepressant-induced increases in growth and survival-associated signaling molecules are IGF-dependent. Growth Factors 25, 118–131 (2007).
Müller, P., Duderstadt, Y., Lessmann, V. & Müller, N. G. Lactate and BDNF: key mediators of exercise induced neuroplasticity? J. Clin. Med. 9, 1136 (2020).
Cechinel, L. R. et al. Treadmill exercise induces age and protocol-dependent epigenetic changes in prefrontal cortex of Wistar rats. Behav. Brain Res. 313, 82–87 (2016).
Segabinazi, E. et al. Effects of maternal physical exercise on global DNA methylation and hippocampal plasticity of rat male offspring. Neuroscience 418, 218–230 (2019).
Goli, P., Yazdi, M., Poursafa, P. & Kelishadi, R. Intergenerational influence of paternal physical activity on the offspring’s brain: a systematic review and meta-analysis. Int. J. Devel. Neurosci. https://doi.org/10.1002/jdn.10081 (2020).
Denham, J., O’Brien, B. J., Harvey, J. T. & Charchar, F. J. Genome-wide sperm DNA methylation changes after 3 months of exercise training in humans. Epigenomics 7, 717–731 (2015).
Nystoriak, M. A. & Bhatnagar, A. Cardiovascular effects and benefits of exercise. Front. Cardiovasc. Med. 5, 135 (2018).
Ho, Q. T. & Kuo, C. J. Vascular endothelial growth factor: biology and therapeutic applications. Int. J. Biochem. Cell Biol. 39, 1349–1357 (2007).
Fabel, K. et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 18, 2803–2812 (2003).
Kiuchi, T., Lee, H. & Mikami, T. Regular exercise cures depression-like behavior via VEGF-Flk-1 signaling in chronically stressed mice. Neuroscience 207, 208–217 (2012).
Pianta, S. et al. A short bout of exercise prior to stroke improves functional outcomes by enhancing angiogenesis. NeuroMolecular Med. 21, 517–528 (2019).
Rezaei, R. et al. High intensity exercise preconditioning provides differential protection against brain injury following experimental stroke. Life Sci. 207, 30–35 (2018).
Pang, Q. et al. Role of caveolin-1/vascular endothelial growth factor pathway in basic fibroblast growth factor-induced angiogenesis and neurogenesis after treadmill training following focal cerebral ischemia in rats. Brain Res. 1663, 9–19 (2017).
Zhao, Y. et al. Treadmill exercise promotes neurogenesis in ischemic rat brains via caveolin-1/VEGF signaling pathways. Neurochem. Res. 42, 389–397 (2017).
Gao, Y. et al. Treadmill exercise promotes angiogenesis in the ischemic penumbra of rat brains through caveolin-1/VEGF signaling pathways. Brain Res. 1585, 83–90 (2014).
Hawley, J. A., Joyner, M. J. & Green, D. J. Mimicking exercise: what matters most and where to next? J. Physiol. 599, 791–802 (2021).
Kobilo, T., Yuan, C. & van Praag, H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn. Mem. 18, 103–107 (2011).
Kobilo, T. et al. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn. Mem. 21, 119–126 (2014).
Lauritzen, H. P. M. M. et al. Contraction and AICAR stimulate IL-6 vesicle depletion from skeletal muscle fibers in vivo. Diabetes 62, 3081–3092 (2013).
Moon, H. Y. et al. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 24, 332–340 (2016).
Marangos, P. J. et al. Adenosinergic modulation of homocysteine-induced seizures in mice. Epilepsia 31, 239–246 (1990).
Suwa, M., Nakano, H., Radak, Z. & Kumagai, S. Short-term adenosine monophosphate–activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside treatment increases the sirtuin 1 protein expression in skeletal muscle. Metabolism 60, 394–403 (2011).
Wang, J. et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell 11, 23–35 (2012).
