Abstract
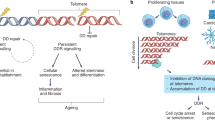
Telomeres are specialized nucleoprotein structures at the ends of linear chromosomes that prevent the activation of DNA damage response and repair pathways. Numerous factors localize at telomeres to regulate their length, structure and function, to avert replicative senescence or genome instability and cell death. In humans, Mendelian defects in several of these factors can result in abnormally short or dysfunctional telomeres, causing a group of rare heterogeneous premature-ageing diseases, termed telomeropathies, short-telomere syndromes or telomere biology disorders (TBDs). Here, we review the TBD-causing genes identified so far and describe their main functions associated with telomere biology. We present molecular aspects of TBDs, including genetic anticipation, phenocopy, incomplete penetrance and somatic genetic rescue, which underlie the complexity of these diseases. We also discuss the implications of phenotypic and genetic features of TBDs on fundamental aspects related to human telomere biology, ageing and cancer, as well as on diagnostic, therapeutic and clinical approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
de Lange, T. Shelterin-mediated telomere protection. Annu. Rev. Genet. 52, 223–247 (2018).
Gilson, E. & Geli, V. How telomeres are replicated. Nat. Rev. Mol. Cell Biol. 8, 825–838 (2007).
Rossiello, F., Jurk, D., Passos, J. F. & d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 24, 135–147 (2022).
Bertuch, A. A. The molecular genetics of the telomere biology disorders. RNA Biol. 13, 696–706 (2016).
Alder, J. K. & Armanios, M. Telomere-mediated lung disease. Physiol. Rev. 102, 1703–1720 (2022).
Demanelis, K. et al. Determinants of telomere length across human tissues. Science 369, eaaz6876 (2020).
Blasco, M. A. Mice with bad ends: mouse models for the study of telomeres and telomerase in cancer and aging. EMBO J. 24, 1095–1103 (2005).
Greenberg, R. A., Allsopp, R. C., Chin, L., Morin, G. B. & DePinho, R. A. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene 16, 1723–1730 (1998).
Kipling, D. & Cooke, H. J. Hypervariable ultra-long telomeres in mice. Nature 347, 400–402 (1990).
Heiss, N. S. et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat. Genet. 19, 32–38 (1998).
Lai, T. P., Wright, W. E. & Shay, J. W. Comparison of telomere length measurement methods. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160451 (2018).
Alter, B. P. et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood 110, 1439–1447 (2007).
Alder, J. K. et al. Diagnostic utility of telomere length testing in a hospital-based setting. Proc. Natl Acad. Sci. USA 115, E2358–E2365 (2018). By examining telomere length and clinical features of a cohort of 100 patients with TBD, this work demonstrated that the degree of telomere shortening inversely correlated with the age at diagnosis and the TBD phenotype.
Roake, C. M. & Artandi, S. E. Regulation of human telomerase in homeostasis and disease. Nat. Rev. Mol. Cell Biol. 21, 384–397 (2020).
Meyerson, M. et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90, 785–795 (1997).
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998). This study demonstrates that ectopic expression of TERT is sufficient to immortalize human somatic cells.
Nault, J. C., Ningarhari, M., Rebouissou, S. & Zucman-Rossi, J. The role of telomeres and telomerase in cirrhosis and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 16, 544–558 (2019).
Lorbeer, F. K. & Hockemeyer, D. TERT promoter mutations and telomeres during tumorigenesis. Curr. Opin. Genet. Dev. 60, 56–62 (2020).
Greider, C. W. Telomerase is processive. Mol. Cell. Biol. 11, 4572–4580 (1991).
Armanios, M. Y. et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 356, 1317–1326 (2007).
Tsakiri, K. D. et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc. Natl Acad. Sci. USA 104, 7552–7557 (2007).
Du, H. Y. et al. Complex inheritance pattern of dyskeratosis congenita in two families with 2 different mutations in the telomerase reverse transcriptase gene. Blood 111, 1128–1130 (2008).
Aspesi, A. et al. Compound heterozygosity for two new TERT mutations in a patient with aplastic anemia. Pediatr. Blood Cancer 55, 550–553 (2010).
Gramatges, M. M., Qi, X., Sasa, G. S., Chen, J. J. & Bertuch, A. A. A homozygous telomerase T-motif variant resulting in markedly reduced repeat addition processivity in siblings with Hoyeraal Hreidarsson syndrome. Blood 121, 3586–3593 (2013).
Niaz, A. et al. Functional interaction between compound heterozygous TERT mutations causes severe telomere biology disorder. Blood Adv. 6, 3779–3791 (2022).
Cepni, E., Satkin, N. B., Moheb, L. A., Rocha, M. E. & Kayserili, H. Biallelic TERT variant leads to Hoyeraal-Hreidarsson syndrome with additional dyskeratosis congenita findings. Am. J. Med. Genet. Part A 188, 1226–1232 (2022).
Stockklausner, C. et al. A novel autosomal recessive TERT T1129P mutation in a dyskeratosis congenita family leads to cellular senescence and loss of CD34+ hematopoietic stem cells not reversible by mTOR-inhibition. Aging 7, 911–927 (2015).
Marrone, A. et al. Telomerase reverse-transcriptase homozygous mutations in autosomal recessive dyskeratosis congenita and Hoyeraal-Hreidarsson syndrome. Blood 110, 4198–4205 (2007).
Roake, C. M. et al. Disruption of telomerase RNA maturation kinetics precipitates disease. Mol. Cell 74, 688–700.e683 (2019). This work demonstrates that a feedforward pathway of hTR oligoadenylation by PADP5 and deadenylation by PARN regulates the rate of hTR maturation.
Qin, J. & Autexier, C. Regulation of human telomerase RNA biogenesis and localization. RNA Biol. 18, 305–315 (2021).
Tseng, C. K. et al. Human telomerase RNA processing and quality control. Cell Rep. 13, 2232–2243 (2015). This work demonstrates that the nuclear exosome competes with PARN to regulate the maturation of hTR molecule.
Nguyen, T. H. D. et al. Cryo-EM structure of substrate-bound human telomerase holoenzyme. Nature 557, 190–195 (2018). Using cryo-electron microscopy, this study reveals a flexible bi-lobed structure of human telomerase holoenzyme bound to its DNA substrate and provides new insights into the impact of disease-associated dyskerin mutations.
Vulliamy, T. et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature 413, 432–435 (2001).
Collopy, L. C. et al. Triallelic and epigenetic-like inheritance in human disorders of telomerase. Blood 126, 176–184 (2015).
Egan, E. D. & Collins, K. Biogenesis of telomerase ribonucleoproteins. RNA 18, 1747–1759 (2012).
Shukla, S., Schmidt, J. C., Goldfarb, K. C., Cech, T. R. & Parker, R. Inhibition of telomerase RNA decay rescues telomerase deficiency caused by dyskerin or PARN defects. Nat. Struct. Mol. Biol. 23, 286–292 (2016).
Knight, S. W. et al. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am. J. Hum. Genet. 65, 50–58 (1999).
Ghanim, G. E. et al. Structure of human telomerase holoenzyme with bound telomeric DNA. Nature 593, 449–453 (2021).
Alder, J. K. et al. Telomere phenotypes in females with heterozygous mutations in the dyskeratosis congenita 1 (DKC1) gene. Hum. Mutat. 34, 1481–1485 (2013).
Xu, J. et al. Investigation of chromosome X inactivation and clinical phenotypes in female carriers of DKC1 mutations. Am. J. Hematol. 91, 1215–1220 (2016).
