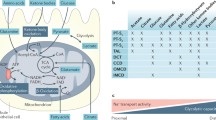
Abstract
Preclinical models of human disease provide powerful tools for therapeutic discovery but have limitations. This problem is especially apparent in the field of acute kidney injury (AKI), in which clinical trial failures have been attributed to inaccurate modelling performed largely in rodents. Multidisciplinary efforts such as the Kidney Precision Medicine Project are now starting to identify molecular subtypes of human AKI. In addition, over the past decade, there have been developments in human pluripotent stem cell-derived kidney organoids as well as zebrafish, rodent and large animal models of AKI. These organoid and AKI models are being deployed at different stages of preclinical therapeutic development. However, the traditionally siloed, preclinical investigator-driven approaches that have been used to evaluate AKI therapeutics to date rarely account for the limitations of the model systems used and have given rise to false expectations of clinical efficacy in patients with different AKI pathophysiologies. To address this problem, there is a need to develop more flexible and integrated approaches, involving teams of investigators with expertise in a range of different model systems, working closely with clinical investigators, to develop robust preclinical evidence to support more focused interventions in patients with AKI.
Key points
-
Human induced pluripotent stem cell-derived kidney organoid models of toxin-induced acute kidney injury (AKI) are amenable to high-throughput drug discovery and may provide insight into inter-individual variations in responses to therapeutic interventions.
-
Zebrafish models of toxin-induced AKI can be used for high-throughput, rapid therapeutic discovery before translation into mammalian systems.
-
Ischaemic, cardiac, toxin and sepsis-associated rodent models of AKI can be used to reflect diverse pathophysiologies in human AKI, validate therapeutic targets using genetic studies and explore distant organ effects of AKI.
-
Large animal models provide opportunities to more closely model human AKI pathophysiology and pharmacology, with increasingly complex, layered models of injury.
-
The discovery of molecular subtypes of human AKI will drive the development of focused preclinical therapeutic strategies to target defined AKI pathophysiologies.
-
We recommend multidisciplinary, bench-to-bedside approaches to the development and design of preclinical research pipelines using multiple models and species to optimize the potential for translation of findings into therapies for human AKI.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Al-Jaghbeer, M., Dealmeida, D., Bilderback, A., Ambrosino, R. & Kellum, J. A. Clinical Decision Support for In-Hospital AKI. J. Am. Soc. Nephrol. 29, 654–660 (2018).
Kellum, J. A., Lameire, N. & Group, K. A. G. W. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit. Care 17, 204 (2013).
Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Prim. 7, 52 (2021).
Ronco, C., Bellomo, R. & Kellum, J. A. Acute kidney injury. Lancet 394, 1949–1964 (2019).
Hoste, E. A. J. et al. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 14, 607–625 (2018).
Tenzi, J. et al. Renal histopathology in critically ill patients with septic acute kidney injury (S-AKI). J. Crit. Care 68, 38–41 (2021).
Neugarten, J. & Golestaneh, L. Female sex reduces the risk of hospital-associated acute kidney injury: a meta-analysis. BMC Nephrol. 19, 314 (2018).
Neugarten, J. & Golestaneh, L. Influence of sex on the progression of chronic kidney disease. Mayo Clin. Proc. 94, 1339–1356 (2019).
Skrypnyk, N. I., Siskind, L. J., Faubel, S. & de Caestecker, M. P. Bridging translation for acute kidney injury with better preclinical modeling of human disease. Am. J. Physiol. Renal Physiol. 310, F972–F984 (2016).
Przepiorski, A., Crunk, A. E., Espiritu, E. B., Hukriede, N. A. & Davidson, A. J. The utility of human kidney organoids in modeling kidney disease. Semin. Nephrol. 40, 188–198 (2020).
Combes, A. N., Zappia, L., Er, P. X., Oshlack, A. & Little, M. H. Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med. 11, 3 (2019).
Freedman, B. S. et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun. 6, 8715 (2015).
Homan, K. A. et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, 255–262 (2019).
Morizane, R. et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 33, 1193–1200 (2015).
Przepiorski, A. et al. A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Rep. 11, 470–484 (2018).
Subramanian, A. et al. Single cell census of human kidney organoids shows reproducibility and diminished off-target cells after transplantation. Nat. Commun. 10, 5462 (2019).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Wu, H. et al. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23, 869–881.e8 (2018).
Taguchi, A. & Nishinakamura, R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 21, 730–746.e6 (2017).
Tsujimoto, H. et al. A modular differentiation system maps multiple human kidney lineages from pluripotent stem cells. Cell Rep. 31, 107476 (2020).
Uchimura, K., Wu, H., Yoshimura, Y. & Humphreys, B. D. Human pluripotent stem cell-derived kidney organoids with improved collecting duct maturation and injury modeling. Cell Rep. 33, 108514 (2020).
Bajaj, P. et al. Human pluripotent stem cell-derived kidney model for nephrotoxicity studies. Drug Metab. Dispos. 46, 1703–1711 (2018).
Czerniecki, S. M. et al. High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 22, 929–940.e4 (2018).
Kang, H. M. et al. Effective reconstruction of functional organotypic kidney spheroid for in vitro nephrotoxicity studies. Sci. Rep. 9, 17610 (2019).
Digby, J. L. M., Vanichapol, T., Przepiorski, A., Davidson, A. J. & Sander, V. Evaluation of cisplatin-induced injury in human kidney organoids. Am. J. Physiol. Renal Physiol. 318, F971–F978 (2020).
Lemos, D. R. et al. Interleukin-1beta Activates a MYC-dependent metabolic switch in kidney stromal cells necessary for progressive tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 29, 1690–1705 (2018).
Sweeney, D. E. et al. Functional maturation of drug transporters in the developing, neonatal, and postnatal kidney. Mol. Pharmacol. 80, 147–154 (2011).
