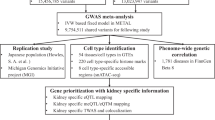
Abstract
The kidney is an organ of key relevance to blood pressure (BP) regulation, hypertension and antihypertensive treatment. However, genetically mediated renal mechanisms underlying susceptibility to hypertension remain poorly understood. We integrated genotype, gene expression, alternative splicing and DNA methylation profiles of up to 430 human kidneys to characterize the effects of BP index variants from genome-wide association studies (GWASs) on renal transcriptome and epigenome. We uncovered kidney targets for 479 (58.3%) BP-GWAS variants and paired 49 BP-GWAS kidney genes with 210 licensed drugs. Our colocalization and Mendelian randomization analyses identified 179 unique kidney genes with evidence of putatively causal effects on BP. Through Mendelian randomization, we also uncovered effects of BP on renal outcomes commonly affecting patients with hypertension. Collectively, our studies identified genetic variants, kidney genes, molecular mechanisms and biological pathways of key relevance to the genetic regulation of BP and inherited susceptibility to hypertension.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the results presented in this article are either available in the Supplementary Information (Supplementary Tables and Supplementary Note) or can be obtained from the authors upon reasonable request. The normalized gene expression, splice junction usage and DNA methylation data are archived at the Dryad digital repository (https://doi.org/10.5061/dryad.15dv41nvx). The e-, s- and mQTL summary statistics are available in the Supplementary Tables.
Code availability
Our studies make use of well-established computational and statistical analysis software and these are fully referenced in the Methods. All custom code used to orchestrate these analyses is available on request.
References
Beaney, T. et al. May Measurement Month 2017: an analysis of blood pressure screening results worldwide. Lancet Glob. Health 6, e736–e743 (2018).
Lim, S. S. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260 (2012).
Doris, P. A. The genetics of blood pressure and hypertension: the role of rare variation. Cardiovasc. Ther. 29, 37–45 (2011).
Evangelou, E. et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 50, 1412–1425 (2018).
Warren, H. R. et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat. Genet. 49, 403–415 (2017).
Giri, A. et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat. Genet. 51, 51–62 (2019).
Cabrera, C. P. et al. Exploring hypertension genome-wide association studies findings and impact on pathophysiology, pathways, and pharmacogenetics. Wiley Interdiscip. Rev. Syst. Biol. Med. 7, 73–90 (2015).
Ehret, G. B. et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478, 103–109 (2011).
Surendran, P. et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat. Genet. 48, 1151–1161 (2016).
Ehret, G. B. et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat. Genet. 48, 1171–1184 (2016).
Liu, C. et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat. Genet. 48, 1162–1170 (2016).
Hoffmann, T. J. et al. Genome-wide association analyses using electronic health records identify new loci influencing blood pressure variation. Nat. Genet. 49, 54–64 (2017).
Wain, L. V. et al. Novel blood pressure locus and gene discovery using genome-wide association study and expression data sets from blood and the kidney. Hypertension 70, e4–e19 (2017).
Do, C. et al. Mechanisms and disease associations of haplotype-dependent allele-specific DNA methylation. Am. J. Hum. Genet. 98, 934–955 (2016).
Do, C. et al. Genetic–epigenetic interactions in cis: a major focus in the post-GWAS era. Genome Biol. 18, 120 (2017).
Park, E., Pan, Z., Zhang, Z., Lin, L. & Xing, Y. The expanding landscape of alternative splicing variation in human populations. Am. J. Hum. Genet. 102, 11–26 (2018).
Smith, A. K. et al. Methylation quantitative trait loci (meQTLs) are consistently detected across ancestry, developmental stage, and tissue type. BMC Genomics 15, 145 (2014).
Raj, T. et al. Integrative transcriptome analyses of the aging brain implicate altered splicing in Alzheimer’s disease susceptibility. Nat. Genet. 50, 1584–1592 (2018).
Jiang, X. et al. Hypertension and renin-angiotensin system blockers are not associated with expression of angiotensin-converting enzyme 2 (ACE2) in the kidney. Eur. Heart J. 41, 4580–4588 (2020).
