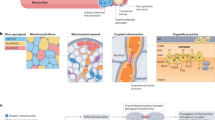
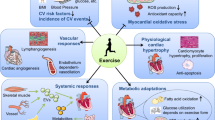
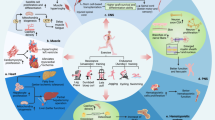
Abstract
Exercise is fundamental for good health, whereas physical inactivity underpins many chronic diseases of modern society. It is well appreciated that regular exercise improves metabolism and the metabolic phenotype in a number of tissues. The phenotypic alterations observed in skeletal muscle are partly mediated by transcriptional responses that occur following each individual bout of exercise. This adaptive response increases oxidative capacity and influences the function of myokines and extracellular vesicles that signal to other tissues. Our understanding of the epigenetic and transcriptional mechanisms that mediate the skeletal muscle gene expression response to exercise as well as of their upstream signalling pathways has advanced substantially in the past 10 years. With this knowledge also comes the opportunity to design new therapeutic strategies based on the biology of exercise for a variety of chronic conditions where regular exercise might be a challenge. This Review provides an overview of the beneficial adaptive responses to exercise and details the molecular mechanisms involved. The possibility of designing therapeutic interventions based on these molecular mechanisms is addressed, using relevant examples that have exploited this approach.
Key points
-
Exercise is effective in the primary prevention of 35 chronic diseases.
-
The adaptive response to exercise, which is mediated in part by transcriptional alterations in metabolic and other genes, is an important contributor to these health benefits.
-
Identifying the mechanisms that mediate the exercise adaptive response could uncover molecular targets to guide the design of new medicines to better treat chronic diseases.
-
A number of signalling, epigenetic and transcriptional molecules identified as contributing to the exercise adaptive response have been targeted pharmacologically to deliver health benefits in proof-of-concept studies.
-
A further understanding of the complexities of the molecular responses to exercise will provide new opportunities to engage these mechanisms therapeutically.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Booth, F. W., Roberts, C. K. & Laye, M. J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2, 1143–1211 (2012).
Egan, B. & Zierath, J. R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 17, 162–184 (2013).
Febbraio, M. A. Exercise metabolism in 2016: health benefits of exercise - more than meets the eye! Nat. Rev. Endocrinol. 13, 72–74 (2017).
Williams, R. S. & Neufer, P. D. in Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems (eds Rowell, L. B. & Shepherd, J. T.) (American Physiological Society, 1996).
Hawley, J. A., Hargreaves, M., Joyner, M. J. & Zierath, J. R. Integrative biology of exercise. Cell 159, 738–749 (2014).
Baar, K. Training for endurance and strength: lessons from cell signaling. Med. Sci. Sports Exerc. 38, 1939–1944 (2006).
Hoppeler, H., Baum, O., Lurman, G. & Mueller, M. Molecular mechanisms of muscle plasticity with exercise. Compr. Physiol. 1, 1383–1412 (2011).
Coffey, V. G. & Hawley, J. A. Concurrent exercise training: do opposites distract? J. Physiol. 595, 2883–2896 (2017).
Gibala, M. J. & Little, J. P. Physiological basis of brief vigorous exercise to improve health. J. Physiol. 598, 61–69 (2020).
Konopka, A. R. & Harber, M. P. Skeletal muscle hypertrophy after aerobic exercise training. Exerc. Sport. Sci. Rev. 42, 53–61 (2014).
Wilkinson, S. B. et al. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J. Physiol. 586, 3701–3717 (2008).
Chen, Z. P. et al. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 52, 2205–2212 (2003). This study uncovered the exercise intensity-dependent activation of AMPK in human skeletal muscle.
Hardie, D. G., Schaffer, B. E. & Brunet, A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 26, 190–201 (2016).
Hanson, P. I., Meyer, T., Stryer, L. & Schulman, H. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron 12, 943–956 (1994).
Rose, A. J. & Hargreaves, M. Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle. J. Physiol. 553, 303–309 (2003). This was the first study to quantify CaMKII activation in human skeletal muscle in response to exercise.