Liu, Y., Tang, G., Zhang, Z., Wang, Y. & Yang, G.-Y. Metformin promotes focal angiogenesis and neurogenesis in mice following middle cerebral artery occlusion. Neurosci. Lett. 579, 46–51 (2014).
DiTacchio, K. A., Heinemann, S. F. & Dziewczapolski, G. Metformin treatment alters memory function in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 44, 43–48 (2015).
Hervás, D. et al. Metformin intake associates with better cognitive function in patients with Huntington’s disease. PLoS ONE 12, e0179283 (2017).
Moore, E. M. et al. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 36, 2981–2987 (2013).
Imfeld, P., Bodmer, M., Jick, S. S. & Meier, C. R. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J. Am. Geriatrics Soc. 60, 916–921 (2012).
Deschemin, J.-C., Foretz, M., Viollet, B. & Vaulont, S. AMPK is not required for the effect of metformin on the inhibition of BMP6-induced hepcidin gene expression in hepatocytes. Sci. Rep. 7, 12679 (2017).
Pedersen, B. K. & Febbraio, M. A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 88, 1379–1406 (2008).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Pedersen, B. K. The diseasome of physical inactivity - and the role of myokines in muscle-fat cross talk. J. Physiol. 587, 5559–5568 (2009).
Pedersen, B. K. Exercise-induced myokines and their role in chronic diseases. Brain Behav. Immun. 25, 811–816 (2011).
Pedersen, B. K. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 15, 383–392 (2019).
Murphy, R. M., Watt, M. J. & Febbraio, M. A. Metabolic communication during exercise. Nat. Metab. 2, 805–816 (2020).
Rai, M. & Demontis, F. Systemic nutrient and stress signaling via myokines and myometabolites. Annu. Rev. Physiol. 78, 85–107 (2016).
Giudice, J. & Taylor, J. M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 34, 49–55 (2017).
Pedersen, B. K. & Febbraio, M. Muscle-derived interleukin-6—a possible link between skeletal muscle, adipose tissue, liver, and brain. Brain Behav. Immun. 19, 371–376 (2005).
Rao, R. R. et al. Meteorin-like Is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157, 1279–1291 (2014).
Barlow, J. P. et al. Beta-aminoisobutyric acid is released by contracting human skeletal muscle and lowers insulin release from INS-1 832/3 cells by mediating mitochondrial energy metabolism. Metab. Open 7, 100053 (2020).
Severinsen, M. C. K. & Pedersen, B. K. Muscle–organ crosstalk: the emerging roles of myokines. Endocr. Rev. 41, 594–609 (2020).
Akira, S., Taga, T. & Kishimoto, T. Interleukin-6 in biology and medicine. Adv. Immunol. 54, 1–78 (1993).
Storer, M. A. et al. Interleukin-6 regulates adult neural stem cell numbers during normal and abnormal post-natal development. Stem Cell Rep. 10, 1464–1480 (2018).
Bowen, K. K., Dempsey, R. J. & Vemuganti, R. Adult interleukin-6 knockout mice show compromisedneurogenesis. NeuroReport 22, 126–130 (2011).
Vallières, L., Campbell, I. L., Gage, F. H. & Sawchenko, P. E. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J. Neurosci. 22, 486–492 (2002).
Señarís, R. M. et al. Interleukin-6 regulates the expression of hypothalamic neuropeptides involved in body weight in a gender-dependent way: IL-6 and hypothalamic neuropeptides. J. Neuroendocrinol. 23, 675–686 (2011).
Agudelo, L. Z. et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159, 33–45 (2014).
Jager, S., Handschin, C., St.-Pierre, J. & Spiegelman, B. M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1. Proc. Natl Acad. Sci. USA 104, 12017–12022 (2007).
Roy, T. & Lloyd, C. E. Epidemiology of depression and diabetes: a systematic review. J. Affect. Disord. 142, S8–S21 (2012).
Berg, U. & Bang, P. Exercise and circulating insulin-like growth factor I. Horm. Res. Paediatrics 62, 50–58 (2004).