Walne, A. J. et al. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum. Mol. Genet. 16, 1619–1629 (2007).
Trahan, C., Martel, C. & Dragon, F. Effects of dyskeratosis congenita mutations in dyskerin, NHP2 and NOP10 on assembly of H/ACA pre-RNPs. Hum. Mol. Genet. 19, 825–836 (2010).
Kannengiesser, C. et al. First heterozygous NOP10 mutation in familial pulmonary fibrosis. Eur. Respir. J. 55, 1902465 (2020).
Manali, E. D. et al. Genotype–phenotype relationships in inheritable idiopathic pulmonary fibrosis: a Greek national cohort study. Respiration 101, 531–543 (2022).
Vulliamy, T. et al. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc. Natl Acad. Sci. USA 105, 8073–8078 (2008).
Benyelles, M. et al. NHP2 deficiency impairs rRNA biogenesis and causes pulmonary fibrosis and Hoyeraal-Hreidarsson syndrome. Hum. Mol. Genet. 29, 907–922 (2020).
Balogh, E. et al. Pseudouridylation defect due to DKC1 and NOP10 mutations causes nephrotic syndrome with cataracts, hearing impairment, and enterocolitis. Proc. Natl Acad. Sci. USA 117, 15137–15147 (2020).
Venteicher, A. S. et al. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science 323, 644–648 (2009).
Chen, L. et al. An activity switch in human telomerase based on RNA conformation and shaped by TCAB1. Cell 174, 218–230 e213 (2018).
Zhong, F. et al. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev. 25, 11–16 (2010).
Shao, Y. et al. A unique homozygous WRAP53 Arg298Trp mutation underlies dyskeratosis congenita in a Chinese Han family. BMC Med. Genet. 19, 40 (2018).
Bergstrand, S. et al. Biallelic mutations in WRAP53 result in dysfunctional telomeres, Cajal bodies and DNA repair, thereby causing Hoyeraal-Hreidarsson syndrome. Cell Death Dis. 11, 238 (2020).
Brailovski, E. et al. Previously unreported WRAP53 gene variants in a patient with dyskeratosis congenita. Ann. Hematol. 101, 907–909 (2022).
Freund, A. et al. Proteostatic control of telomerase function through TRiC-mediated folding of TCAB1. Cell 159, 1389–1403 (2014).
Henriksson, S. et al. The scaffold protein WRAP53beta orchestrates the ubiquitin response critical for DNA double-strand break repair. Genes Dev. 28, 2726–2738 (2014).
Stanley, S. E. et al. Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema. Sci. Transl. Med. 8, 351ra107 (2016).
Hoareau-Aveilla, C., Bonoli, M., Caizergues-Ferrer, M. & Henry, Y. hNaf1 is required for accumulation of human box H/ACA snoRNPs, scaRNPs, and telomerase. RNA 12, 832–840 (2006).
Nguyen, D. et al. A polyadenylation-dependent 3′ end maturation pathway is required for the synthesis of the human telomerase RNA. Cell Rep. 13, 2244–2257 (2015).
Boyraz, B. et al. Posttranscriptional manipulation of TERC reverses molecular hallmarks of telomere disease. J. Clin. Invest. 126, 3377–3382 (2016).
Moon, D. H. et al. Poly(A)-specific ribonuclease (PARN) mediates 3′-end maturation of the telomerase RNA component. Nat. Genet. 47, 1482–1488 (2015).
Dhanraj, S. et al. Bone marrow failure and developmental delay caused by mutations in poly(A)-specific ribonuclease (PARN). J. Med. Genet. 52, 738–748 (2015).
Dodson, L. M. et al. From incomplete penetrance with normal telomere length to severe disease and telomere shortening in a family with monoallelic and biallelic PARN pathogenic variants. Hum. Mutat. 40, 2414–2429 (2019).
Tummala, H. et al. Poly(A)-specific ribonuclease deficiency impacts telomere biology and causes dyskeratosis congenita. J. Clin. Invest. 125, 2151–2160 (2015).
Burris, A. M. et al. Hoyeraal-hreidarsson syndrome due to PARN mutations: fourteen years of follow-up. Pediatr. Neurol. 56, 62–68.e61 (2016).
Benyelles, M. et al. Impaired telomere integrity and rRNA biogenesis in PARN-deficient patients and knock-out models. EMBO Mol. Med. 11, e10201 (2019).
Stuart, B. D. et al. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat. Genet. 47, 512–517 (2015).
Lata, S. et al. Whole-exome sequencing in adults with chronic kidney disease: a pilot study. Ann. Intern. Med. 168, 100–109 (2018).
Schmid, M. & Jensen, T. H. Controlling nuclear RNA levels. Nat. Rev. Genet. 19, 518–529 (2018).
Garland, W. et al. Chromatin modifier HUSH co-operates with RNA decay factor NEXT to restrict transposable element expression. Mol. Cell 82, 1691–1707 (2022).
Gable, D. L. et al. ZCCHC8, the nuclear exosome targeting component, is mutated in familial pulmonary fibrosis and is required for telomerase RNA maturation. Genes Dev. 33, 1381–1396 (2019).
Lingaraju, M. et al. The MTR4 helicase recruits nuclear adaptors of the human RNA exosome using distinct arch-interacting motifs. Nat. Commun. 10, 3393 (2019).
Gerlach, P. et al. Structure and regulation of the nuclear exosome targeting complex guides RNA substrates to the exosome. Mol. Cell 82, 2505–2518.e7 (2022).
Puno, M. R. & Lima, C. D. Structural basis for RNA surveillance by the human nuclear exosome targeting (NEXT) complex. Cell 185, 2132–2147.e2126 (2022).
Najmabadi, H. et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 478, 57–63 (2011).
Schmutz, I. et al. TINF2 is a haploinsufficient tumor suppressor that limits telomere length. eLife 9, e61235 (2020).
Savage, S. A. et al. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am. J. Hum. Genet. 82, 501–509 (2008).
Walne, A. J., Vulliamy, T., Beswick, R., Kirwan, M. & Dokal, I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood 112, 3594–3600 (2008).
Touzot, F. et al. Heterogeneous telomere defects in patients with severe forms of dyskeratosis congenita. J. Allergy Clin. Immunol. 129, 473–482 (2012).
Karremann, M. et al. Revesz syndrome revisited. Orphanet J. Rare Dis. 15, 299 (2020).
Alder, J. K. et al. Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest 147, 1361–1368 (2015).
Sasa, G. S., Ribes-Zamora, A., Nelson, N. D. & Bertuch, A. A. Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood. Clin. Genet. 81, 470–478 (2012).
Choo, S. et al. Editing TINF2 as a potential therapeutic approach to restore telomere length in dyskeratosis congenita. Blood 140, 608–918 (2022). CRISPR–Cas9 editing of the TINF2 locus in pluripotent stem cells bearing a heterozygous TBD-associated TINF2 mutation demonstrates that the mutant TIN2 protein markedly shortens telomeres via a GoF effect.
Nandakumar, J. et al. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature 492, 285–289 (2012). This work demonstrates the crucial role of a patch of residues on the surface of TPP1, known as the TEL patch, in the recruitment and stimulation of telomerase at telomeres.
Sekne, Z., Ghanim, G. E., van Roon, A. M. & Nguyen, T. H. D. Structural basis of human telomerase recruitment by TPP1–POT1. Science 375, 1173–1176 (2022).
Kocak, H. et al. Hoyeraal-Hreidarsson syndrome caused by a germline mutation in the TEL patch of the telomere protein TPP1. Genes Dev. 28, 2090–2102 (2014).