Ojuka, E. O., Goyaram, V. & Smith, J. A. The role of CaMKII in regulating GLUT4 expression in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 303, E322–E331 (2012).
Richter, E. A. & Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 93, 993–1017 (2013).
Watt, M. J., Howlett, K. F., Febbraio, M. A., Spriet, L. L. & Hargreaves, M. Adrenaline increases skeletal muscle glycogenolysis, pyruvate dehydrogenase activation and carbohydrate oxidation during moderate exercise in humans. J. Physiol. 534, 269–278 (2001).
Warren, J. B., Dalton, N., Turner, C., Clark, T. J. & Toseland, P. A. Adrenaline secretion during exercise. Clin. Sci. 66, 87–90 (1984).
Goldstein, D. S. Plasma catecholamines and essential hypertension. An analytical review. Hypertension 5, 86–99 (1983).
Wojtaszewski, J. F., Nielsen, P., Hansen, B. F., Richter, E. A. & Kiens, B. Isoform-specific and exercise intensity-dependent activation of 5’-AMP-activated protein kinase in human skeletal muscle. J. Physiol. 528, 221–226 (2000).
Blair, E. et al. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum. Mol. Genet. 10, 1215–1220 (2001).
Burwinkel, B. et al. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the gamma 2-subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am. J. Hum. Genet. 76, 1034–1049 (2005).
Hood, D. A., Memme, J. M., Oliveira, A. N. & Triolo, M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu. Rev. Physiol. 81, 19–41 (2019).
Kraniou, G. N., Cameron-Smith, D. & Hargreaves, M. Effect of short-term training on GLUT-4 mRNA and protein expression in human skeletal muscle. Exp. Physiol. 89, 559–563 (2004).
Burgomaster, K. A. et al. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1970–R1976 (2007).
Stuart, C. A., Lee, M. L., South, M. A., Howell, M. E. A. & Stone, M. H. Muscle hypertrophy in prediabetic men after 16 wk of resistance training. J. Appl. Physiol. 123, 894–901 (2017).
Jorgensen, S. B. et al. Role of AMPKα2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am. J. Physiol. Endocrinol. Metab. 292, E331–E339 (2007).
Jorgensen, S. B. et al. Effects of α-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 19, 1146–1148 (2005).
Maarbjerg, S. J. et al. Genetic impairment of AMPKα2 signaling does not reduce muscle glucose uptake during treadmill exercise in mice. Am. J. Physiol. Endocrinol. Metab. 297, E924–E934 (2009).
McGee, S. L. et al. Compensatory regulation of HDAC5 in muscle maintains metabolic adaptive responses and metabolism in response to energetic stress. FASEB J. 28, 3384–3395 (2014).
Hoffman, N. J. et al. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab. 22, 922–935 (2015). This was one of the first studies to quantify the extensive phosphorylation events that occur in human skeletal muscle in response to exercise.
Potts, G. K. et al. A map of the phosphoproteomic alterations that occur after a bout of maximal-intensity contractions. J. Physiol. 595, 5209–5226 (2017).
Nelson, M. E. et al. Phosphoproteomics reveals conserved exercise-stimulated signaling and AMPK regulation of store-operated calcium entry. EMBO J. 38, e102578 (2019).
Gollnick, P. D. & Saltin, B. Significance of skeletal muscle oxidative enzyme enhancement with endurance training. Clin. Physiol. 2, 1–12 (1982).
Holloszy, J. O. & Coyle, E. F. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 56, 831–838 (1984).
Hesselink, M. K., Schrauwen-Hinderling, V. & Schrauwen, P. Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus. Nat. Rev. Endocrinol. 12, 633–645 (2016).
McGlory, C., van Vliet, S., Stokes, T., Mittendorfer, B. & Phillips, S. M. The impact of exercise and nutrition on the regulation of skeletal muscle mass. J. Physiol. 597, 1251–1258 (2019).
Wackerhage, H., Schoenfeld, B. J., Hamilton, D. L., Lehti, M. & Hulmi, J. J. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J. Appl. Physiol. 126, 30–43 (2019).