Nakajima, S., Ohsawa, I., Ohta, S., Ohno, M. & Mikami, T. Regular voluntary exercise cures stress-induced impairment of cognitive function and cell proliferation accompanied by increases in cerebral IGF-1 and GST activity in mice. Behav. Brain Res. 211, 178–184 (2010).
Carro, E., Nuñez, A., Busiguina, S. & Torres-Aleman, I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 20, 2926–2933 (2000).
Ding, Q., Vaynman, S., Akhavan, M., Ying, Z. & Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 140, 823–833 (2006).
Ferris, L. T., Williams, J. S. & Shen, C.-L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 39, 728–734 (2007).
Schiffer, T. et al. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci. Lett. 488, 234–237 (2011).
El Hayek, L. et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 39, 2369–2382 (2019).
Margineanu, M. B., Mahmood, H., Fiumelli, H. & Magistretti, P. J. L-Lactate regulates the expression of synaptic plasticity and neuroprotection genes in cortical neurons: a transcriptome analysis. Front. Mol. Neurosci. 11, 375 (2018).
Erickson, H. P. Irisin and FNDC5 in retrospect: an exercise hormone or a transmembrane receptor? Adipocyte 2, 289–293 (2013).
Wrann, C. D. FNDC5/Irisin – their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plasticity 1, 55–61 (2015).
Schnyder, S. & Handschin, C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 80, 115–125 (2015).
Jin, Y. et al. Molecular and functional interaction of the myokine irisin with physical exercise and Alzheimer’s disease. Molecules 23, 3229 (2018).
Lourenco, M. V. et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 25, 165–175 (2019).
Liu, Y., Zhu, C., Guo, J., Chen, Y. & Meng, C. The neuroprotective effect of irisin in ischemic stroke. Front. Aging Neurosci. 12, 588958 (2020).
Hofmann, T. et al. The exercise-induced myokine irisin does not show an association with depressiveness, anxiety and perceived stress in obese women. J. Physiol. Pharmacol. 67, 195–203 (2016).
Wrann, C. D. et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 18, 649–659 (2013).
Iizuka, K., Machida, T. & Hirafuji, M. Skeletal muscle is an endocrine organ. J. Pharmacol. Sci. 125, 125–131 (2014).
Clow, C. & Jasmin, B. J. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol. Biol. Cell 21, 2182–2190 (2010).
Matthews, V. B. et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 52, 1409–1418 (2009).
Rasmussen, P. et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise: Brain-derived neurotrophic factor release during exercise. Exp. Physiol. 94, 1062–1069 (2009).
Wang, B., Yao, M., Lv, L., Ling, Z. & Li, L. The human microbiota in health and disease. Engineering 3, 71–82 (2017).
Weinstock, G. M. Genomic approaches to studying the human microbiota. Nature 489, 250–256 (2012).
Marchesi, J. R. et al. The gut microbiota and host health: a new clinical frontier. Gut 65, 330–339 (2016).
Cryan, J. F. et al. The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013 (2019).
Scotti, E. et al. Exploring the microbiome in health and disease: Implications for toxicology. Toxicol. Res. Appl. 1, 239784731774188 (2017).
Cerdá, B. et al. Gut microbiota modification: another piece in the puzzle of the benefits of physical exercise in health? Front. Physiol. 7, 51 (2016).
Gubert, C., Kong, G., Renoir, T. & Hannan, A. J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 134, 104621 (2020).
Dalton, A., Mermier, C. & Zuhl, M. Exercise influence on the microbiome–gut–brain axis. Gut Microbes 10, 555–568 (2019).
Cronin, O. et al. Gut microbiota: implications for sports and exercise medicine. Br. J. Sports Med. 51, 700–701 (2017).
Monda, V. et al. Exercise modifies the gut microbiota with positive health effects. Oxid. Med. Cell. Longev. 2017, 1–8 (2017).
Mitchell, C. M. et al. Does exercise alter gut microbial composition? — a systematic review. Med. Sci. Sports Exerc. 51, 160–167 (2019).