Soo, J. Y., Jansen, J., Masereeuw, R. & Little, M. H. Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat. Rev. Nephrol. 14, 378–393 (2018).
Bantounas, I. et al. Generation of functioning nephrons by implanting human pluripotent stem cell-derived kidney progenitors. Stem Cell Rep. 10, 766–779 (2018).
Garreta, E. et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 18, 397–405 (2019).
Sharmin, S. et al. Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J. Am. Soc. Nephrol. 27, 1778–1791 (2016).
van den Berg, C. W. et al. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Rep. 10, 751–765 (2018).
Lin, N. Y. C. et al. Renal reabsorption in 3D vascularized proximal tubule models. Proc. Natl Acad. Sci. USA 116, 5399–5404 (2019).
Reimschuessel, R., Bennett, R. O., May, E. B. & Lipsky, M. M. Development of newly formed nephrons in the goldfish kidney following hexachlorobutadiene-induced nephrotoxicity. Toxicol. Pathol. 18, 32–38 (1990).
Reimschuessel, R. & Williams, D. Development of new nephrons in adult kidneys following gentamicin-induced nephrotoxicity. Ren. Fail. 17, 101–106 (1995).
Reimschuessel, R. A fish model of renal regeneration and development. ILAR J. 42, 285–291 (2001).
Wingert, R. A. & Davidson, A. J. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 73, 1120–1127 (2008).
Davidson, A. J. Uncharted waters: nephrogenesis and renal regeneration in fish and mammals. Pediatr. Nephrol. 26, 1435–1443 (2011).
Diep, C. Q. et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 470, 95–100 (2011).
Zhou, W., Boucher, R. C., Bollig, F., Englert, C. & Hildebrandt, F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am. J. Physiol. Renal Physiol. 299, F1040–F1047 (2010).
Brilli Skvarca, L. et al. Enhancing regeneration after acute kidney injury by promoting cellular dedifferentiation in zebrafish. Dis. Model Mech. 12, dmm037390 (2019).
Cianciolo Cosentino, C. et al. Histone deacetylase inhibitor enhances recovery after AKI. J. Am. Soc. Nephrol. 24, 943–953 (2013).
Hentschel, D. M. et al. Acute renal failure in zebrafish: a novel system to study a complex disease. Am. J. Physiol. Renal Physiol. 288, F923–F929 (2005).
Chiba, T. et al. Retinoic acid signaling coordinates macrophage-dependent injury and repair after AKI. J. Am. Soc. Nephrol. 27, 495–508 (2016).
Yin, W. et al. Mammalian target of rapamycin mediates kidney injury molecule 1-dependent tubule injury in a surrogate model. J. Am. Soc. Nephrol. 27, 1943–1957 (2016).
Sanker, S. et al. Development of high-content assays for kidney progenitor cell expansion in transgenic zebrafish. J. Biomol. Screen. 18, 1193–1202 (2013).
Skrypnyk, N. I. et al. Delayed treatment with PTBA analogs reduces postinjury renal fibrosis after kidney injury. Am. J. Physiol. Renal Physiol. 310, F705–F716 (2016).
Wen, X. et al. Time-dependent effects of histone deacetylase inhibition in sepsis-associated acute kidney injury. Intensive Care Med. Exp. 8, 9 (2020).
Clatworthy, A. E. et al. Pseudomonas aeruginosa infection of zebrafish involves both host and pathogen determinants. Infect. Immun. 77, 1293–1303 (2009).
Wen, X. et al. A zebrafish model of infection-associated acute kidney injury. Am. J. Physiol. Renal Physiol. 315, F291–F299 (2018).
Emmerich, C. H. et al. Improving target assessment in biomedical research: the GOT-IT recommendations. Nat. Rev. Drug Discov. 20, 64–81 (2021).
Opsahl, J. A., Abraham, P. A. & Keane, W. F. Angiotensin-converting enzyme inhibitors in chronic renal failure. Drugs 39 (Suppl. 2), 23–32 (1990).
Sharfuddin, A. A. & Molitoris, B. A. Pathophysiology of ischemic acute kidney injury. Nat. Rev. Nephrol. 7, 189–200 (2011).
Heyman, S. N., Rosen, S. & Rosenberger, C. Animal models of renal dysfunction: acute kidney injury. Expert Opin. Drug Discov. 4, 629–641 (2009).
Kirita, Y., Wu, H., Uchimura, K., Wilson, P. C. & Humphreys, B. D. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc. Natl Acad. Sci. USA 117, 15874–15883 (2020).
Cippa, P. E. et al. Transcriptional trajectories of human kidney injury progression. JCI Insight 3, e123151 (2018).
Wei, Q. & Dong, Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am. J. Physiol. Renal Physiol. 303, F1487–F1494 (2012).
Skrypnyk, N. I., Harris, R. C. & de Caestecker, M. P. Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. J. Vis. Exp. https://doi.org/10.3791/50495 (2013).
Zager, R. A. & Altschuld, R. Body temperature: an important determinant of severity of ischemic renal injury. Am. J. Physiol. 251, F87–F93 (1986).
Shanley, P. F. et al. Topography of focal proximal tubular necrosis after ischemia with reflow in the rat kidney. Am. J. Pathol. 122, 462–468 (1986).
Lee, H. T., Ota-Setlik, A., Fu, Y., Nasr, S. H. & Emala, C. W. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology 101, 1313–1324 (2004).
Ferenbach, D. A. & Bonventre, J. V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 11, 264–276 (2015).
Soranno, D. E. et al. Acute kidney injury results in long-term diastolic dysfunction that is prevented by histone deacetylase inhibition. JACC Basic. Transl. Sci. 6, 119–133 (2021).
Le Clef, N., Verhulst, A., D’Haese, P. C. & Vervaet, B. A. Unilateral renal ischemia-reperfusion as a robust model for acute to chronic kidney injury in mice. PLoS One 11, e0152153 (2016).
Thompson, R. H. et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur. Urol. 58, 340–345 (2010).