Fiuza-Luces, C. et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 15, 731–743 (2018).
Vettor, R. et al. Exercise training boosts eNOS-dependent mitochondrial biogenesis in mouse heart: role in adaptation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 306, E519–E528 (2014).
Brouwers, B., Hesselink, M. K., Schrauwen, P. & Schrauwen-Hinderling, V. B. Effects of exercise training on intrahepatic lipid content in humans. Diabetologia 59, 2068–2079 (2016).
Stanford, K. I. & Goodyear, L. J. Exercise regulation of adipose tissue. Adipocyte 5, 153–162 (2016).
Thompson, D., Karpe, F., Lafontan, M. & Frayn, K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol. Rev. 92, 157–191 (2012).
Kantartzis, K. et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut 58, 1281–1288 (2009).
Younossi, Z. M., Marchesini, G., Pinto-Cortez, H. & Petta, S. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Transplantation 103, 22–27 (2019).
van der Windt, D. J., Sud, V., Zhang, H., Tsung, A. & Huang, H. The effects of physical exercise on fatty liver disease. Gene Expr. 18, 89–101 (2018).
Trevellin, E. et al. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 63, 2800–2811 (2014).
Flores-Opazo, M. et al. Exercise and GLUT4 in human subcutaneous adipose tissue. Physiol. Rep. 6, e13918 (2018).
Tsiloulis, T. et al. No evidence of white adipocyte browning after endurance exercise training in obese men. Int. J. Obes. 42, 721–727 (2018).
Camera, D. M., Anderson, M. J., Hawley, J. A. & Carey, A. L. Short-term endurance training does not alter the oxidative capacity of human subcutaneous adipose tissue. Eur. J. Appl. Physiol. 109, 307–316 (2010).
Larsen, S. et al. The effect of high-intensity training on mitochondrial fat oxidation in skeletal muscle and subcutaneous adipose tissue. Scand. J. Med. Sci. Sports 25, e59–e69 (2015).
Herz, C. T. & Kiefer, F. W. Adipose tissue browning in mice and humans. J. Endocrinol. 241, R97–R109 (2019).
Tyndall, A. V. et al. Protective effects of exercise on cognition and brain health in older adults. Exerc. Sport. Sci. Rev. 46, 215–223 (2018).
Mailing, L. J., Allen, J. M., Buford, T. W., Fields, C. J. & Woods, J. A. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc. Sport. Sci. Rev. 47, 75–85 (2019).
Pedersen, B. K., Steensberg, A. & Schjerling, P. Muscle-derived interleukin-6: possible biological effects. J. Physiol. 536, 329–337 (2001).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Whitham, M. & Febbraio, M. A. The ever-expanding myokinome: discovery challenges and therapeutic implications. Nat. Rev. Drug. Discov. 15, 719–729 (2016).
Barlow, J. P. & Solomon, T. P. Do skeletal muscle-secreted factors influence the function of pancreatic β-cells? Am. J. Physiol. Endocrinol. Metab. 314, E297–E307 (2018).
Pedersen, B. K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 15, 383–392 (2019).
Takahashi, H. et al. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 1, 291–303 (2019).
Frodermann, V. et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 25, 1761–1771 (2019).
Ennequin, G., Sirvent, P. & Whitham, M. Role of exercise-induced hepatokines in metabolic disorders. Am. J. Physiol. Endocrinol. Metab. 317, E11–E24 (2019).
Scheiman, J. et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 25, 1104–1109 (2019). This paper uncovered a muscle–gut–muscle signalling axis that regulates adaptation to exercise and exercise performance.
Hawley, J. A. Microbiota and muscle highway – two-way traffic. Nat. Rev. Endocrinol. 16, 71–72 (2020).
Parker, B. L. et al. Multiplexed temporal quantification of the exercise-regulated plasma peptidome. Mol. Cell Proteom. 16, 2055–2068 (2017).