Allen, J. M. et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med. Sci. Sports Exerc. 50, 747–757 (2018).
Allen, J. M. et al. Exercise training-induced modification of the gut microbiota persists after microbiota colonization and attenuates the response to chemically-induced colitis in gnotobiotic mice. Gut Microbes 9, 115–130 (2018).
Batacan, R. B. et al. A gut reaction: the combined influence of exercise and diet on gastrointestinal microbiota in rats. J. Appl. Microbiol. 122, 1627–1638 (2017).
Campbell, S. C. et al. The effect of diet and exercise on intestinal integrity and microbial diversity in mice. PLoS ONE 11, e0150502 (2016).
Scheiman, J. et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 25, 1104–1109 (2019).
Kang, S. S. et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol. Neurodegener. 9, 36 (2014).
Feng, X. et al. Exercise prevents enhanced postoperative neuroinflammation and cognitive decline and rectifies the gut microbiome in a rat model of metabolic syndrome. Front. Immunol. 8, 1768 (2017).
Abraham, D. et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 115, 122–131 (2019).
Denham, J. & Spencer, S. J. Emerging roles of extracellular vesicles in the intercellular communication for exercise-induced adaptations. Am. J. Physiol. Endocrinol. Metab. 319, E320–E329 (2020).
Safdar, A. & Tarnopolsky, M. A. Exosomes as mediators of the systemic adaptations to endurance exercise. Cold Spring Harb. Perspect. Med. 8, a029827 (2018).
Veziroglu, E. M. & Mias, G. I. Characterizing extracellular vesicles and their diverse RNA contents. Front. Genet. 11, 700 (2020).
Whitham, M. et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 27, 237–251.e4 (2018).
Frühbeis, C., Helmig, S., Tug, S., Simon, P. & Krämer-Albers, E.-M. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J. Extracell. Vesicles 4, 28239 (2015).
Garner, R. T. et al. Multivesicular body and exosome pathway responses to acute exercise. Exp. Physiol. 105, 511–521 (2020).
Nair, V. D. et al. Sedentary and trained older men have distinct circulating exosomal microRNA profiles at baseline and in response to acute exercise. Front. Physiol. 11, 605 (2020).
Xiang, H. et al. Characterization of blood-derived exosomal proteins after exercise. J. Int. Med. Res. 48, 030006052095754 (2020).
Kirchmair, R. et al. Secretoneurin, an angiogenic neuropeptide, induces postnatal vasculogenesis. Circulation 110, 1121–1127 (2004).
Wang, J. et al. Moderate exercise has beneficial effects on mouse ischemic stroke by enhancing the functions of circulating endothelial progenitor cell-derived exosomes. Exp. Neurol. 330, 113325 (2020).
Okamoto, M. et al. Mild exercise increases dihydrotestosterone in hippocampus providing evidence for androgenic mediation of neurogenesis. Proc. Natl Acad. Sci. USA 109, 13100–13105 (2012).
Kozareva, D. A., O’Leary, O. F., Cryan, J. F. & Nolan, Y. M. Deletion of TLX and social isolation impairs exercise-induced neurogenesis in the adolescent hippocampus. Hippocampus 28, 3–11 (2018).
Chen, C. et al. The exercise-glucocorticoid paradox: how exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front. Neuroendocrinol. 44, 83–102 (2017).
Lin, L., Wu, J., Yuan, Y., Sun, X. & Zhang, L. Working memory predicts hypothalamus-pituitary-adrenal axis response to psychosocial stress in males. Front. Psychiatry 11, 142 (2020).
Young, A. H. The effects of HPA axis function on cognition and its implications for the pathophysiology of bipolar disorder. Harv. Rev. Psychiatry 22, 331–333 (2014).
Gardner, M. et al. Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and cognitive capability at older ages: individual participant meta-analysis of five cohorts. Sci. Rep. 9, 4555 (2019).