Guo, Y. et al. Inherited bone marrow failure associated with germline mutation of ACD, the gene encoding telomere protein TPP1. Blood 124, 2767–2774 (2014).
Bisht, K., Smith, E. M., Tesmer, V. M. & Nandakumar, J. Structural and functional consequences of a disease mutation in the telomere protein TPP1. Proc. Natl Acad. Sci. USA 113, 13021–13026 (2016).
Hoffman, T. W. et al. Pulmonary fibrosis linked to variants in the ACD gene, encoding the telomere protein TPP1. Eur. Respir. J. 54, 1900809 (2019).
Henslee, G., Williams, C. L., Liu, P. & Bertuch, A. A. Identification and characterization of novel ACD variants: modulation of TPP1 protein level offsets the impact of germline loss-of-function variants on telomere length. Cold Spring Harb. Mol. Case Stud. 7, a005454 (2021).
Tummala, H. et al. Homozygous OB-fold variants in telomere protein TPP1 are associated with dyskeratosis congenita-like phenotypes. Blood 132, 1349–1353 (2018).
Graniel, J. V. et al. Differential impact of a dyskeratosis congenita mutation in TPP1 on mouse hematopoiesis and germline. Life Sci. Alliance 5, e202101208 (2021).
Aoude, L. G. et al. Nonsense mutations in the shelterin complex genes ACD and TERF2IP in familial melanoma. J. Natl Cancer Inst. 107, dju408 (2015).
Speedy, H. E. et al. Germ line mutations in shelterin complex genes are associated with familial chronic lymphocytic leukemia. Blood 128, 2319–2326 (2016).
Wang, Y. et al. Identification of rare variants predisposing to thyroid cancer. Thyroid 29, 946–955 (2019).
Wang, F. et al. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510 (2007).
Glousker, G., Briod, A. S., Quadroni, M. & Lingner, J. Human shelterin protein POT1 prevents severe telomere instability induced by homology-directed DNA repair. EMBO J. 39, e104500 (2020).
Zaug, A. J., Goodrich, K. J., Song, J. J., Sullivan, A. E. & Cech, T. R. Reconstitution of a telomeric replicon organized by CST. Nature https://doi.org/10.1038/s41586-022-04930-8 (2022). In this work, reconstitution of telomere replication in vitro provides new insight into the roles of the CST complex in orchestrating the initiation of C-strand synthesis.
Chen, L. Y., Redon, S. & Lingner, J. The human CST complex is a terminator of telomerase activity. Nature 488, 540–544 (2012).
Takai, H. et al. A POT1 mutation implicates defective telomere end fill-in and telomere truncations in Coats plus. Genes Dev. 30, 812–826 (2016).
Kelich, J. et al. Telomere dysfunction implicates POT1 in patients with idiopathic pulmonary fibrosis. J. Exp. Med. 219, e20211681 (2022).
Shi, J. et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat. Genet. 46, 482–486 (2014).
Robles-Espinoza, C. D. et al. POT1 loss-of-function variants predispose to familial melanoma. Nat. Genet. 46, 478–481 (2014).
Shen, E. et al. POT1 mutation spectrum in tumour types commonly diagnosed among POT1-associated hereditary cancer syndrome families. J. Med. Genet. 57, 664–670 (2020).
Youds, J. L. et al. RTEL-1 enforces meiotic crossover interference and homeostasis. Science 327, 1254–1258 (2010).
Vannier, J. B. et al. RTEL1 is a replisome-associated helicase that promotes telomere and genome-wide replication. Science 342, 239–242 (2013).
Wu, W. et al. RTEL1 suppresses G-quadruplex-associated R-loops at difficult-to-replicate loci in the human genome. Nat. Struct. Mol. Biol. 27, 424–437 (2020).
Takedachi, A. et al. SLX4 interacts with RTEL1 to prevent transcription-mediated DNA replication perturbations. Nat. Struct. Mol. Biol. 27, 438–449 (2020).
Bjorkman, A. et al. Human RTEL1 associates with Poldip3 to facilitate responses to replication stress and R-loop resolution. Genes. Dev. 34, 1065–1074 (2020).
Kotsantis, P. et al. RTEL1 regulates G4/R-loops to avert replication-transcription collisions. Cell Rep. 33, 108546 (2020).
Sarek, G. et al. CDK phosphorylation of TRF2 controls t-loop dynamics during the cell cycle. Nature 575, 523–527 (2019).
Sarek, G., Vannier, J. B., Panier, S., Petrini, J. H. & Boulton, S. J. TRF2 recruits RTEL1 to telomeres in S phase to promote T-loop unwinding. Mol. Cell 57, 622–635 (2015).
Vannier, J. B., Pavicic-Kaltenbrunner, V., Petalcorin, M. I., Ding, H. & Boulton, S. J. RTEL1 dismantles T loops and counteracts telomeric G4-DNA to maintain telomere integrity. Cell 149, 795–806 (2012).
Ghisays, F. et al. RTEL1 influences the abundance and localization of TERRA RNA. Nat. Commun. 12, 3016 (2021).
Schertzer, M. et al. Human regulator of telomere elongation helicase 1 (RTEL1) is required for the nuclear and cytoplasmic trafficking of pre-U2 RNA. Nucleic Acids Res. 43, 1834–1847 (2015).
Walne, A. J., Vulliamy, T., Kirwan, M., Plagnol, V. & Dokal, I. Constitutional mutations in RTEL1 cause severe dyskeratosis congenita. Am. J. Hum. Genet. 92, 448–453 (2013).
Deng, Z. et al. Inherited mutations in the helicase RTEL1 cause telomere dysfunction and Hoyeraal-Hreidarsson syndrome. Proc. Natl Acad. Sci. USA 110, E3408–E3416 (2013).
Le Guen, T. et al. Human RTEL1 deficiency causes Hoyeraal-Hreidarsson syndrome with short telomeres and genome instability. Hum. Mol. Genet. 22, 3239–3249 (2013).
Ballew, B. J. et al. A recessive founder mutation in regulator of telomere elongation helicase 1, RTEL1, underlies severe immunodeficiency and features of Hoyeraal Hreidarsson syndrome. PLoS Genet. 9, e1003695 (2013).
Ballew, B. J. et al. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in Dyskeratosis congenita. Hum. Genet. 132, 473–480 (2013).
Jullien, L. et al. Mutations of the RTEL1 helicase in a Hoyeraal-Hreidarsson syndrome patient highlight the importance of the ARCH domain. Hum. Mutat. 37, 469–472 (2016).
Touzot, F. et al. Extended clinical and genetic spectrum associated with biallelic RTEL1 mutations. Blood Adv. 1, 36–46 (2016).
Speckmann, C. et al. Clinical and molecular heterogeneity of RTEL1 deficiency. Front. Immunol. 8, 449 (2017).
Awad, A. et al. Full length RTEL1 is required for the elongation of the single-stranded telomeric overhang by telomerase. Nucleic acids Res. 48, 7239–7251 (2020).
Cogan, J. D. et al. Rare variants in RTEL1 are associated with familial interstitial pneumonia. Am. J. Respir. Crit. Care Med. 191, 646–655 (2015).
Kannengiesser, C. et al. Heterozygous RTEL1 mutations are associated with familial pulmonary fibrosis. Eur. Respir. J. 46, 474–485 (2015).
Kropski, J. A. & Loyd, J. E. Telomeres revisited: RTEL1 variants in pulmonary fibrosis. Eur. Respir. J. 46, 312–314 (2015).