Thompson, R. H. et al. Renal function after partial nephrectomy: effect of warm ischemia relative to quantity and quality of preserved kidney. Urology 79, 356–360 (2012).
Scarfe, L. et al. Long-term outcomes in mouse models of ischemia-reperfusion-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 317, F1068–F1080 (2019).
Menshikh, A. et al. Capillary rarefaction is more closely associated with CKD progression after cisplatin, rhabdomyolysis, and ischemia-reperfusion-induced AKI than renal fibrosis. Am. J. Physiol. Renal Physiol. 317, F1383–F1397 (2019).
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010). 1p following 143.
Finn, W. F. Enhanced recovery from postischemic acute renal failure. Micropuncture studies in the rat. Circ. Res. 46, 440–448 (1980).
Soranno, D. E. et al. Matching human unilateral AKI, a reverse translational approach to investigate kidney recovery after ischemia. J. Am. Soc. Nephrol. 30, 990–1005 (2019).
Clements, M. E., Chaber, C. J., Ledbetter, S. R. & Zuk, A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS One 8, e70464 (2013).
Kim, J. et al. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice. A role for manganese superoxide dismutase. J. Biol. Chem. 281, 20349–20356 (2006).
Aufhauser, D. D. Jr et al. Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J. Clin. Invest. 126, 1968–1977 (2016).
Tannenbaum, C., Ellis, R. P., Eyssel, F., Zou, J. & Schiebinger, L. Sex and gender analysis improves science and engineering. Nature 575, 137–146 (2019).
Peng, J. et al. Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int. 87, 137–150 (2015).
Gao, G. et al. TNF-alpha mediates increased susceptibility to ischemic AKI in diabetes. Am. J. Physiol. Renal Physiol. 304, F515–F521 (2013).
Shi, H., Patschan, D., Epstein, T., Goligorsky, M. S. & Winaver, J. Delayed recovery of renal regional blood flow in diabetic mice subjected to acute ischemic kidney injury. Am. J. Physiol. Renal Physiol. 293, F1512–F1517 (2007).
Polichnowski, A. J. et al. Severe renal mass reduction impairs recovery and promotes fibrosis after AKI. J. Am. Soc. Nephrol. 25, 1496–1507 (2014).
Vandenberghe, W. et al. Acute kidney injury in cardiorenal syndrome type 1 patients: a systematic review and meta-analysis. Cardiorenal Med. 6, 116–128 (2016).
Uduman, J. Epidemiology of cardiorenal syndrome. Adv. Chronic Kidney Dis. 25, 391–399 (2018).
Vallabhajosyula, S. et al. Sex disparities in acute kidney injury complicating acute myocardial infarction with cardiogenic shock. ESC Heart Fail. 6, 874–877 (2019).
Neugarten, J., Sandilya, S., Singh, B. & Golestaneh, L. Sex and the risk of AKI following cardio-thoracic surgery: a meta-analysis. Clin. J. Am. Soc. Nephrol. 11, 2113–2122 (2016).
Chang, D. et al. Noninvasive identification of renal hypoxia in experimental myocardial infarctions of different sizes by using BOLD MR imaging in a mouse model. Radiology 286, 129–139 (2018).
Lekawanvijit, S. et al. Myocardial infarction impairs renal function, induces renal interstitial fibrosis, and increases renal KIM-1 expression: implications for cardiorenal syndrome. Am. J. Physiol. Heart Circ. Physiol. 302, H1884–H1893 (2012).
Lu, J. et al. Abrogation of lectin-like oxidized LDL receptor-1 attenuates acute myocardial ischemia-induced renal dysfunction by modulating systemic and local inflammation. Kidney Int. 82, 436–444 (2012).
Ranganathan, P. et al. MicroRNA-150 deletion in mice protects kidney from myocardial infarction-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 309, F551–F558 (2015).
Rutledge, C. A. et al. A novel ultrasound-guided mouse model of sudden cardiac arrest. PLoS One 15, e0237292 (2020).
Matsushita, K. et al. The acute kidney injury to chronic kidney disease transition in a mouse model of acute cardiorenal syndrome emphasizes the role of inflammation. Kidney Int. 97, 95–105 (2020).
Li, X. et al. Acute renal venous obstruction is more detrimental to the kidney than arterial occlusion: implication for murine models of acute kidney injury. Am. J. Physiol. Renal Physiol. 302, F519–F525 (2012).
Hutchens, M. P. et al. Estrogen is renoprotective via a non-receptor dependent mechanism after cardiac arrest in vivo. Anesthesiology 112, 395–405 (2010).
Burne-Taney, M. J. et al. Acute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injury. Am. J. Physiol. Renal Physiol. 285, F87–F94 (2003).
Zhang, Q. et al. Tolllike receptor 4 contributes to acute kidney injury after cardiopulmonary resuscitation in mice. Mol. Med. Rep. 14, 2983–2990 (2016).
Ikeda, M. et al. Estrogen administered after cardiac arrest and cardiopulmonary resuscitation ameliorates acute kidney injury in a sex- and age-specific manner. Crit. Care 19, 332 (2015).
Zeiner, A. et al. The effect of mild therapeutic hypothermia on renal function after cardiopulmonary resuscitation in men. Resuscitation 60, 253–261 (2004).
Neyra, J. A. et al. Kidney tubular damage and functional biomarkers in acute kidney injury following cardiac surgery. Kidney Int. Rep. 4, 1131–1142 (2019).
Ballaux, P. K., Gourlay, T., Ratnatunga, C. P. & Taylor, K. M. A literature review of cardiopulmonary bypass models for rats. Perfusion 14, 411–417 (1999).
Yu, L. et al. The deep hypothermic circulatory arrest causes more kidney malfunctions based on a novel rabbit model. Ann. Saudi Med. 34, 532–540 (2014).