Nisoli, E. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299, 896–899 (2003).
Nisoli, E. et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc. Natl Acad. Sci. USA 101, 16507–16512 (2004).
Engeli, S. et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J. Clin. Invest. 122, 4675–4679 (2012).
Fedele, E. & Ricciarelli, R. Memory enhancers for Alzheimer’s dementia: focus on cGMP. Pharmaceuticals 14, 61 (2021).
Amoasii, L. et al. NURR1 activation in skeletal muscle controls systemic energy homeostasis. Proc. Natl Acad. Sci. USA 116, 11299–11308 (2019).
Jakaria, M. D. et al. Molecular insights into NR4A2(Nurr1): an emerging target for neuroprotective therapy against neuroinflammation and neuronal cell death. Mol. Neurobiol. 56, 5799–5814 (2019).
Moon, M. et al. Nurr1 (NR4A2) regulates Alzheimer’s disease-related pathogenesis and cognitive function in the 5XFAD mouse model. Aging Cell 18, e12866 (2019).
Jeon, S. G. et al. The critical role of Nurr1 as a mediator and therapeutic target in Alzheimer’s disease-related pathogenesis. Aging Dis. 11, 705 (2020).
Shim, J.-W. et al. Generation of functional dopamine neurons from neural precursor cells isolated from the subventricular zone and white matter of the adult rat brain using nurr1 overexpression. Stem Cell 25, 1252–1262 (2007).
Lee, C. et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 21, 443–454 (2015).
Kim, K. H., Son, J. M., Benayoun, B. A. & Lee, C. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab. 28, 516–524.e7 (2018).
Zarse, K. & Ristow, M. A mitochondrially encoded hormone ameliorates obesity and insulin resistance. Cell Metab. 21, 355–356 (2015).
Alis, R., Lucia, A., Blesa, J. R. & Sanchis-Gomar, F. The role of mitochondrial derived peptides (MDPs) in metabolism: MOTS-C a new mitokine. J. Cell. Physiol. 230, 2903–2904 (2015).
Yong, C. & Tang, B. A mitochondrial encoded messenger at the nucleus. Cells 7, 105 (2018).
Reynolds, J. C. et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat. Commun. 12, 470 (2021).
Kim, S.-J. et al. Mitochondrial-derived peptides in aging and age-related diseases. GeroScience https://doi.org/10.1007/s11357-020-00262-5 (2020).
Cummings, J., Lee, G., Ritter, A. & Zhong, K. Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement. 4, 195–214 (2018).
Cummings, J. et al. Drug development in Alzheimer’s disease: the path to 2025. Alzheimers Res. Ther. 8, 39 (2016).
Koprich, J. B., Kalia, L. V. & Brotchie, J. M. Animal models of α-synucleinopathy for Parkinson disease drug development. Nat. Rev. Neurosci. 18, 515–529 (2017).
Bates, G. P. et al. Huntington disease. Nat. Rev. Dis. Prim. 1, 15005 (2015).
Keshavan, M. S., Lawler, A. N., Nasrallah, H. A. & Tandon, R. New drug developments in psychosis: challenges, opportunities and strategies. Prog. Neurobiol. 152, 3–20 (2017).
Ionescu, D. F. & Papakostas, G. I. Experimental medication treatment approaches for depression. Transl. Psychiatry 7, e1068–e1068 (2017).
Robbins, T. W. Cross-species studies of cognition relevant to drug discovery: a translational approach: cross-species cognitive studies and drug discovery. Br. J. Pharmacol. 174, 3191–3199 (2017).
Gomez-Pinilla, F., Zhuang, Y., Feng, J., Ying, Z. & Fan, G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation: exercise and epigenetics. Eur. J. Neurosci. 33, 383–390 (2011).
Intlekofer, K. A. et al. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology 38, 2027–2034 (2013).
Sleiman, S. F. et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 5, e15092 (2016).