Juge, P. A. et al. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur. Respir. J. 49, 1602314 (2017).
Borie, R. et al. Regulator of telomere length 1 (RTEL1) mutations are associated with heterogeneous pulmonary and extra-pulmonary phenotypes. Eur. Respir. J. 53, 1800508 (2019).
Marsh, J. C. W. et al. Heterozygous RTEL1 variants in bone marrow failure and myeloid neoplasms. Blood Adv. 2, 36–48 (2018).
Cardoso, S. R. et al. Myelodysplasia and liver disease extend the spectrum of RTEL1 related telomeropathies. Haematologica 102, e293–e296 (2017).
Margalef, P. et al. Stabilization of reversed replication forks by telomerase drives telomere catastrophe. Cell 172, 439–453.e414 (2018).
Bhat, K. P. & Cortez, D. RPA and RAD51: fork reversal, fork protection, and genome stability. Nat. Struct. Mol. Biol. 25, 446–453 (2018).
Audry, J. et al. RPA prevents G-rich structure formation at lagging-strand telomeres to allow maintenance of chromosome ends. EMBO J. 34, 1942–1958 (2015).
Codd, V. et al. Polygenic basis and biomedical consequences of telomere length variation. Nat. Genet. 53, 1425–1433 (2021).
Sharma, R. et al. Gain-of-function mutations in RPA1 cause a syndrome with short telomeres and somatic genetic rescue. Blood 139, 1039–1051 (2022). This study introduces RPA1 as a TBD gene associated with direct SGR when the germline mutation enhances binding to telomeric DNA.
Flynn, R. L. et al. TERRA and hnRNPA1 orchestrate an RPA-to-POT1 switch on telomeric single-stranded DNA. Nature 471, 532–536 (2011).
Schmiester, M. & Demuth, I. SNM1B/Apollo in the DNA damage response and telomere maintenance. Oncotarget 8, 48398–48409 (2017).
Lenain, C. et al. The Apollo 5′ exonuclease functions together with TRF2 to protect telomeres from DNA repair. Curr. Biol. 16, 1303–1310 (2006).
van Overbeek, M. & de Lange, T. Apollo, an Artemis-related nuclease, interacts with TRF2 and protects human telomeres in S phase. Curr. Biol. 16, 1295–1302 (2006).
Quesada, V. et al. Giant tortoise genomes provide insights into longevity and age-related disease. Nat. Ecol. Evol. 3, 87–95 (2019).
Kolora, S. R. R. et al. Origins and evolution of extreme life span in Pacific ocean rockfishes. Science 374, 842–847 (2021).
Ye, J. et al. TRF2 and apollo cooperate with topoisomerase 2alpha to protect human telomeres from replicative damage. Cell 142, 230–242 (2010).
Lam, Y. C. et al. SNMIB/Apollo protects leading-strand telomeres against NHEJ-mediated repair. EMBO J. 29, 2230–2241 (2010).
Wu, P., van Overbeek, M., Rooney, S. & de Lange, T. Apollo contributes to G overhang maintenance and protects leading-end telomeres. Mol. Cell 39, 606–617 (2010).
Wu, P., Takai, H. & de Lange, T. Telomeric 3′ overhangs derive from resection by Exo1 and Apollo and fill-in by POT1b-associated CST. Cell 150, 39–52 (2012).
Taub, M. A. et al. Genetic determinants of telomere length from 109,122 ancestrally diverse whole-genome sequences in TOPMed. Cell Genom. 2, 100084 (2022).
Kermasson, L. et al. Inherited human Apollo deficiency causes severe bone marrow failure and developmental defects. Blood 139, 2427–2440 (2022). This work introduces biallelic mutations in the Apollo gene, DCLRE1B, as a cause of telomere dysfunction and clinical features of TBDs without global reduction in telomere length.
Akhter, S., Lam, Y. C., Chang, S. & Legerski, R. J. The telomeric protein SNM1B/Apollo is required for normal cell proliferation and embryonic development. Aging Cell 9, 1047–1056 (2010).
Fouquerel, E. et al. Targeted and persistent 8-oxoguanine base damage at telomeres promotes telomere loss and crisis. Mol. Cell 75, 117–130.e116 (2019).
Baddock, H. T. et al. A phosphate binding pocket is a key determinant of exo- versus endo-nucleolytic activity in the SNM1 nuclease family. Nucleic Acids Res. 49, 9294–9309 (2021).
Barnes, R. P. et al. Telomeric 8-oxo-guanine drives rapid premature senescence in the absence of telomere shortening. Nat. Struct. Mol. Biol. 29, 639–652 (2021).
Wan, M., Qin, J., Songyang, Z. & Liu, D. OB fold-containing protein 1 (OBFC1), a human homolog of yeast Stn1, associates with TPP1 and is implicated in telomere length regulation. J. Biol. Chem. 284, 26725–26731 (2009).
Miyake, Y. et al. RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol. Cell 36, 193–206 (2009).
Surovtseva, Y. V. et al. Conserved telomere maintenance component 1 interacts with STN1 and maintains chromosome ends in higher eukaryotes. Mol. Cell 36, 207–218 (2009).
Zaug, A. J. et al. CST does not evict elongating telomerase but prevents initiation by ssDNA binding. Nucleic Acids Res. 49, 11653–11665 (2021).
Mirman, Z. et al. 53BP1–RIF1–shieldin counteracts DSB resection through CST- and Polα-dependent fill-in. Nature 560, 112–116 (2018).
Stewart, J. A. et al. Human CST promotes telomere duplex replication and general replication restart after fork stalling. EMBO J. 31, 3537–3549 (2012).
Wang, F., Stewart, J. & Price, C. M. Human CST abundance determines recovery from diverse forms of DNA damage and replication stress. Cell Cycle 13, 3488–3498 (2014).
Wang, Y. & Chai, W. Pathogenic CTC1 mutations cause global genome instabilities under replication stress. Nucleic Acids Res. 46, 3981–3992 (2018).
Anderson, B. H. et al. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nat. Genet. 44, 338–342 (2012).
Polvi, A. et al. Mutations in CTC1, encoding the CTS telomere maintenance complex component 1, cause cerebroretinal microangiopathy with calcifications and cysts. Am. J. Hum. Genet. 90, 540–549 (2012).
Keller, R. B. et al. CTC1 Mutations in a patient with dyskeratosis congenita. Pediatr. Blood Cancer 59, 311–314 (2012).
Walne, A. et al. Mutations in the telomere capping complex in bone marrow failure and related syndromes. Haematologica 98, 334–338 (2013).
Lin, H., Gong, L., Zhan, S., Wang, Y. & Liu, A. Novel biallelic missense mutations in CTC1 gene identified in a Chinese family with Coats plus syndrome. J. Neurol. Sci. 382, 142–145 (2017).
Gu, P. et al. CTC1-STN1 coordinates G- and C-strand synthesis to regulate telomere length. Aging Cell 17, e12783 (2018).
Feng, X., Hsu, S. J., Kasbek, C., Chaiken, M. & Price, C. M. CTC1-mediated C-strand fill-in is an essential step in telomere length maintenance. Nucleic Acids Res. 45, 4281–4293 (2017).
Chen, L. Y., Majerska, J. & Lingner, J. Molecular basis of telomere syndrome caused by CTC1 mutations. Genes Dev. 27, 2099–2108 (2013).
Sargolzaeiaval, F. et al. CTC1 mutations in a Brazilian family with progeroid features and recurrent bone fractures. Mol. Genet. Genom. Med. 6, 1148–1156 (2018).