Madrahimov, N. et al. Cardiopulmonary bypass in a mouse model: a novel approach. J. Vis. Exp. https://doi.org/10.3791/56017 (2017).
Wang, G. et al. Erythropoietin attenuates cardiopulmonary bypass-induced renal inflammatory injury by inhibiting nuclear factor-kappaB p65 expression. Eur. J. Pharmacol. 689, 154–159 (2012).
Koning, N. J. et al. Impaired microcirculatory perfusion in a rat model of cardiopulmonary bypass: the role of hemodilution. Am. J. Physiol. Heart Circ. Physiol. 310, H550–H558 (2016).
Darby, P. J. et al. Anemia increases the risk of renal cortical and medullary hypoxia during cardiopulmonary bypass. Perfusion 28, 504–511 (2013).
Ohno, K. et al. Diabetes increases the susceptibility to acute kidney injury after myocardial infarction through augmented activation of renal Toll-like receptors in rats. Am. J. Physiol. Heart Circ. Physiol. 313, H1130–H1142 (2017).
Kimura, Y. et al. Canagliflozin, a sodium-glucose cotransporter 2 inhibitor, normalizes renal susceptibility to type 1 cardiorenal syndrome through reduction of renal oxidative stress in diabetic rats. J. Diabetes Investig. 10, 933–946 (2019).
Singh, A. P. et al. Animal models of acute renal failure. Pharmacol. Rep. 64, 31–44 (2012).
Ortiz, A. et al. Translational value of animal models of kidney failure. Eur. J. Pharmacol. 759, 205–220 (2015).
Doi, K. et al. Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome. Kidney Int. 74, 1017–1025 (2008).
Souza, A. C. et al. TLR4 mutant mice are protected from renal fibrosis and chronic kidney disease progression. Physiol. Rep. 3, e12558. (2015).
Novitskaya, T. et al. A PTBA small molecule enhances recovery and reduces postinjury fibrosis after aristolochic acid-induced kidney injury. Am. J. Physiol. Renal Physiol. 306, F496–F504 (2014).
Pabla, N. & Dong, Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 73, 994–1007 (2008).
Sharp, C. N. & Siskind, L. J. Developing better mouse models to study cisplatin-induced kidney injury. Am. J. Physiol. Renal Physiol. 313, F835–F841 (2017).
Ghosh, S. Cisplatin: the first metal based anticancer drug. Bioorg. Chem. 88, 102925 (2019).
Bosch, X., Poch, E. & Grau, J. M. Rhabdomyolysis and acute kidney injury. N. Engl. J. Med. 361, 62–72 (2009).
Stewart, J. H. et al. The pattern of excess cancer in dialysis and transplantation. Nephrol. Dial. Transpl. 24, 3225–3231 (2009).
Latcha, S. et al. Long-term renal outcomes after cisplatin treatment. Clin. J. Am. Soc. Nephrol. 11, 1173–1179 (2016).
Sharp, C. N. et al. Repeated administration of low-dose cisplatin in mice induces fibrosis. Am. J. Physiol. Renal Physiol. 310, F560–F568 (2016).
Sharp, C. N. et al. Subclinical kidney injury induced by repeated cisplatin administration results in progressive chronic kidney disease. Am. J. Physiol. Renal Physiol. 315, F161–F172 (2018).
George, B., Joy, M. S. & Aleksunes, L. M. Urinary protein biomarkers of kidney injury in patients receiving cisplatin chemotherapy. Exp. Biol. Med. 243, 272–282 (2018).
Sears, S. M. et al. C57BL/6 mice require a higher dose of cisplatin to induce renal fibrosis and CCL2 correlates with cisplatin-induced kidney injury. Am. J. Physiol. Renal Physiol. 319, F674–F685 (2020).
Zha, M. et al. The circadian clock gene Bmal1 facilitates cisplatin-induced renal injury and hepatization. Cell Death Dis. 11, 446 (2020).
Ravichandran, K. et al. CD4 T cell knockout does not protect against kidney injury and worsens cancer. J. Mol. Med. 94, 443–455 (2016).
Van Avondt, K., Nur, E. & Zeerleder, S. Mechanisms of haemolysis-induced kidney injury. Nat. Rev. Nephrol. 15, 671–692 (2019).
Panizo, N., Rubio-Navarro, A., Amaro-Villalobos, J. M., Egido, J. & Moreno, J. A. Molecular mechanisms and novel therapeutic approaches to rhabdomyolysis-induced acute kidney injury. Kidney Blood Press. Res. 40, 520–532 (2015).
Boutaud, O. & Roberts, L. J. 2nd Mechanism-based therapeutic approaches to rhabdomyolysis-induced renal failure. Free Radic. Biol. Med. 51, 1062–1067 (2011).
Kerchberger, V. E. & Ware, L. B. The role of circulating cell-free hemoglobin in sepsis-associated acute kidney injury. Semin. Nephrol. 40, 148–159 (2020).
O’Neal, J. B., Shaw, A. D. & Billings, F. T. 4th Acute kidney injury following cardiac surgery: current understanding and future directions. Crit. Care 20, 187 (2016).
Kawai, H. et al. Experimental glycerol myopathy: a histological study. Acta Neuropathol. 80, 192–197 (1990).
McMahon, G. M., Zeng, X. & Waikar, S. S. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern. Med. 173, 1821–1828 (2013).
Okamura, D. M. & Pennathur, S. The balance of powers: redox regulation of fibrogenic pathways in kidney injury. Redox Biol. 6, 495–504 (2015).
Belliere, J. et al. Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury. J. Am. Soc. Nephrol. 26, 1363–1377 (2015).
Soares, T. J., Costa, R. S., Volpini, R. A., Da Silva, C. G. & Coimbra, T. M. Long-term evolution of the acute tubular necrosis (ATN) induced by glycerol: role of myofibroblasts and macrophages. Int. J. Exp. Pathol. 83, 165–172 (2002).