Xu, X. et al. Molecular insights into genome-wide association studies of chronic kidney disease-defining traits. Nat. Commun. 9, 4800 (2018).
Rowland, J. et al. Uncovering genetic mechanisms of kidney aging through transcriptomics, genomics, and epigenomics. Kidney Int. 95, 624–635 (2019).
Young, M. D. et al. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science 361, 594–599 (2018).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Wang, X., Park, J., Susztak, K., Zhang, N. R. & Li, M. Bulk tissue cell type deconvolution with multi-subject single-cell expression reference. Nat. Commun. 10, 380 (2019).
Ardlie, K. G. et al. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348, 648–660 (2015).
Gillies, C. E. et al. An eQTL landscape of kidney tissue in human nephrotic syndrome. Am. J. Hum. Genet. 103, 232–244 (2018).
Crowley, S. D. & Coffman, T. M. The inextricable role of the kidney in hypertension. J. Clin. Invest. 124, 2341–2347 (2014).
Morris, A. P. et al. Trans-ethnic kidney function association study reveals putative causal genes and effects on kidney-specific disease aetiologies. Nat. Commun. 10, 29 (2019).
Wu, H. et al. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23, 869–881.e8 (2018).
Saferali, A. et al. Analysis of genetically driven alternative splicing identifies FBXO38 as a novel COPD susceptibility gene. PLOS Genet. 15, e1008229 (2019).
Han, X. et al. Cardiovascular effects of renal distal tubule deletion of the FGF receptor 1 gene. J. Am. Soc. Nephrol. 29, 69–80 (2018).
Shaw, N., Yang, B., Millward, A., Demaine, A. & Hodgkinson, A. AKR1B10 is induced by hyperglycaemia and lipopolysaccharide in patients with diabetic nephropathy. Cell Stress Chaperones 19, 281–287 (2014).
Hartmannová, H. et al. Acadian variant of Fanconi syndrome is caused by mitochondrial respiratory chain complex I deficiency due to a non-coding mutation in complex I assembly factor NDUFAF6. Hum. Mol. Genet. 25, 4062–4079 (2016).
Zhang, X. et al. Identification of common genetic variants controlling transcript isoform variation in human whole blood. Nat. Genet. 47, 345–352 (2015).
Guantes, R. et al. Global variability in gene expression and alternative splicing is modulated by mitochondrial content. Genome Res. 125, 633–644 (2015).
Marques, F. Z. et al. Signatures of miR-181a on the renal transcriptome and blood pressure. Mol. Med. 21, 739–748 (2015).
Hannon, E. et al. Leveraging DNA-methylation quantitative-trait loci to characterize the relationship between methylomic variation, gene expression, and complex traits. Am. J. Hum. Genet. 103, 654–665 (2018).
Ng, B. et al. An xQTL map integrates the genetic architecture of the human brain’s transcriptome and epigenome. Nat. Neurosci. 20, 1418–1426 (2017).
Schiffrin, E. L., Deng, L. Y., Sventek, P. & Day, R. Enhanced expression of endothelin-1 gene in resistance arteries in severe human essential hypertension. J. Hypertens. 15, 57–63 (1997).
Barbetti, F. & D’Annunzio, G. Genetic causes and treatment of neonatal diabetes and early childhood diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 32, 575–591 (2018).
Haas, M. E. et al. Genetic association of albuminuria with cardiometabolic disease and blood pressure. Am. J. Hum. Gen. 103, 461–473 (2018).
Pierce, B. L. & Burgess, S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am. J. Epidemiol. 178, 1177–1184 (2013).
Hopkins, A. L. & Groom, C. R. The druggable genome. Nat. Rev. Drug Discov. 1, 727–730 (2002).
Finan, C. et al. The druggable genome and support for target identification and validation in drug development. Sci. Transl. Med. 9, eaag1166 (2017).
Kitaba, S., Murota, H., Yahata, Y., Azukizawa, H. & Katayama, I. Novel functional aspect of antihistamines: the impact of bepotastine besilate on substance P-induced events. J. Allergy 2009, 853687 (2009).
Ryu, Y. et al. Class I histone deacetylase inhibitor MS-275 attenuates vasoconstriction and inflammation in angiotensin II-induced hypertension. PLoS ONE 14, e0213186 (2019).