Simon, A. J. et al. Mutations in STN1 cause Coats plus syndrome and are associated with genomic and telomere defects. J. Exp. Med. 213, 1429–1440 (2016).
Passi, G. R. et al. An Indian child with Coats plus syndrome due to mutations in STN1. Am. J. Med. Genet. Part. A 182, 2139–2144 (2020).
Acharya, T. et al. Novel compound heterozygous STN1 variants are associated with Coats plus syndrome. Mol. Genet. Genom. Med. 9, e1708 (2021).
Himes, R. W. et al. Gastrointestinal hemorrhage: a manifestation of the telomere biology disorders. J. Pediatr. 230, 55–61 e54 (2021).
Toufektchan, E. et al. Germline mutation of MDM4, a major p53 regulator, in a familial syndrome of defective telomere maintenance. Sci. Adv. 6, eaay3511 (2020).
Vulliamy, T. J. et al. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cell Mol. Dis. 34, 257–263 (2005).
Yamaguchi, H. et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N. Engl. J. Med. 352, 1413–1424 (2005).
Nachmani, D. et al. Germline NPM1 mutations lead to altered rRNA 2′-O-methylation and cause dyskeratosis congenita. Nat. Genet. 51, 1518–1529 (2019).
Aspesi, A. & Ellis, S. R. Rare ribosomopathies: insights into mechanisms of cancer. Nat. Rev. Cancer 19, 228–238 (2019).
Penev, A. et al. Alternative splicing is a developmental switch for hTERT expression. Mol. Cell 81, 2349–2360.e2346 (2021).
Kim, J. H. et al. De novo mutations in SON disrupt RNA splicing of genes essential for brain development and metabolism, causing an intellectual-disability syndrome. Am. J. Hum. Genet. 99, 711–719 (2016).
Grozdanov, P. N., Roy, S., Kittur, N. & Meier, U. T. SHQ1 is required prior to NAF1 for assembly of H/ACA small nucleolar and telomerase RNPs. RNA 15, 1188–1197 (2009).
Bizarro, J. & Meier, U. T. Inherited SHQ1 mutations impair interaction with NAP57/dyskerin, a major target in dyskeratosis congenita. Mol. Genet. Genom. Med. 5, 805–808 (2017).
Mroczek, S. & Dziembowski, A. U6 RNA biogenesis and disease association. Wiley Interdiscip. Rev. RNA 4, 581–592 (2013).
Trippe, R. et al. Identification, cloning, and functional analysis of the human U6 snRNA-specific terminal uridylyl transferase. RNA 12, 1494–1504 (2006).
Wang, L., Clericuzio, C. & Larizza, L. in GeneReviews((R)) (eds M. P. Adam et al.) (1993).
Walne, A. J. et al. Marked overlap of four genetic syndromes with dyskeratosis congenita confounds clinical diagnosis. Haematologica 101, 1180–1189 (2016).
Shchepachev, V., Wischnewski, H., Missiaglia, E., Soneson, C. & Azzalin, C. M. Mpn1, mutated in poikiloderma with neutropenia protein 1, is a conserved 3′-to-5′ RNA exonuclease processing U6 small nuclear RNA. Cell Rep. 2, 855–865 (2012).
Vulliamy, T. et al. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat. Genet. 36, 447–449 (2004). This is the first report of increasing disease severity and shorter telomeres in succeeding generations in the TBDs.
Armanios, M. et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc. Natl Acad. Sci. Usa. 102, 15960–15964 (2005).
Goldman, F. et al. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc. Natl Acad. Sci. Usa. 102, 17119–17124 (2005).
Newton, C. A. et al. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur. Respir. J. 48, 1710–1720 (2016).
Gutierrez-Rodrigues, F. et al. A novel homozygous RTEL1 variant in a consanguineous Lebanese family: phenotypic heterogeneity and disease anticipation. Hum. Genet. 138, 1323–1330 (2019).
Fernandez, B. A. et al. A Newfoundland cohort of familial and sporadic idiopathic pulmonary fibrosis patients: clinical and genetic features. Respir. Res. 13, 64 (2012).
Hao, L. Y. et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell 123, 1121–1131 (2005).
Armanios, M. et al. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am. J. Hum. Genet. 85, 823–832 (2009).
Xing, C. & Garcia, C. K. Epigenetic inheritance of telomere length obscures identification of causative PARN locus. J. Med. Genet. 53, 356–358 (2016).
van der Vis, J. J. et al. Pulmonary fibrosis in non-mutation carriers of families with short telomere syndrome gene mutations. Respirology 26, 1160–1170 (2021). In a systematic study of 99 families with familial pulmonary fibrosis, the phenomenon of phenocopy is suggested by five individuals who lack the familial TBD gene mutation but developed pulmonary fibrosis.
Alder, J. K. et al. Ancestral mutation in telomerase causes defects in repeat addition processivity and manifests as familial pulmonary fibrosis. PLoS Genet. 7, e1001352 (2011).
Vulliamy, T. J. & Dokal, I. Dyskeratosis congenita: the diverse clinical presentation of mutations in the telomerase complex. Biochimie 90, 122–130 (2008).
Gaysinskaya, V., Stanley, S. E., Adam, S. & Armanios, M. Synonymous mutation in DKC1 causes telomerase RNA insufficiency manifesting as familial pulmonary fibrosis. Chest 158, 2449–2457 (2020).
van der Vis, J. J., van der Smagt, J. J., Hennekam, F. A. M., Grutters, J. C. & van Moorsel, C. H. M. Pulmonary fibrosis and a TERT founder mutation with a latency period of 300 years. Chest 158, 612–619 (2020).
Mustjoki, S. & Young, N. S. Somatic mutations in “Benign” disease. N. Engl. J. Med. 384, 2039–2052 (2021).
Abascal, F. et al. Somatic mutation landscapes at single-molecule resolution. Nature 593, 405–410 (2021).
Lee-Six, H. et al. Population dynamics of normal human blood inferred from somatic mutations. Nature 561, 473–478 (2018).
Revy, P., Kannengiesser, C. & Fischer, A. Somatic genetic rescue in Mendelian haematopoietic diseases. Nat. Rev. Genet. 20, 582–598 (2019).
Gutierrez-Rodrigues, F. et al. Pathogenic TERT promoter variants in telomere diseases. Genet. Med. 21, 1594–1602 (2019). This study extends the detection of haematopoietic somatic TERTpam in individuals with TBDs from those with idiopathic pulmonary fibrosis to those with marrow failure and liver disease and demonstrates an association of these somatic mutations with germline variants associated with telomerase deficiency.
Maryoung, L. et al. Somatic mutations in telomerase promoter counterbalance germline loss-of-function mutations. J. Clin. Invest. 127, 982–986 (2017). This work is the first to demonstrate somatic TERTpam in blood cells of individuals with idiopathic pulmonary fibrosis and germline LoF mutations in TERT or PARN.
Perdigones, N. et al. Clonal hematopoiesis in patients with dyskeratosis congenita. Am. J. Hematol. 91, 1227–1233 (2016).
Jongmans, M. C. et al. Revertant somatic mosaicism by mitotic recombination in dyskeratosis congenita. Am. J. Hum. Genet. 90, 426–433 (2012).
Schratz, K. E. et al. Somatic reversion impacts myelodysplastic syndromes and acute myeloid leukemia evolution in the short telomere disorders. J. Clin. Invest. 131, e147598 (2021). This study extends the genes with potential SGR events in the context of TBD to include RNA decay genes, specifically when the germline mutation reduces the level of mature hTR.