Faulk, T. et al. Rhabdomyolysis among critically Ill combat casualties: long-term outcomes. Am. J. Nephrol. 48, 399–405 (2018).
Bolanos, J. A. et al. Outcomes after post-traumatic AKI requiring RRT in United States military service members. Clin. J. Am. Soc. Nephrol. 10, 1732–1739 (2015).
Peerapornratana, S., Manrique-Caballero, C. L., Gomez, H. & Kellum, J. A. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 96, 1083–1099 (2019).
Manrique-Caballero, C. L., Del Rio-Pertuz, G. & Gomez, H. Sepsis-associated acute kidney injury. Crit. Care Clin. 37, 279–301 (2021).
Iskander, K. N. et al. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol. Rev. 93, 1247–1288 (2013).
Hotchkiss, R. S., Coopersmith, C. M., McDunn, J. E. & Ferguson, T. A. The sepsis seesaw: tilting toward immunosuppression. Nat. Med. 15, 496–497 (2009).
Legrand, M. et al. The role of renal hypoperfusion in development of renal microcirculatory dysfunction in endotoxemic rats. Intensive Care Med. 37, 1534–1542 (2011).
Brealey, D. et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R491–R497 (2004).
Vaure, C. & Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 5, 316 (2014).
El-Achkar, T. M. et al. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am. J. Physiol. Renal Physiol. 290, F1034–F1043 (2006).
Doi, K. et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J. Am. Soc. Nephrol. 20, 1217–1221 (2009).
Street, J. M. et al. The role of adenosine 1a receptor signaling on GFR early after the induction of sepsis. Am. J. Physiol. Renal Physiol. 314, F788–F797 (2018).
Alverdy, J. C., Keskey, R. & Thewissen, R. Can the cecal ligation and puncture model be repurposed to better inform therapy in human sepsis? Infect. Immun. https://doi.org/10.1128/IAI.00942-19 (2020).
Rittirsch, D., Huber-Lang, M. S., Flierl, M. A. & Ward, P. A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36 (2009).
Miyaji, T. et al. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int. 64, 1620–1631 (2003).
Doi, K. et al. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 73, 1266–1274 (2008).
Leelahavanichkul, A. et al. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney Int. 80, 1198–1211 (2011).
Levy, E. M., Viscoli, C. M. & Horwitz, R. I. The effect of acute renal failure on mortality. A cohort analysis. JAMA 275, 1489–1494 (1996).
Faubel, S. Have we reached the limit of mortality benefit with our approach to renal replacement therapy in acute kidney injury? Am. J. Kidney Dis. 62, 1030–1033 (2013).
Faubel, S. & Shah, P. B. Immediate consequences of acute kidney injury: the impact of traditional and nontraditional complications on mortality in acute kidney injury. Adv. Chronic Kidney Dis. 23, 179–185 (2016).
Faubel, S. & Edelstein, C. L. Mechanisms and mediators of lung injury after acute kidney injury. Nat. Rev. Nephrol. 12, 48–60 (2015).
Hoke, T. S. et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J. Am. Soc. Nephrol. 18, 155–164 (2007).
Andres-Hernando, A. et al. Cytokine production increases and cytokine clearance decreases in mice with bilateral nephrectomy. Nephrol. Dial. Transpl. 27, 4339–4347 (2012).
Dennen, P. et al. Urine interleukin-6 is an early biomarker of acute kidney injury in children undergoing cardiac surgery. Crit. Care 14, R181 (2010).
Klein, C. L. et al. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 74, 901–909 (2008).
Hassoun, H. T. et al. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am. J. Physiol. Renal Physiol. 293, F30–F40 (2007).
Karimi, Z. et al. Renal ischemia/reperfusion against nephrectomy for induction of acute lung injury in rats. Ren. Fail. 38, 1503–1515 (2016).
Walcher, A., Faubel, S., Keniston, A. & Dennen, P. In critically Ill patients requiring CRRT, AKI Is associated with increased respiratory failure and death versus ESRD. Ren. Fail. 33, 935–942 (2011).
Waikar, S. S., Liu, K. D. & Chertow, G. M. The incidence and prognostic significance of acute kidney injury. Curr. Opin. Nephrol. Hypertens. 16, 227–236 (2007).
Bhargava, R. et al. Acute lung injury and acute kidney injury are established by four hours in experimental sepsis and are improved with pre, but not post, sepsis administration of TNF-alpha antibodies. PLoS One 8, e79037 (2013).
Teixeira, J. P., Ambruso, S., Griffin, B. R. & Faubel, S. Pulmonary consequences of acute kidney injury. Semin. Nephrol. 39, 3–16 (2019).
Andres-Hernando, A. et al. Prolonged acute kidney injury exacerbates lung inflammation at 7 days post-acute kidney injury. Physiol. Rep. 2, e12084 (2014).
Cruz, D. N., Gheorghiade, M., Palazzuoli, A., Ronco, C. & Bagshaw, S. M. Epidemiology and outcome of the cardio-renal syndrome. Heart Fail. Rev. 16, 531–542 (2011).
Ronco, C., Haapio, M., House, A. A., Anavekar, N. & Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 52, 1527–1539 (2008).
Ronco, C., House, A. A. & Haapio, M. Cardiorenal and renocardiac syndromes: the need for a comprehensive classification and consensus. Nat. Clin. Pract. Nephrol. 4, 310–311 (2008).
Gammelager, H. et al. Three-year risk of cardiovascular disease among intensive care patients with acute kidney injury: a population-based cohort study. Crit. Care 18, 492 (2014).
Sumida, M. et al. Regulation of mitochondrial dynamics by dynamin-related protein-1 in acute cardiorenal syndrome. J. Am. Soc. Nephrol. 26, 2378–2387 (2015).
Fox, B. M. et al. Metabolomics assessment reveals oxidative stress and altered energy production in the heart after ischemic acute kidney injury in mice. Kidney Int. 95, 590–610 (2019).