Whitham, M. et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 27, 237–251.e4 (2018). This study quantified the extensive number of proteins released from skeletal muscle in extracellular vesicles during exercise and showed that they signal to the liver.
Lewis, G. D. et al. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2, 33ra37 (2010). This work defined the numerous metabolites altered in the plasma in response to exercise and showed that a number of these metabolites have transcriptional effects in skeletal muscle cells.
Brennan, A. M. et al. Plasma metabolite profiles in response to chronic exercise. Med. Sci. Sports Exerc. 50, 1480–1486 (2018).
Flores-Opazo, M., Raajendiran, A., Watt, M. J. & Hargreaves, M. Exercise serum increases GLUT4 in human adipocytes. Exp. Physiol. 104, 630–634 (2019).
Neufer, P. D. & Dohm, G. L. Exercise induces a transient increase in transcription of the GLUT-4 gene in skeletal muscle. Am. J. Physiol. 265, C1597–C1603 (1993).
Kraniou, Y., Cameron-Smith, D., Misso, M., Collier, G. & Hargreaves, M. Effects of exercise on GLUT-4 and glycogenin gene expression in human skeletal muscle. J. Appl. Physiol. 88, 794–796 (2000).
Pilegaard, H., Ordway, G. A., Saltin, B. & Neufer, P. D. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am. J. Physiol. Endocrinol. Metab. 279, E806–E814 (2000). One of the first studies to characterize the widespread transcriptional response to exercise in the immediate post-exercise period.
Pilegaard, H., Saltin, B. & Neufer, P. D. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J. Physiol. 546, 851–858 (2003).
Perry, C. G. et al. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 588, 4795–4810 (2010).
Egan, B., O’Connor, P. L., Zierath, J. R. & O’Gorman, D. J. Time course analysis reveals gene-specific transcript and protein kinetics of adaptation to short-term aerobic exercise training in human skeletal muscle. PLoS One 8, e74098 (2013). This paper provided an extensive time course analysis of the transcriptional and protein expression responses to exercise training in human skeletal muscle.
Robinson, M. M. et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 25, 581–592 (2017).
Miller, B. F., Konopka, A. R. & Hamilton, K. L. The rigorous study of exercise adaptations: why mRNA might not be enough. J. Appl. Physiol. 121, 594–596 (2016).
McGee, S. L. & Hargreaves, M. Epigenetics and exercise. Trends Endocrinol. Metab. 30, 636–645 (2019).
McGee, S. L. & Walder, K. R. Exercise and the skeletal muscle epigenome. Cold Spring Harb. Perspect. Med. 7, a029876 (2017).
Bird, A. P. & Wolffe, A. P. Methylation-induced repression–belts, braces, and chromatin. Cell 99, 451–454 (1999).
Barres, R. et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 15, 405–411 (2012). The first study to show that methylation of DNA linked to exercise-responsive genes is reduced in response to exercise.
Rasmussen, K. D. & Helin, K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 30, 733–750 (2016).
Bannister, A. J. & Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 21, 381–395 (2011).
Jenuwein, T. & Allis, C. D. Translating the histone code. Science 293, 1074–1080 (2001).
Lee, J. S., Smith, E. & Shilatifard, A. The language of histone crosstalk. Cell 142, 682–685 (2010).
Gorisch, S. M., Wachsmuth, M., Toth, K. F., Lichter, P. & Rippe, K. Histone acetylation increases chromatin accessibility. J. Cell Sci. 118, 5825–5834 (2005).
Venkatesh, S. & Workman, J. L. Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell Biol. 16, 178–189 (2015).
Smith, J. A., Kohn, T. A., Chetty, A. K. & Ojuka, E. O. CaMK activation during exercise is required for histone hyperacetylation and MEF2A binding at the MEF2 site on the Glut4 gene. Am. J. Physiol. Endocrinol. Metab. 295, E698–E704 (2008). This study linked the activation of CaMKII during exercise to lysine acetylation of histone 3 at the GLUT4 promoter and provided one of the first examples of epigenetic control of exercise-induced transcriptional responses.
