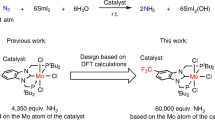
Abstract
The production of ammonia from nitrogen gas is one of the most important industrial processes, owing to the use of ammonia as a raw material for nitrogen fertilizers. Currently, the main method of ammonia production is the Haber–Bosch process, which operates under very high temperatures and pressures and is therefore very energy-intensive1. The transition-metal-catalysed reduction of nitrogen gas2,3,4,5,6 is an alternative method for the formation of ammonia. In these reaction systems, metallocenes or potassium graphite are typically used as the reducing reagent, and conjugate acids of pyridines or related compounds are used as a proton source. To develop a next-generation nitrogen-fixation system, these reagents should be low cost, readily available and environmentally friendly. Here we show that the combination of samarium(ii) diiodide (SmI2) with alcohols or water enables the fixation of nitrogen to be catalysed by molybdenum complexes under ambient conditions. Up to 4,350 equivalents of ammonia can be produced (based on the molybdenum catalyst), with a turnover frequency of around 117 per minute. The amount of ammonia produced and its rate of formation are one and two orders of magnitude larger, respectively, than those achieved in artificial reaction systems reported so far, and the formation rate approaches that observed with nitrogenase enzymes. The high reactivity is achieved by a proton-coupled electron-transfer process that is enabled by weakening of the O–H bonds of alcohols and water coordinated to SmI2. Although the current reaction is not suitable for use on an industrial scale, this work demonstrates an opportunity for further research into catalytic nitrogen fixation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for the reported structures have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers: CCDC 185998 (5), 1857999 (6), 1858000 (7a) and 1858001 (7b·2C4H8O). All other data supporting the findings of this study are available within the paper or from the corresponding author upon reasonable request.
References
Liu, H. Ammonia Synthesis Catalysts: Innovation and Practice (World Scientific, Beijing, 2013).
Yandulov, D. V. & Schrock, R. R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 301, 76–78 (2003).
Nishibayashi, Y. (ed.) Nitrogen Fixation (Topics in Organometallic Chemistry) 1st edn, Vol. 60 (Springer, Heidelberg, 2017).
Arashiba, K., Miyake, Y. & Nishibayashi, Y. A molybdenum complex bearing PNP-type pincer ligands leads to the catalytic reduction of dinitrogen into ammonia. Nat. Chem. 3, 120–125 (2011).
Anderson, J. S., Rittle, J. & Peters, J. C. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature 501, 84–87 (2013).
Fajardo, J. Jr & Peters, J. C. Catalytic nitrogen-to-ammonia conversion by osmium and ruthenium complexes. J. Am. Chem. Soc. 139, 16105–16108 (2017).
Arashiba, K. et al. Catalytic nitrogen fixation via direct cleavage of nitrogen–nitrogen triple bond of molecular dinitrogen under ambient reaction conditions. Bull. Chem. Soc. Jpn 90, 1111–1118 (2017).
Karunadasa, H. I., Chang, C. J. & Long, J. R. A molecular molybdenum-oxo catalyst for generating hydrogen from water. Nature 464, 1329–1333 (2010).
Chatt, J., Pearman, A. J. & Richards, R. L. The reduction of mono-coordinated molecular nitrogen to ammonia in a protic environment. Nature 253, 39–40 (1975).
Ohki, Y., Aoyagi, K. & Seino, H. Synthesis and protonation of N-heterocyclic-carbene-supported dinitrogen complexes of molybdenum(0). Organometallics 34, 3414–3420 (2015).
Li, H., Shang, J., Ai, Z. & Zhang, L. Efficient visible light nitrogen fixation with BiOBr nanosheets of oxygen vacancies on the exposed {001} facets. J. Am. Chem. Soc. 137, 6393–6399 (2015).
Hirakawa, H., Hashimoto, M., Shiraishi, Y. & Hirai, T. Photocatalytic conversion of nitrogen to ammonia with water on surface oxygen vacancies of titanium dioxide. J. Am. Chem. Soc. 139, 10929–10936 (2017).
Szostak, M., Fazakerley, N. J., Parmar, D. & Procter, D. J. Cross-coupling reactions using samarium(ii) iodide. Chem. Rev. 114, 5959–6039 (2014).
Choquette, K. A. & Flowers, R. A. in Comprehensive Organic Synthesis II Vol. 1 (eds Knochel, P. & Molander, G. A.) Ch. 9, 278–343 (Elsevier, Amsterdam, 2014).