Kelly, K. J. Distant effects of experimental renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 14, 1549–1558 (2003).
Martinez-Martinez, E. et al. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension 66, 767–775 (2015).
Go, A. S. et al. Acute kidney injury and risk of heart failure and atherosclerotic events. Clin. J. Am. Soc. Nephrol. 13, 833–841 (2018).
Giraud, S. et al. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: the preclinical model. J. Biomed. Biotechnol. 2011, 532127 (2011).
Fairbairn, L., Kapetanovic, R., Sester, D. P. & Hume, D. A. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J. Leukoc. Biol. 89, 855–871 (2011).
Seok, J. et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl Acad. Sci. USA 110, 3507–3512 (2013).
Packialakshmi, B., Stewart, I. J., Burmeister, D. M., Chung, K. K. & Zhou, X. Large animal models for translational research in acute kidney injury. Ren. Fail. 42, 1042–1058 (2020).
Adams, P. L., Adams, F. F., Bell, P. D. & Navar, L. G. Impaired renal blood flow autoregulation in ischemic acute renal failure. Kidney Int. 18, 68–76 (1980).
Snoeijs, M. G. et al. Addition of a water-soluble propofol formulation to preservation solution in experimental kidney transplantation. Transplantation 92, 296–302 (2011).
Ekser, B., Rigotti, P., Gridelli, B. & Cooper, D. K. Xenotransplantation of solid organs in the pig-to-primate model. Transpl. Immunol. 21, 87–92 (2009).
Xu, M. et al. Anti-CD47 monoclonal antibody therapy reduces ischemia-reperfusion injury of renal allografts in a porcine model of donation after cardiac death. Am. J. Transpl. 18, 855–867 (2018).
Neumayer, H. H., Blossei, N., Seherr-Thohs, U. & Wagner, K. Amelioration of postischaemic acute renal failure in conscious dogs by human atrial natriuretic peptide. Nephrol. Dial. Transpl. 5, 32–38 (1990).
Nilsson, K. F., Sandin, J., Gustafsson, L. E. & Frithiof, R. The novel nitric oxide donor PDNO attenuates ovine ischemia-reperfusion induced renal failure. Intensive Care Med. Exp. 5, 29 (2017).
Zahran, M. H. et al. Renoprotective effect of local sildenafil administration in renal ischaemia-reperfusion injury: a randomised controlled canine study. Arab. J. Urol. 17, 150–159 (2019).
O’Kane, D. et al. Zinc preconditioning protects against renal ischaemia reperfusion injury in a preclinical sheep large animal model. Biometals 31, 821–834 (2018).
Woolley, J. L. et al. Effect of the calcium entry blocker verapamil on renal ischemia. Crit. Care Med. 16, 48–51 (1988).
Favreau, F. et al. Expression and modulation of translocator protein and its partners by hypoxia reoxygenation or ischemia and reperfusion in porcine renal models. Am. J. Physiol. Renal Physiol. 297, F177–F190 (2009).
Hunter, J. P., Hosgood, S. A., Barlow, A. D. & Nicholson, M. L. Ischaemic conditioning reduces kidney injury in an experimental large-animal model of warm renal ischaemia. Br. J. Surg. 102, 1517–1525 (2015).
Amdisen, C. et al. Testing danegaptide effects on kidney function after ischemia/reperfusion injury in a new porcine two week model. PLoS One 11, e0164109 (2016).
Castellano, G. et al. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia-reperfusion-induced renal damage. Am. J. Pathol. 176, 1648–1659 (2010).
Sølling, C. et al. Erythropoietin administration is associated with short-term improvement in glomerular filtration rate after ischemia-reperfusion injury. Acta Anaesthesiol. Scand. 55, 185–195 (2011).
GIBBON, J. H. Jr Artificial maintenance of circulation during experimental occlusion of pulmonary artery. Arch. Surg. 34, 1105–1131 (1937).
DiVincenti, L. Jr, Westcott, R. & Lee, C. Sheep (Ovis aries) as a model for cardiovascular surgery and management before, during, and after cardiopulmonary bypass. J. Am. Assoc. Lab. Anim. Sci. 53, 439–448 (2014).
Lankadeva, Y. R. et al. Reversal of renal tissue hypoxia during experimental cardiopulmonary bypass in sheep by increased pump flow and arterial pressure. Acta Physiol. 231, e13596 (2021).
Lannemyr, L. et al. Effects of cardiopulmonary bypass on renal perfusion, filtration, and oxygenation in patients undergoing cardiac surgery. Anesthesiology 126, 205–213 (2017).
Lankadeva, Y. R. et al. Strategies that improve renal medullary oxygenation during experimental cardiopulmonary bypass may mitigate postoperative acute kidney injury. Kidney Int. 95, 1338–1346 (2019).
Lankadeva, Y. R. et al. Influence of blood hemoglobin concentration on renal hemodynamics and oxygenation during experimental cardiopulmonary bypass in sheep. Acta Physiol. 231, e13583 (2020).
Qureshi, S. H., Patel, N. N. & Murphy, G. J. Vascular endothelial cell changes in postcardiac surgery acute kidney injury. Am. J. Physiol. Renal Physiol. 314, F726–F735 (2018).
Murphy, G. J. et al. An initial evaluation of post-cardiopulmonary bypass acute kidney injury in swine. Eur. J. Cardiothorac. Surg. 36, 849–855 (2009).
Shi, N. et al. The association between obesity and risk of acute kidney injury after cardiac surgery. Front. Endocrinol. 11, 534294 (2020).
O’Sullivan, K. E. et al. The effect of obesity on acute kidney injury after cardiac surgery. J. Thorac. Cardiovasc. Surg. 150, 1622–1628 (2015).
Billings, F. T. et al. Obesity and oxidative stress predict AKI after cardiac surgery. J. Am. Soc. Nephrol. 23, 1221–1228 (2012).