McKinsey, T. A., Zhang, C. L. & Olson, E. N. Control of muscle development by dueling HATs and HDACs. Curr. Opin. Genet. Dev. 11, 497–504 (2001).
McGee, S. L., Fairlie, E., Garnham, A. P. & Hargreaves, M. Exercise-induced histone modifications in human skeletal muscle. J. Physiol. 587, 5951–5958 (2009).
McGee, S. L. & Hargreaves, M. Exercise and myocyte enhancer factor 2 regulation in human skeletal muscle. Diabetes 53, 1208–1214 (2004).
Akimoto, T., Li, P. & Yan, Z. Functional interaction of regulatory factors with the Pgc-1α promoter in response to exercise by in vivo imaging. Am. J. Physiol. Cell Physiol. 295, C288–C292 (2008).
Gaur, V. et al. Disruption of the class IIa HDAC corepressor complex increases energy expenditure and lipid oxidation. Cell Rep. 16, 2802–2810 (2016). One of the first studies to show that manipulation of protein interactions similar to exercise could induce exercise-like transcriptional and metabolic effects.
Ali, I., Conrad, R. J., Verdin, E. & Ott, M. Lysine acetylation goes global: from epigenetics to metabolism and therapeutics. Chem. Rev. 118, 1216–1252 (2018).
Howlett, K. F. & McGee, S. L. Epigenetic regulation of skeletal muscle metabolism. Clin. Sci. 130, 1051–1063 (2016).
Dent, J. R. et al. Muscle-specific knockout of general control of amino acid synthesis 5 (GCN5) does not enhance basal or endurance exercise-induced mitochondrial adaptation. Mol. Metab. 6, 1574–1584 (2017).
LaBarge, S. A. et al. p300 is not required for metabolic adaptation to endurance exercise training. FASEB J. 30, 1623–1633 (2016).
Segal, E. & Widom, J. From DNA sequence to transcriptional behaviour: a quantitative approach. Nat. Rev. Genet. 10, 443–456 (2009).
Wilson, S. & Filipp, F. V. A network of epigenomic and transcriptional cooperation encompassing an epigenomic master regulator in cancer. NPJ Syst. Biol. Appl. 4, 24 (2018).
Lemon, B. & Tjian, R. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev. 14, 2551–2569 (2000).
Palstra, R. J. & Grosveld, F. Transcription factor binding at enhancers: shaping a genomic regulatory landscape in flux. Front. Genet. 3, 195 (2012).
Kupr, B., Schnyder, S. & Handschin, C. Role of nuclear receptors in exercise-induced muscle adaptations. Cold Spring Harb. Perspect. Med. 7, a029835 (2017).
Reibe, S., Hjorth, M., Febbraio, M. A. & Whitham, M. GeneXX: an online tool for the exploration of transcript changes in skeletal muscle associated with exercise. Physiol. Genomics 50, 376–384 (2018).
Pillon, N. J. et al. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat. Commun. 11, 470 (2020). An extensive meta-analysis that has characterized the skeletal muscle transcriptional response to different modes of exercise.
Goode, J. M. et al. The nuclear receptor, Nor-1, induces the physiological responses associated with exercise. Mol. Endocrinol. 30, 660–676 (2016).
Pearen, M. A. et al. The nuclear receptor, Nor-1, markedly increases type II oxidative muscle fibers and resistance to fatigue. Mol. Endocrinol. 26, 372–384 (2012).
Pearen, M. A. et al. Transgenic muscle-specific Nor-1 expression regulates multiple pathways that effect adiposity, metabolism, and endurance. Mol. Endocrinol. 27, 1897–1917 (2013).
Potthoff, M. J. et al. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J. Clin. Invest. 117, 2459–2467 (2007).
Michael, L. F. et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl Acad. Sci. USA 98, 3820–3825 (2001).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Vega, R. B., Huss, J. M. & Kelly, D. P. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 20, 1868–1876 (2000).
McKinsey, T. A., Zhang, C. L., Lu, J. & Olson, E. N. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408, 106–111 (2000).