Teprovich, J. A. Jr, Balili, M. N., Pintauer, T. & Flowers, R. A. II. Mechanistic studies of proton-donor coordination to samarium diiodide. Angew. Chem. Int. Ed. 46, 8160–8163 (2007).
Robbins, J. L., Edelstein, N., Spencer, B. & Smart, J. C. Syntheses and electronic structures of decamethylmetallocenes. J. Am. Chem. Soc. 104, 1882–1893 (1982).
Kuhlman, M. L. & Flowers R. A. II. Aggregation state and reducing power of the samarium diiodide–DMPU complex in acetonitrile. Tetrahedron Lett. 41, 8049–8052 (2000).
Chciuk, T. V., Anderson, W. R. Jr & Flowers, R. A. II. High-affinity proton donors promote proton-coupled electron transfer by samarium diiodide. Angew. Chem. Int. Ed. 55, 6033–6036 (2016).
Chciuk, T. V., Anderson, W. R. Jr & Flowers, R. A. II. Proton-coupled electron transfer in the reduction of carbonyls by samarium diiodide–water complexes. J. Am. Chem. Soc. 138, 8738–8741 (2016).
Chciuk, T. V., Anderson, W. R. Jr & Flowers, R. A. II. Interplay between substrate and proton donor coordination in reductions of carbonyls by SmI2–water through proton-coupled electron-transfer. J. Am. Chem. Soc. 140, 15342–15352 (2018).
Kolmar, S. S. & Mayer, J. M. SmI2(H2O)n reduction of electron rich enamines by proton-coupled electron transfer. J. Am. Chem. Soc. 139, 10687–10692 (2017).
Bezdek, M. J., Guo, S. & Chirik, P. J. Coordination-induced weakening of ammonia, water, and hydrazine X–H bonds in a molybdenum complex. Science 354, 730–733 (2016).
Bezdek, M. J. & Chirik, P. J. Interconversion of molybdenum imido and amido complexes by proton-coupled electron transfer. Angew. Chem. Int. Ed. 57, 2224–2228 (2018).
Jonas, R. T. & Stack, T. D. P. C–H bond activation by a ferric methoxide complex: a model for the rate-determining step in the mechanism of lipoxygenase. J. Am. Chem. Soc. 119, 8566–8567 (1997).
Spiegel, D. A., Wiberg, K. B., Schacherer, L. N., Medeiros, M. R. & Wood, J. L. Deoxygenation of alcohols employing water as the hydrogen atom source. J. Am. Chem. Soc. 127, 12513–12515 (2005).
Pozzi, D., Scanlan, E. M. & Renaud, P. A mild radical procedure for the reduction of B-alkylcatecholboranes to alkanes. J. Am. Chem. Soc. 127, 14204–14205 (2005).
Eizawa, A. et al. Remarkable catalytic activity of dinitrogen-bridged dimolybdenum complexes bearing NHC-based PCP-pincer ligands toward nitrogen fixation. Nat. Commun. 8, 14874 (2017).
Brown, K. A. et al. Light-driven dinitrogen reduction catalyzed by a CdS:nitrogenase MoFe protein biohybrid. Science 352, 448–450 (2016).
Pappas, I. & Chirik, P. J. Catalytic proton coupled electron transfer from metal hydrides to titanocene amides, hydrazides and imides: determination of thermodynamic parameters relevant to nitrogen fixation. J. Am. Chem. Soc. 138, 13379–13389 (2016).
Warren, J. J., Tronic, T. A. & Mayer, J. M. Thermochemistry of proton-coupled electron transfer reagents and its implications. Chem. Rev. 110, 6961–7001 (2010).
Pavlishchuk, V. V. & Addison, A. W. Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 °C. Inorg. Chim. Acta 298, 97–102 (2000).
Kaljurand, I. et al. Extension of the self-consistent spectrophotometric basicity scale in acetonitrile to a full span of 28 pK a units: unification of different basicity scales. J. Org. Chem. 70, 1019–1028 (2005).
Arashiba, K. et al. Catalytic reduction of dinitrogen to ammonia by use of molybdenum–nitride complexes bearing a tridentate triphosphine as catalysts. J. Am. Chem. Soc. 137, 5666–5669 (2015).
Watson, P. L., Tulip, T. H. & Williams, I. Defluorination of perfluoroolefins by divalent lanthanoid reagents: activating C–F bonds. Organometallics 9, 1999–2009 (1990).