Stamou, S. C. et al. Effect of body mass index on outcomes after cardiac surgery: is there an obesity paradox? Ann. Thorac. Surg. 91, 42–47 (2011).
Sleeman, P. et al. High fat feeding promotes obesity and renal inflammation and protects against post cardiopulmonary bypass acute kidney injury in swine. Crit. Care 17, R262 (2013).
Santiago, M. J. et al. Cisplatin-induced non-oliguric acute kidney injury in a pediatric experimental animal model in piglets. PLoS One 11, e0149013 (2016).
Wang, S. Y., Zhang, C. Y., Cai, G. Y. & Chen, X. M. Method used to establish a large animal model of drug-induced acute kidney injury. Exp. Biol. Med. 246, 986–995 (2021).
Zheng, J. S. et al. Screening of early diagnostic markers of gentamicin-induced acute kidney injury in canines. J. Vet. Res. 63, 405–411 (2019).
Garry, F., Chew, D. J. & Hoffsis, G. F. Urinary indices of renal function in sheep with induced aminoglycoside nephrotoxicosis. Am. J. Vet. Res. 51, 420–427 (1990).
Cui, J. et al. Rapamycin protects against gentamicin-induced acute kidney injury via autophagy in mini-pig models. Sci. Rep. 5, 11256 (2015).
Margulies, K. B., McKinley, L. J., Cavero, P. G. & Burnett, J. C. Jr. Induction and prevention of radiocontrast-induced nephropathy in dogs with heart failure. Kidney Int. 38, 1101–1108 (1990).
Wu, J. et al. Retinoic acid attenuates contrast-induced acute kidney injury in a miniature pig model. Biochem. Biophys. Res. Commun. 512, 163–169 (2019).
Tang, W. et al. Renal protective effects of early continuous venovenous hemofiltration in rhabdomyolysis: improved renal mitochondrial dysfunction and inhibited apoptosis. Artif. Organs 37, 390–400 (2013).
Rajagopalan, P. R., Reines, H. D., Pulliam, C., Fitts, C. T. & LeVeen, H. H. Reversal of acute renal failure using hemodilution with hydroxyethyl starch. J. Trauma. 23, 795–800 (1983).
van Griensven, M. et al. Protective effects of the complement inhibitor compstatin cp40 in hemorrhagic shock. Shock 51, 78–87 (2019).
Sondeen, J. L., Gonzaludo, G. A., Loveday, J. A., Rodkey, W. G. & Wade, C. E. Hypertonic saline/dextran improves renal function after hemorrhage in conscious swine. Resuscitation 20, 231–241 (1990).
Hoareau, G. L. et al. Renal effects of three endoaortic occlusion strategies in a swine model of hemorrhagic shock. Injury 50, 1908–1914 (2019).
Smith, S. et al. Aggressive treatment of acute kidney injury and hyperkalemia improves survival in a combat relevant trauma model in swine. Am. J. Surg. 219, 860–864 (2020).
Wong, Y. C. et al. Potential biomarker panel for predicting organ dysfunction and acute coagulopathy in a polytrauma porcine model. Shock 43, 157–165 (2015).
Fröhlich, M. et al. Induced hypothermia reduces the hepatic inflammatory response in a swine multiple trauma model. J. Trauma. Acute Care Surg. 76, 1425–1432 (2014).
Hasslacher, J. et al. Acute kidney injury and mild therapeutic hypothermia in patients after cardiopulmonary resuscitation — a post hoc analysis of a prospective observational trial. Crit. Care 22, 154 (2018).
Sullivan, T. P., Eaglstein, W. H., Davis, S. C. & Mertz, P. The pig as a model for human wound healing. Wound Repair. Regen. 9, 66–76 (2001).
Khorram-Sefat, R. et al. The therapeutic effect of C1-inhibitor on gut-derived bacterial translocation after thermal injury. Shock 9, 101–108 (1998).
Guenther, T. M. et al. High versus low volume fluid resuscitation strategies in a porcine model (sus scrofa) of combined thermal and traumatic brain injury. Shock 55, 536–544 (2020).
Burmeister, D. M. et al. Impact of isolated burns on major organs: a large animal model characterized. Shock 46, 137–147 (2016).
Gómez, B. I. et al. Enteral resuscitation with oral rehydration solution to reduce acute kidney injury in burn victims: evidence from a porcine model. PLoS One 13, e0195615 (2018).
Mason, S. A., Nathens, A. B. & Jeschke, M. G. “Hold the pendulum: rates of acute kidney injury are increased in patients who receive resuscitation volumes less than predicted by the Parkland Equation”. Ann. Surg. 266, e108 (2017).
Christenson, J. T. & Owunwanne, A. Leucocyte sequestration in endotoxemia and the effect of low-molecular-weight dextran. Eur. J. Nucl. Med. 17, 28–33 (1990).
Kampmeier, T. G. et al. Effects of resuscitation with human albumin 5%, hydroxyethyl starch 130/0.4 6%, or crystalloid on kidney damage in an ovine model of septic shock. Br. J. Anaesth. 121, 581–587 (2018).
Linton, A. L., Walker, J. F., Lindsay, R. M. & Sibbald, W. J. Acute renal failure and tubular damage due to sepsis in an animal model. Proc. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 21, 837–842 (1985).
Di Giantomasso, D., Morimatsu, H., May, C. N. & Bellomo, R. Intrarenal blood flow distribution in hyperdynamic septic shock: Effect of norepinephrine. Crit. Care Med. 31, 2509–2513 (2003).
Langenberg, C., Gobe, G., Hood, S., May, C. N. & Bellomo, R. Renal histopathology during experimental septic acute kidney injury and recovery. Crit. Care Med. 42, e58–e67 (2014).