Merrill, G. F., Kurth, E. J., Hardie, D. G. & Winder, W. W. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 273, E1107–E1112 (1997). One of the first studies to show that pharmacological activation of AMPK could induce metabolic effects similar to exercise.
Aschenbach, W. G. et al. Effect of AICAR treatment on glycogen metabolism in skeletal muscle. Diabetes 51, 567–573 (2002).
McGee, S. L. et al. Exercise increases nuclear AMPKα2 in human skeletal muscle. Diabetes 52, 926–928 (2003).
McGee, S. L. & Hargreaves, M. AMPK and transcriptional regulation. Front. Biosci. 13, 3022–3033 (2008).
McGee, S. L. et al. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes 57, 860–867 (2008).
Lo, W. S. et al. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol. Cell 5, 917–926 (2000).
Backs, J., Backs, T., Bezprozvannaya, S., McKinsey, T. A. & Olson, E. N. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol. Cell. Biol. 28, 3437–3445 (2008).
Corton, J. M., Gillespie, J. G., Hawley, S. A. & Hardie, D. G. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 229, 558–565 (1995).
Winder, W. W. et al. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. 88, 2219–2226 (2000).
Narkar, V. A. et al. AMPK and PPARδ agonists are exercise mimetics. Cell 134, 405–415 (2008).
Myers, R. W. et al. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science 357, 507–511 (2017).
Muise, E. S. et al. Pharmacological AMPK activation induces transcriptional responses congruent to exercise in skeletal and cardiac muscle, adipose tissues and liver. PLoS One 14, e0211568 (2019).
Zhou, X. et al. PAN-AMPK activation improves renal function in a rat model of progressive diabetic nephropathy. J. Pharmacol. Exp. Ther. 371, 45–55 (2019).
Arad, M. et al. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J. Clin. Invest. 109, 357–362 (2002).
Fan, W. et al. PPARδ promotes running endurance by preserving glucose. Cell Metab. 25, 1186–1193.e4 (2017).
Tanaka, T. et al. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl Acad. Sci. USA 100, 15924–15929 (2003).
Chen, W., Wang, L. L., Liu, H. Y., Long, L. & Li, S. Peroxisome proliferator-activated receptor δ-agonist, GW501516, ameliorates insulin resistance, improves dyslipidaemia in monosodium L-glutamate metabolic syndrome mice. Basic Clin. Pharmacol. Toxicol. 103, 240–246 (2008).
Bernardo, B. L. et al. Postnatal PPARδ activation and myostatin inhibition exert distinct yet complimentary effects on the metabolic profile of obese insulin-resistant mice. PLoS One 5, e11307 (2010).
Dimopoulos, N., Watson, M., Green, C. & Hundal, H. S. The PPARδ agonist, GW501516, promotes fatty acid oxidation but has no direct effect on glucose utilisation or insulin sensitivity in rat L6 skeletal muscle cells. FEBS Lett. 581, 4743–4748 (2007).
Doktorova, M. et al. Intestinal PPARδ protects against diet-induced obesity, insulin resistance and dyslipidemia. Sci. Rep. 7, 846 (2017).
Miura, P. et al. Pharmacological activation of PPARβ/δ stimulates utrophin A expression in skeletal muscle fibers and restores sarcolemmal integrity in mature mdx mice. Hum. Mol. Genet. 18, 4640–4649 (2009).
Gupta, R. A. et al. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-δ accelerates intestinal adenoma growth. Nat. Med. 10, 245–247 (2004).
Borland, M. G. et al. Inhibition of tumorigenesis by peroxisome proliferator-activated receptor (PPAR)-dependent cell cycle blocks in human skin carcinoma cells. Toxicology 404–405, 25–32 (2018).
Ji, Y., Li, H., Wang, F. & Gu, L. PPARβ/δ agonist GW501516 inhibits tumorigenicity of undifferentiated nasopharyngeal carcinoma in C666-1 cells by promoting apoptosis. Front. Pharmacol. 9, 648 (2018).