Yandulov, D. V. & Schrock, R. R. Synthesis of tungsten complexes that contain hexaisopropylterphenyl-substituted triamidoamine ligands, and reactions relevant to the reduction of dinitrogen to ammonia. Can. J. Chem. 83, 341–357 (2005).
Weatherburn, M. W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39, 971–974 (1967).
Watt, G. W. & Chrisp, J. D. Spectrophotometric method for determination of hydrazine. Anal. Chem. 24, 2006–2008 (1952).
CrystalStructure v.4.3.: Single Crystal Structure Analysis Software (Rigaku Corp., Tokyo and MSC, Texas, 2018).
Altomare, A. et al. SIR97: a new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 32, 115–119 (1999).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Acknowledgements
This project is supported by CREST, JST (JPMJCR1541). We thank Grants-in-Aid for Scientific Research (numbers JP17H01201, JP15H05798 and JP18K19093) from JSPS and MEXT. Y.A. is a recipient of the JSPS Predoctoral Fellowships for Young Scientists. We also thank J. C. Peters and S. Schneider for helpful discussions.
Reviewer information
Nature thanks Robert Flowers and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Y.N. directed and conceived this project. Y.A., K.A. and K.N. conducted the experimental work including X-ray analysis. All authors discussed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
Y.A., K.N. and Y.N. have filed a patent based on the work described here (Japanese patent application number 2018-036967).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Reactions via direct nitrogen cleavage pathway.
a, A reaction pathway via direct cleavage of the nitrogen–nitrogen triple bond. b, Synthesis of the molybdenum oxo complex (5) from 1a and water, and reduction of 5 to 4 via direct nitrogen cleavage of the nitrogen–nitrogen triple bond. SmI2(THF)2 was used as the source of SmI2.aYield based on NMR.
Extended Data Fig. 2 1H NMR spectra of catalytic reduction of dinitrogen to ammonia under 15N2.
a–c, 1H NMR (DMSO-d6) spectra of product from catalytic reaction with 1c under 15N2 and [ColH]OTf (a), authentic sample of the mixture of 14NH4Cl and [ColH]OTf (b) and authentic sample of the mixture of 15NH4Cl and [ColH]OTf (c).
Extended Data Fig. 3 Kinetic study of stoichiometric reactions.
A kinetic study of the stoichiometric reaction was carried out by monitoring the formation of 4 by UV–vis spectroscopy at room temperature. A typical procedure is described below. A THF solution containing 1a (1.2 μmol) was added into a quartz glass cell (1 cm × 1 cm × 4 cm) with a septum in a nitrogen-filled glove box. The THF solution of SmI2 (3.0 μmol) was added to the quartz glass cell using a syringe with stirring. The total amount of solution was adjusted to be 3.0 ml. The spectra were measured every 0.4 s, and the rate was determined from the time profile of the initial 100 s. The reaction rate (kobs, in abs s−1) was determined from the formation rate of the absorbance of 4 at 828 nm. a, Typical time profile of the formation of 4 observed at 828 nm with SmI2 (1.0 mM) and 1a (0.4 mM) in THF at room temperature. b, Rate of formation of 4 at various concentrations of 1a. c, Rate dependence of the formation of 4 on concentration of 1a in THF. d, UV–vis absorption spectra between 380 and 1,100 nm of 1a (0.48 mM in THF; blue line), 4 (0.60 mM in THF; red line), and SmI2 (1.2 mM in THF; green line).
Source data
Rights and permissions
About this article
Cite this article
Ashida, Y., Arashiba, K., Nakajima, K. et al. Molybdenum-catalysed ammonia production with samarium diiodide and alcohols or water. Nature 568, 536–540 (2019). https://doi.org/10.1038/s41586-019-1134-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1134-2
This article is cited by
-
A blueprint for catalysis
Nature Chemistry (2024)
-
Spin polarized Fe1−Ti pairs for highly efficient electroreduction nitrate to ammonia
Nature Communications (2024)
-
Oxygen-Coordinated Single Mn Sites for Efficient Electrocatalytic Nitrate Reduction to Ammonia
Nano-Micro Letters (2024)
-
Photocatalytic nitrogen fixation under an ambient atmosphere using a porous coordination polymer with bridging dinitrogen anions
Nature Chemistry (2023)
-
Cyclopentadienyl ring activation in organometallic chemistry and catalysis
Nature Reviews Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.