Gutierrez-Rodrigues, F. et al. Clonal hematopoiesis in telomere biology disorders associates with the underlying germline defect and somatic mutations in POT1, PPM1D, and TERT promoter. Blood 138 (Suppl. 1), 1111–1112 (2021).
Horn, S. et al. TERT promoter mutations in familial and sporadic melanoma. Science 339, 959–961 (2013).
Huang, F. W. et al. Highly recurrent TERT promoter mutations in human melanoma. Science 339, 957–959 (2013).
Chiba, K. et al. Cancer-associated TERT promoter mutations abrogate telomerase silencing. eLife 4, e07918 (2015).
Kennedy, A. L. et al. Distinct genetic pathways define pre-malignant versus compensatory clonal hematopoiesis in Shwachman–Diamond syndrome. Nat. Commun. 12, 1334 (2021).
Tan, S. et al. Somatic genetic rescue of a germline ribosome assembly defect. Nat. Commun. 12, 5044 (2021).
Ramsay, A. J. et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat. Genet. 45, 526–530 (2013).
Wu, Y., Poulos, R. C. & Reddel, R. R. Role of POT1 in human cancer. Cancers 12, 2739 (2020).
Bojesen, S. E. et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat. Genet. 45, 371–384 (2013).
Dionne, I. & Wellinger, R. J. Cell cycle-regulated generation of single-stranded G-rich DNA in the absence of telomerase. Proc. Natl Acad. Sci. Usa. 93, 13902–13907 (1996).
Lai, T. P. et al. A method for measuring the distribution of the shortest telomeres in cells and tissues. Nat. Commun. 8, 1356 (2017).
Kahl, V. F. S. et al. Telomere length measurement by molecular combing. Front. Cell Dev. Biol. 8, 493 (2020).
Baird, D. M., Rowson, J., Wynford-Thomas, D. & Kipling, D. Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat. Genet. 33, 203–207 (2003).
Lee, M. et al. Systematic computational identification of variants that activate exonic and intronic cryptic splice sites. Am. J. Hum. Genet. 100, 751–765 (2017).
Arthur, J. W. et al. A novel cause of DKC1-related bone marrow failure: partial deletion of the 3′ untranslated region. EJHaem 2, 157–166 (2021).
Guo, Q. et al. Intron retention by a novel intronic mutation in DKC1 gene caused recurrent still birth and early death in a Chinese family. Mol. Genet. Genom. Med. 10, e1934 (2022).
Walne, A. J. & Dokal, I. Dyskeratosis congenita: a historical perspective. Mech. Ageing Dev. 129, 48–59 (2008).
Gorgy, A. I. et al. Hepatopulmonary syndrome is a frequent cause of dyspnea in the short telomere disorders. Chest 148, 1019–1026 (2015).
Bhala, S. et al. CNS manifestations in patients with telomere biology disorders. Neurol. Genet. 5, 370 (2019).
Catto, L. F. B. et al. Somatic genetic rescue in hematopoietic cells in GATA2 deficiency. Blood 136, 1002–1005 (2020).
Le Guen, T. et al. An in vivo genetic reversion highlights the crucial role of Myb-Like, SWIRM, and MPN domains 1 (MYSM1) in human hematopoiesis and lymphocyte differentiation. J. Allergy Clin. Immunol. 136, 1619–1626.e5 (2015).
Kannengiesser, C., Borie, R. & Revy, P. Pulmonary fibrosis associated with TINF2 gene mutation: is somatic reversion required? Eur. Respir. J. 44, 269–270 (2014).
Peffault de Latour, R. et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Bone Marrow Transpl. 50, 1168–1172 (2015).
Phillips-Houlbracq, M. et al. Determinants of survival after lung transplantation in telomerase-related gene mutation carriers: a retrospective cohort. Am. J. Transpl. 22, 1236–1244 (2022).
Silhan, L. L. et al. Lung transplantation in telomerase mutation carriers with pulmonary fibrosis. Eur. Respir. J. 44, 178–187 (2014).
Tokman, S. et al. Clinical outcomes of lung transplant recipients with telomerase mutations. J. Heart Lung Transplant. 34, 1318–1324 (2015).
Stuart, B. D. et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir. Med. 2, 557–565 (2014).
Fok, W. C. et al. Posttranscriptional modulation of TERC by PAPD5 inhibition rescues hematopoietic development in dyskeratosis congenita. Blood 133, 1308–1312 (2019). This study establishes that reducing hTR 3′-end oligoadenylaton by silencing PAPD5 can increase hTR and telomerase activity, and restore definitive haematopoiesis in DKC1 mutant cells.
Nagpal, N. et al. Small-molecule PAPD5 inhibitors restore telomerase activity in patient stem cells. Cell Stem Cell 26, 896–909.e898 (2020). This study further develops PAPD5 inhibition as a therapeutical approach in TBDs by demonstrating rescue of telomere homeostasis in PARN-deficient human CD34+ cells in a mouse xenotransplantation model.
Shukla, S., Jeong, H. C., Sturgeon, C. M., Parker, R. & Batista, L. F. Z. Chemical inhibition of PAPD5/7 rescues telomerase function and hematopoiesis in dyskeratosis congenita. Blood Adv. 4, 2717–2722 (2020).
Calado, R. T. et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 114, 2236–2243 (2009).
Norberg, A. et al. Novel variants in Nordic patients referred for genetic testing of telomere-related disorders. Eur. J. Hum. Genet. 26, 858–867 (2018).
Vulliamy, T. J. et al. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood 107, 2680–2685 (2006).
Vulliamy, T. J. et al. Dyskeratosis congenita caused by a 3′ deletion: germline and somatic mosaicism in a female carrier. Blood 94, 1254–1260 (1999).
Knight, S. W. et al. Identification of novel DKC1 mutations in patients with dyskeratosis congenita: implications for pathophysiology and diagnosis. Hum. Genet. 108, 299–303 (2001).
Hiramatsu, H. et al. A novel missense mutation in the DKC1 gene in a Japanese family with X-linked dyskeratosis congenita. Pediatr. Hematol. Oncol. 19, 413–419 (2002).
Lin, J. H., Lee, J. Y., Tsao, C. J. & Chao, S. C. DKC1 gene mutation in a Taiwanese kindred with X-linked dyskeratosis congenita. Kaohsiung J. Med. Sci. 18, 573–577 (2002).
Kanegane, H. et al. Identification of DKC1 gene mutations in Japanese patients with X-linked dyskeratosis congenita. Br. J. Haematol. 129, 432–434 (2005).
Hisata, S. et al. A novel missense mutation of DKC1 in dyskeratosis congenita with pulmonary fibrosis. Sarcoidosis Vasc. Diffus. Lung Dis. 30, 221–225 (2013).
Kraemer, D. M. & Goebeler, M. Missense mutation in a patient with X-linked dyskeratosis congenita. Haematologica 88, ECR11 (2003).
Ding, Y. G. et al. Identification of a novel mutation and a de novo mutation in DKC1 in two Chinese pedigrees with dyskeratosis congenita. J. Invest. Dermatol. 123, 470–473 (2004).
Ratnasamy, V. et al. Dyskeratosis congenita with a novel genetic variant in the DKC1 gene: a case report. BMC Med. Genet. 19, 85 (2018).
Parry, E. M. et al. Decreased dyskerin levels as a mechanism of telomere shortening in X-linked dyskeratosis congenita. J. Med. Genet. 48, 327–333 (2011).