Lankadeva, Y. R., Kosaka, J., Evans, R. G., Bellomo, R. & May, C. N. Urinary oxygenation as a surrogate measure of medullary oxygenation during angiotensin II therapy in septic acute kidney injury. Crit. Care Med. 46, e41–e48 (2018).
Lankadeva, Y. R. et al. Effects of fluid bolus therapy on renal perfusion, oxygenation, and function in early experimental septic kidney injury. Crit. Care Med. 47, e36–e43 (2019).
Benes, J. et al. Searching for mechanisms that matter in early septic acute kidney injury: an experimental study. Crit. Care 15, R256 (2011).
Wang, H. et al. Improvement of cytokine response and survival time by bioartificial kidney therapy in acute uremic pigs with multi-organ dysfunction. Int. J. Artif. Organs 33, 526–534 (2010).
Matejovic, M. et al. Molecular differences in susceptibility of the kidney to sepsis-induced kidney injury. BMC Nephrol. 18, 183 (2017).
Vassal, O. et al. Renal haemodynamic response to amino acids infusion in an experimental porcine model of septic shock. Acta Anaesthesiol. Scand. 59, 598–608 (2015).
Matejovic, M. et al. Renal proteomic responses to severe sepsis and surgical trauma: dynamic analysis of porcine tissue biopsies. Shock 46, 453–464 (2016).
Merz, T. et al. Cystathionine-γ-lyase expression is associated with mitochondrial respiration during sepsis-induced acute kidney injury in swine with atherosclerosis. Intensive Care Med. Exp. 6, 43 (2018).
Post, E. H. et al. The effects of acute renal denervation on kidney perfusion and metabolism in experimental septic shock. BMC Nephrol. 18, 182 (2017).
Kubiak, B. D. et al. A clinically applicable porcine model of septic and ischemia/reperfusion-induced shock and multiple organ injury. J. Surg. Res. 166, e59–e69 (2011).
Maybauer, M. O. et al. Recombinant human activated protein C attenuates cardiovascular and microcirculatory dysfunction in acute lung injury and septic shock. Crit. Care 14, R217 (2010).
Lange, M. et al. Effects of early neuronal and delayed inducible nitric oxide synthase blockade on cardiovascular, renal, and hepatic function in ovine sepsis. Anesthesiology 113, 1376–1384 (2010).
Fenhammar, J. et al. Endothelin receptor A antagonism attenuates renal medullary blood flow impairment in endotoxemic pigs. PLoS One 6, e21534 (2011).
Fenhammar, J. et al. Toll-like receptor 4 inhibitor TAK-242 attenuates acute kidney injury in endotoxemic sheep. Anesthesiology 114, 1130–1137 (2011).
Mazzola, S. et al. Carbon monoxide pretreatment prevents respiratory derangement and ameliorates hyperacute endotoxic shock in pigs. FASEB J. 19, 2045–2047 (2005).
Netti, G. S. et al. LPS removal reduces CD80-mediated albuminuria in critically ill patients with Gram-negative sepsis. Am. J. Physiol. Renal Physiol. 316, F723–F731 (2019).
Sander, V. et al. Protocol for large-scale production of kidney organoids from human pluripotent stem cells. Star. Protoc. 1, 100150 (2020).
Hukriede, N., Vogt, A. & de Caestecker, M. Drug discovery to halt the progression of acute kidney injury to chronic kidney disease: a case for phenotypic drug discovery in acute kidney injury. Nephron 137, 268–272 (2017).
Martignoni, M., Groothuis, G. M. & de Kanter, R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert. Opin. Drug Metab. Toxicol. 2, 875–894 (2006).
de Caestecker, M. et al. Bridging translation by improving preclinical study design in AKI. J. Am. Soc. Nephrol. 26, 2905–2916 (2015).
Weisbord, S. D. & Palevsky, P. M. Design of clinical trials in acute kidney injury: lessons from the past and future directions. Semin. Nephrol. 36, 42–52 (2016).
Faubel, S. et al. Ongoing clinical trials in AKI. Clin. J. Am. Soc. Nephrol. 7, 861–873 (2012).
de Caestecker, M. P., Siew, E. D., Harris, R. C. & Hukriede, N. A. Introduction: The 2019 Federation of American societies for experimental biology acute kidney injury from bench to bedside conference. Semin. Nephrol. 40, 99–100 (2020).
Hale, L. J. et al. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat. Commun. 9, 5167 (2018).
Kumar, S. V. et al. Kidney micro-organoids in suspension culture as a scalable source of human pluripotent stem cell-derived kidney cells. Development 146, dev172361 (2019).
Lawlor, K. T. et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat. Mater. 20, 260–271 (2021).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article. M.P.D.C., N.A.H., D.E.S., V.S., T.P., P.S.T.Y., L.J.S., M.P.H., A.J.D., D.M.B. and S.F. wrote the text. M.P.D.C., D.M.B. and S.F. made substantial contributions to discussions of the content. M.P.D.C., N.A.H., D.E.S., V.S., M.C.S., P.S.T.Y., L.J.S., M.P.H., A.J.D., D.M.B. and S.F. reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks the anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hukriede, N.A., Soranno, D.E., Sander, V. et al. Experimental models of acute kidney injury for translational research. Nat Rev Nephrol 18, 277–293 (2022). https://doi.org/10.1038/s41581-022-00539-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00539-2
This article is cited by
-
Targeting ferroptosis for treating kidney disease
Clinical and Experimental Nephrology (2024)
-
Advances in pediatric acute kidney injury pathobiology: a report from the 26th Acute Disease Quality Initiative (ADQI) conference
Pediatric Nephrology (2024)
-
Multiorgan recovery in a cadaver body using mild hypothermic ECMO treatment in a murine model
Intensive Care Medicine Experimental (2023)
-
Acute kidney injury decreases pulmonary vascular growth and alveolarization in neonatal rat pups
Pediatric Research (2023)
-
Precision management of acute kidney injury in the intensive care unit: current state of the art
Intensive Care Medicine (2023)