Lahm, A. et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc. Natl Acad. Sci. USA 104, 17335–17340 (2007).
Fischle, W. et al. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol. Cell 9, 45–57 (2002).
Hudson, G. M., Watson, P. J., Fairall, L., Jamieson, A. G. & Schwabe, J. W. Insights into the recruitment of class IIa histone deacetylases (HDACs) to the SMRT/NCoR transcriptional repression complex. J. Biol. Chem. 290, 18237–18244 (2015).
Gaur, V. et al. Scriptaid enhances skeletal muscle insulin action and cardiac function in obese mice. Diabetes Obes. Metab. 19, 936–943 (2017).
Backs, J., Song, K., Bezprozvannaya, S., Chang, S. & Olson, E. N. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J. Clin. Invest. 116, 1853–1864 (2006).
Zhang, C. L. et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110, 479–488 (2002).
Vega, R. B. et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol. Cell. Biol. 24, 8374–8385 (2004).
Seidel, C., Schnekenburger, M., Dicato, M. & Diederich, M. Histone deacetylase 6 in health and disease. Epigenomics 7, 103–118 (2015).
Fan, W. & Evans, R. M. Exercise mimetics: impact on health and performance. Cell Metab. 25, 242–247 (2017).
Hawley, J. A., Joyner, M. J. & Green, D. J. Mimicking exercise: what matters most and where to next? J. Physiol. https://doi.org/10.1113/jp278761 (2019).
Even-Faitelson, L., Hassan-Zadeh, V., Baghestani, Z. & Bazett-Jones, D. P. Coming to terms with chromatin structure. Chromosoma 125, 95–110 (2016).
Dultz, E. et al. Quantitative imaging of chromatin decompaction in living cells. Mol. Biol. Cell 29, 1763–1777 (2018).
Talbert, P. B., Meers, M. P. & Henikoff, S. Old cogs, new tricks: the evolution of gene expression in a chromatin context. Nat. Rev. Genet. 20, 283–297 (2019).
Li, E. & Zhang, Y. DNA methylation in mammals. Cold Spring Harb. Perspect. Biol. 6, a019133 (2014).
Nicolas, D., Zoller, B., Suter, D. M. & Naef, F. Modulation of transcriptional burst frequency by histone acetylation. Proc. Natl Acad. Sci. USA 115, 7153–7158 (2018).
Acknowledgements
The authors acknowledge the many researchers whose work they have not been able to cite in this Review. Original work by the authors was supported by the Diabetes Australia Research Program and the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
Both authors hold equity in Imitex Pty Ltd., a drug discovery start-up that was spun out from papers published by the authors that have identified the MEF2-class IIa HDAC axis as a druggable target to replicate aspects of the exercise adaptive response. This pathway is discussed in the current manuscript, along with many others that have been studied within the field.
Additional information
Peer review information
Nature Reviews Endocrinology thanks M.H. Laughlin and M. Tarnopolsky for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Type II fibres
-
Muscle fibres that are fast twitch, are able to produce large amounts of tension, have a fairly high reliance on anaerobic ATP production and fatigue easily.
Rights and permissions
About this article
Cite this article
McGee, S.L., Hargreaves, M. Exercise adaptations: molecular mechanisms and potential targets for therapeutic benefit. Nat Rev Endocrinol 16, 495–505 (2020). https://doi.org/10.1038/s41574-020-0377-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-020-0377-1
This article is cited by
-
Effect of combined Kinesiotaping and resistive exercise on muscle strength and quality of life in breast cancer survivors: a randomized clinical trial
Journal of the Egyptian National Cancer Institute (2024)
-
Exercise mimetics: a novel strategy to combat neuroinflammation and Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
One episode of low intensity aerobic exercise prior to systemic AAV9 administration augments transgene delivery to the heart and skeletal muscle
Journal of Translational Medicine (2023)
-
Mitochondrial transplantation as a possible therapeutic option for sarcopenia
Journal of Molecular Medicine (2023)
-
Signs of aging in midlife: physical function and sex differences in microbiota
GeroScience (2023)