Sznajer, Y. et al. Further delineation of the congenital form of X-linked dyskeratosis congenita (Hoyeraal-Hreidarsson syndrome). Eur. J. Pediatr. 162, 863–867 (2003).
Cossu, F. et al. A novel DKC1 mutation, severe combined immunodeficiency (T+B−NK− SCID) and bone marrow transplantation in an infant with Hoyeraal–Hreidarsson syndrome. Br. J. Haematol. 119, 765–768 (2002).
Vulliamy, T. J. et al. Differences in disease severity but similar telomere lengths in genetic subgroups of patients with telomerase and shelterin mutations. PLoS ONE 6, e24383 (2011).
Knight, S. W. et al. Unexplained aplastic anaemia, immunodeficiency, and cerebellar hypoplasia (Hoyeraal–Hreidarsson syndrome) due to mutations in the dyskeratosis congenita gene, DKC1. Br. J. Haematol. 107, 335–339 (1999).
Kropski, J. A. et al. A novel dyskerin (DKC1) mutation is associated with familial interstitial pneumonia. Chest 146, e1–e7 (2014).
Du, H. Y. et al. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood 113, 309–316 (2009).
Ly, H. et al. Identification and functional characterization of 2 variant alleles of the telomerase RNA template gene (TERC) in a patient with dyskeratosis congenita. Blood 106, 1246–1252 (2005).
Fogarty, P. F. et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet 362, 1628–1630 (2003).
Feurstein, S. et al. Telomere biology disorder prevalence and phenotypes in adults with familial hematologic and/or pulmonary presentations. Blood Adv. 4, 4873–4886 (2020).
Alder, J. K. et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Natl Acad. Sci. USA 105, 13051–13056 (2008).
Alder, J. K. et al. Telomere length is a determinant of emphysema susceptibility. Am. J. Respir. Crit. Care Med. 184, 904–912 (2011).
Borie, R. et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur. Respir. J. 48, 1721–1731 (2016).
Parry, E. M., Alder, J. K., Qi, X., Chen, J. J. & Armanios, M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood 117, 5607–5611 (2011).
Philippot, Q. et al. Interstitial lung diseases associated with mutations of poly(A)-specific ribonuclease: a multicentre retrospective study. Respirology 27, 226–235 (2022).
Calado, R. T. et al. A spectrum of severe familial liver disorders associate with telomerase mutations. PLoS ONE 4, e7926 (2009).
Boyraz, B., Bellomo, C. M., Fleming, M. D., Cutler, C. S. & Agarwal, S. A novel TERC CR4/CR5 domain mutation causes telomere disease via decreased TERT binding. Blood 128, 2089–2092 (2016).
Hartmann, D. et al. Telomerase gene mutations are associated with cirrhosis formation. Hepatology 53, 1608–1617 (2011).
Ueda, Y. et al. A mutation in the H/ACA box of telomerase RNA component gene (TERC) in a young patient with myelodysplastic syndrome. BMC Med. Genet. 15, 68 (2014).
Kirwan, M. et al. Defining the pathogenic role of telomerase mutations in myelodysplastic syndrome and acute myeloid leukemia. Hum. Mutat. 30, 1567–1573 (2009).
Ly, H. et al. Functional characterization of telomerase RNA variants found in patients with hematologic disorders. Blood 105, 2332–2339 (2005).
Ortmann, C. A. et al. TERC mutations in children with refractory cytopenia. Haematologica 91, 707–708 (2006).
Han, B. et al. Telomerase gene mutation screening in Chinese patients with aplastic anemia. Leuk. Res. 34, 258–260 (2010).
Vogiatzi, P., Perdigones, N., Mason, P. J., Wilson, D. B. & Bessler, M. A family with Hoyeraal–Hreidarsson syndrome and four variants in two genes of the telomerase core complex. Pediatr. Blood Cancer 60, E4–E6 (2013).
Dressen, A. et al. Analysis of protein-altering variants in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: a candidate gene sequencing study. Lancet Respir. Med. 6, 603–614 (2018).
Erdem, M., Tufekci, O., Yilmaz, S., Alacacioglu, I. & Oren, H. Long-term follow-up of a case with dyskeratosis congenita caused by NHP2-V126M/X154R mutation: genotype-phenotype association. Acta Haematol. 141, 28–31 (2018).
Sarper, N., Zengin, E. & Kiliç, S. C. A child with severe form of dyskeratosis congenita and TINF2 mutation of shelterin complex. Pediatr. Blood Cancer 55, 1185–1186 (2010).
Gleeson, M. et al. Retinal vasculopathy in autosomal dominant dyskeratosis congenita due to TINF2 mutation. Br. J. Haematol. 159, 498 (2012).
Panichareon, B. et al. Novel mutation of the TINF2 gene in a patient with dyskeratosis congenita. Case Rep. Dermatol. 7, 212–219 (2015).
Du, H. et al. A case report of heterozygous TINF2 gene mutation associated with pulmonary fibrosis in a patient with dyskeratosis congenita. Medicine 97, e0724 (2018).
Roake, C. M., Juntilla, M., Agarwal-Hashmi, R., Artandi, S. & Kuo, C. S. Tissue-specific telomere shortening and degenerative changes in a patient with TINF2 mutation and dyskeratosis congenita. Hum. Pathol. 25, 200517 (2021).
Tsangaris, E. et al. Ataxia and pancytopenia caused by a mutation in TINF2. Hum. Genet. 124, 507–513 (2008).
Gupta, M. P., Talcott, K. E., Kim, D. Y., Agarwal, S. & Mukai, S. Retinal findings and a novel TINF2 mutation in Revesz syndrome: Clinical and molecular correlations with pediatric retinal vasculopathies. Ophthalmic Genet. 38, 51–60 (2017).
Moussa, K., Huang, J. N. & Moore, A. T. Revesz syndrome masquerading as traumatic retinal detachment. J. AAPOS 21, 422–425 e421 (2017).
Sakwit, A. et al. Novel mutation of the TINF2 gene resulting in severe phenotypic Revesz syndrome. Pediatr. Blood Cancer 66, e27557 (2019).
McElnea, E. M. et al. Revesz syndrome masquerading as bilateral cicatricial retinopathy of prematurity. J. AAPOS 17, 634–636 (2013).
Du, H. Y., Mason, P. J., Bessler, M. & Wilson, D. B. TINF2 mutations in children with severe aplastic anemia. Pediatr. Blood Cancer 52, 687 (2009).
Yamaguchi, H. et al. Identification of TINF2 gene mutations in adult Japanese patients with acquired bone marrow failure syndromes. Br. J. Haematol. 150, 725–727 (2010).
Keel, S. B. et al. Genetic features of myelodysplastic syndrome and aplastic anemia in pediatric and young adult patients. Haematologica 101, 1343–1350 (2016).
Magnusson, T., Godby, R. C., Bachiashvili, K. & Jamy, O. First report of novel heterozygous WRAP53 p.Ala522Glyfs*8 mutation associated dyskeratosis congenita. Br. J. Haematol. 196, e27–e29 (2022).
Han, E. et al. A unique case of coats plus syndrome and dyskeratosis congenita in a patient with CTC1 mutations. Ophthalmic Genet. 41, 363–367 (2020).
Riquelme, J. et al. Primary ovarian failure in addition to classical clinical features of coats plus syndrome in a female carrying 2 truncating variants of CTC1. Horm. Res. Paediatr. 94, 448–455 (2021).
Bisserbe, A. et al. Cerebro-retinal microangiopathy with calcifications and cysts due to recessive mutations in the CTC1 gene. Rev. Neurol. 171, 445–449 (2015).