Sølvsten, C. A. E., de Paoli, F., Christensen, J. H. & Nielsen, A. L. Voluntary physical exercise induces expression and epigenetic remodeling of VegfA in the rat hippocampus. Mol. Neurobiol. 55, 567–582 (2018).
Villarroya, F. Irisin, turning up the heat. Cell Metab. 15, 277–278 (2012).
Hoffman-Goetz, L., Pervaiz, N., Packer, N. & Guan, J. Freewheel training decreases pro- and increases anti-inflammatory cytokine expression in mouse intestinal lymphocytes. Brain Behav. Immun. 24, 1105–1115 (2010).
Packer, N. & Hoffman-Goetz, L. Apoptotic and inflammatory cytokine protein expression in intestinal lymphocytes after acute treadmill exercise in young and old mice. J. Sports Med. Phys. Fit. 52, 202–211 (2012).
Ticinesi, A. et al. Exercise and immune system as modulators of intestinal microbiome: implications for the gut-muscle axis hypothesis. Exerc. Immunol. Rev. 25, 84–95 (2019).
Clark, A. & Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J. Int. Soc. Sports Nutr. 13, 43 (2016).
Lambert, C. P., Wright, N. R., Finck, B. N. & Villareal, D. T. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J. Appl. Physiol. 105, 473–478 (2008).
Lamprecht, M. & Frauwallner, A. Exercise, intestinal barrier dysfunction and probiotic supplementation. Med. Sport Sci. 59, 47–56 (2012).
van Wijck, K. et al. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G155–G168 (2012).
McGee, S. L. & Hargreaves, M. Exercise adaptations: molecular mechanisms and potential targets for therapeutic benefit. Nat. Rev. Endocrinol. 16, 495–505 (2020).
Fontana, L. Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat. Rev. Cardiol. 15, 566–577 (2018).
Jaspers, R. T. et al. Exercise, fasting, and mimetics: toward beneficial combinations? FASEB J. 31, 14–28 (2017).
Marcinko, K. et al. The AMPK activator R419 improves exercise capacity and skeletal muscle insulin sensitivity in obese mice. Mol. Metab. 4, 643–651 (2015).
Muise, E. S. et al. Pharmacological AMPK activation induces transcriptional responses congruent to exercise in skeletal and cardiac muscle, adipose tissues and liver. PLoS ONE 14, e0211568 (2019).
Duggal, N. A., Niemiro, G., Harridge, S. D. R., Simpson, R. J. & Lord, J. M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 19, 563–572 (2019).
Otero-Díaz, B. et al. Exercise induces white adipose tissue browning across the weight spectrum in humans. Front. Physiol. 9, 1781 (2018).
Slentz, C. A., Houmard, J. A. & Kraus, W. E. Exercise, abdominal obesity, skeletal muscle, and metabolic risk: evidence for a dose response. Obesity 17, S27–S33 (2009).
Acknowledgements
The authors thank past and present members of the Hannan Laboratory for experimental data and discussions that have informed the ideas presented in this article. A.J.H. is a National Health and Medical Research Council (NHMRC) Principal Research Fellow and is supported by Projects Grants and an Ideas Grant from the NHMRC and a Discovery Project Grant from the Australian Research Council, as well as the DHB Foundation, Equity Trustees.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gubert, C., Hannan, A.J. Exercise mimetics: harnessing the therapeutic effects of physical activity. Nat Rev Drug Discov 20, 862–879 (2021). https://doi.org/10.1038/s41573-021-00217-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-021-00217-1
This article is cited by
-
Exercise mimetics: a novel strategy to combat neuroinflammation and Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
Cannabinoid type 2 receptor inhibition enhances the antidepressant and proneurogenic effects of physical exercise after chronic stress
Translational Psychiatry (2024)
-
Molecular mechanisms of exercise contributing to tissue regeneration
Signal Transduction and Targeted Therapy (2022)
-
Exercise training maintains cardiovascular health: signaling pathways involved and potential therapeutics
Signal Transduction and Targeted Therapy (2022)