Netravathi, M. et al. Whole exome sequencing in an Indian family links Coats plus syndrome and dextrocardia with a homozygous novel CTC1 and a rare HES7 variation. BMC Med. Genet. 16, 5 (2015).
Moriya, K. et al. Novel compound heterozygous RTEL1 gene mutations in a patient with Hoyeraal-Hreidarsson syndrome. Pediatr. Blood Cancer 63, 1683–1684 (2016).
Ziv, A. et al. An RTEL1 mutation links to infantile-onset ulcerative colitis and severe immunodeficiency. J. Clin. Immunol. 40, 1010–1019 (2020).
Belaya, Z. et al. Multiple bilateral hip fractures in a patient with dyskeratosis congenita caused by a novel mutation in the PARN gene. Osteoporos. Int. 32, 1227–1231 (2021).
Kropski, J. A. et al. Rare genetic variants in PARN are associated with pulmonary fibrosis in families. Am. J. Respir. Crit. Care Med. 196, 1481–1484 (2017).
Verduyn, M., Rigaud, M. & Dromer, C. [A rare familial form of idiopathic pulmonary fibrosis with poly(A)-specific ribonuclease (PARN) mutation]. Rev. Pneumol. Clin. 73, 272–275 (2017).
Zhang, D. et al. Homozygous rare PARN missense mutation in familial pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 199, 797–799 (2019).
Techer, H., Koundrioukoff, S., Nicolas, A. & Debatisse, M. The impact of replication stress on replication dynamics and DNA damage in vertebrate cells. Nat. Rev. Genet. 18, 535–550 (2017).
Bryan, T. M. G-Quadruplexes at telomeres: friend or foe? Molecules 25, 3686 (2020).
Azzalin, C. M. & Lingner, J. Telomere functions grounding on TERRA firma. Trends Cell Biol. 25, 29–36 (2015).
Blackburn, E. H., Epel, E. S. & Lin, J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350, 1193–1198 (2015).
Glousker, G., Touzot, F., Revy, P., Tzfati, Y. & Savage, S. A. Unraveling the pathogenesis of Hoyeraal-Hreidarsson syndrome, a complex telomere biology disorder. Br. J. Haematol. 170, 457–471 (2015).
Niewisch, M. R. & Savage, S. A. An update on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert Rev. Hematol. 12, 1037–1052 (2019).
Niewisch, M. R. et al. Disease progression and clinical outcomes in telomere biology disorders. Blood 139, 1807–1819 (2022).
Savage, S. A. Beginning at the ends: telomeres and human disease. F1000Res 7, F1000 Faculty Rev-524 (2018).
Schratz, K. E. & Armanios, M. Cancer and myeloid clonal evolution in the short telomere syndromes. Curr. Opin. Genet. Dev. 60, 112–118 (2020).
Alter, B. P., Giri, N., Savage, S. A. & Rosenberg, P. S. Cancer in dyskeratosis congenita. Blood 113, 6549–6557 (2009).
Schratz, K. E. et al. Cancer spectrum and outcomes in the Mendelian short telomere syndromes. Blood 135, 1946–1956 (2020).
Crow, Y. J. et al. Coats’ plus: a progressive familial syndrome of bilateral Coats’ disease, characteristic cerebral calcification, leukoencephalopathy, slow pre- and post-natal linear growth and defects of bone marrow and integument. Neuropediatrics 35, 10–19 (2004).
Briggs, T. A. et al. Cerebroretinal microangiopathy with calcifications and cysts (CRMCC). Am. J. Med. Genet. A 146A, 182–190 (2008).
Armanios, M. Telomerase mutations and the pulmonary fibrosis-bone marrow failure syndrome complex. N. Engl. J. Med. 367, 384 (2012).
Acknowledgements
The authors apologize to colleagues whose work could not be cited owing to restriction in the number of references allowed. P.R. thanks the members of the GDIS lab and is grateful to A. Fischer for discussion, advice and constant support. P.R. also thanks A. Decottignies and A. Fischer for critical reading of the manuscript. Current work in P.R.’s laboratory is funded by INSERM, Ligue Nationale contre le cancer, INCa and ANR. P.R. is a scientist from Centre National de la Recherche Scientifique (CNRS). Current work in A.A.B.’s laboratory is funded by the National Cancer Institute.
Author information
Authors and Affiliations
Contributions
P.R. and A.A.B. wrote the article. All authors contributed equally to all other aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks Jonathan K. Alder, Marcin W. Wlodarski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- R-loops
-
Nucleic acid structures composed of an RNA–DNA hybrid and a displaced single-stranded DNA that accumulate at specific regions genome-wide, including telomeres. R-loops can cause replication stress and genome instability.
- G-quadruplexes
-
Four DNA stranded secondary structures formed in G-rich sequences in which four guanines form a planar array via Hoogsteen base-pairing. These structures can cause replication stress.
- Somatic genetic rescue
-
In Mendelian disorders, an in vivo somatic genetic event that partially or totally counteracts the deleterious effect of the pathogenic germline mutation and provides a selective advantage over non-somatically modified cells.
- Clones
-
Cells that originate from a common cell ancestor (progenitor) with identical genetic identity.
- Genetic anticipation
-
A phenomenon observed in autosomal dominant diseases in which some clinical manifestations develop earlier and are more severe with successive generations.
- Phenocopy
-
A phenomenon whereby the phenotype dissociates from the genotype. In the present Review, when individuals with familial telomere biology disease exhibit short telomeres and/or premature ageing without carrying the causative germline variant.
- Repeat addition processivity
-
(RAP). The ability of telomerase to synthesize multiple telomeric repeats without dissociating from the telomere.
- Monoallelic
-
Refers to one genetic variant located on one allele of a gene.
- Biallelic
-
Refers to two (possibly different) variants located on both alleles of the same gene.
- Hypomorphic
-
Refers to a variant that results in reduced but not eliminated function of the gene product.
- Compound heterozygous
-
The existence of distinct mutations on opposite alleles of a single gene located on an autosomal chromosome.
- Cajal bodies
-
Distinct sub-nuclear structures present in eukaryotic cells associated with RNA metabolism and ribonucleoprotein biogenesis.
- Incomplete penetrance
-
Refers to the phenomenon of some individuals who carry a pathogenic variant who do not exhibit clinical signs.
- Variable expressivity
-
Refers to the phenomenon of individuals affected by a Mendelian disease who exhibit different clinical features.
- t-circles
-
Extrachromosomal circular DNA molecules that contain telomeric repeat sequences.
- Uniparental isodisomy
-
Refers to both copies of a chromosome originating from one parent (maternal or paternal) and the chromosome from the other parent being absent. Segmental uniparental isodisomy occurs when only part of a chromosome is affected.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Revy, P., Kannengiesser, C. & Bertuch, A.A. Genetics of human telomere biology disorders. Nat Rev Genet 24, 86–108 (2023). https://doi.org/10.1038/s41576-022-00527-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-022-00527-z
This article is cited by
-
Causal relationship between immune cells and telomere length: mendelian randomization analysis
BMC Immunology (2024)
-
Telomere length and cancer risk: finding Goldilocks
Biogerontology (2024)
-
A CRISPR base editing approach for the functional assessment of telomere biology disorder-related genes in human health and aging
Biogerontology (2024)
-
Adaptive and Maladaptive Clonal Hematopoiesis in Telomere Biology Disorders
Current Hematologic Malignancy Reports (2024)
-
Unzipped genome assemblies of polyploid root-knot nematodes reveal unusual and clade-specific telomeric repeats
Nature Communications (2024)