Spritzer, S. D., Bravo, T. P. & Drazkowski, J. F. Topiramate for treatment in patients with migraine and epilepsy. Headache 56, 1081–1085 (2016).
Tonstad, S. et al. Efficacy and safety of topiramate in the treatment of obese subjects with essential hypertension. Am. J. Cardiol. 96, 243–251 (2005).
Moradi, S., Kerman, S. R. J. & Mollabashi, M. The effect of topiramate on weight loss in patients with type 2 diabetes. J. Res. Med. Sci. 18, 297–302 (2013).
Gamazon, E. R. et al. Using an atlas of gene regulation across 44 human tissues to inform complex disease- and trait-associated variation. Nat. Genet. 50, 956–967 (2018).
Kato, N. et al. Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat. Genet. 47, 1282–1293 (2015).
Richard, M. A. et al. DNA methylation analysis identifies loci for blood pressure regulation. Am. J. Hum. Genet. 101, 888–902 (2017).
Tomaszewski, M. et al. Renal mechanisms of association between fibroblast growth factor 1 and blood pressure. J. Am. Soc. Nephrol. 26, 3151–3160 (2015).
Naesens, M. Zero-time renal transplant biopsies. Transplantation 100, 1425–1439 (2016).
Mancia, G. et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 34, 2159–2219 (2013).
Chang, K. et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 45, 1113–1120 (2013).
Creighton, C. J. et al. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–49 (2013).
Ko, Y. A. et al. Genetic-variation-driven gene-expression changes highlight genes with important functions for kidney disease. Am. J. Hum. Genet. 100, 940–953 (2017).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Manichaikul, A. et al. Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867–2873 (2010).
Chen, C. Y. et al. Improved ancestry inference using weights from external reference panels. Bioinformatics 29, 1399–1406 (2013).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Das, S. et al. Next-generation genotype imputation service and methods. Nat. Genet. 48, 1284–1287 (2016).
Deluca, D. S. et al. RNA-SeQC: RNA-seq metrics for quality control and process optimization. Bioinformatics 28, 1530–1532 (2012).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Wright, F. A. et al. Heritability and genomics of gene expression in peripheral blood. Nat. Genet. 46, 430–437 (2014).
Bengtsson, H., Neuvial, P. & Lun, A. aroma-light v. 3.13.0 (2018); https://doi.org/10.18129/B9.bioc.aroma.light
Stegle, O., Parts, L., Piipari, M., Winn, J. & Durbin, R. Using probabilistic estimation of expression residuals (PEER) to obtain increased power and interpretability of gene expression analyses. Nat. Protoc. 7, 500–507 (2012).
Aguet, F. et al. Genetic effects on gene expression across human tissues. Nature 550, 204–213 (2017).
Saha, A. et al. Co-expression networks reveal the tissue-specific regulation of transcription and splicing. Genome Res. 27, 1843–1858 (2017).
Chiang, C. et al. The impact of structural variation on human gene expression. Nat. Genet. 49, 692–699 (2017).
Li, Y. I. et al. Annotation-free quantification of RNA splicing using LeafCutter. Nat. Genet. 50, 151–158 (2018).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Zhao, H. et al. CrossMap: a versatile tool for coordinate conversion between genome assemblies. Bioinformatics 30, 1006–1007 (2014).
Lizio, M. et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol. 16, 22 (2015).
Siggens, L. & Ekwall, K. Epigenetics, chromatin and genome organization: recent advances from the ENCODE project. J. Intern. Med. 276, 201–214 (2014).
Fortin, J. P., Triche, T. J. & Hansen, K. D. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics 33, 558–560 (2017).
Du, P. et al. Comparison of beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics 11, 587 (2010).
Aryee, M. J. et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30, 1363–1369 (2014).
Pidsley, R. et al. A data-driven approach to preprocessing Illumina 450K methylation array data. BMC Genomics 14, 293 (2013).
Ongen, H., Buil, A., Brown, A. A., Dermitzakis, E. T. & Delaneau, O. Fast and efficient QTL mapper for thousands of molecular phenotypes. Bioinformatics 32, 1479–1485 (2016).
Storey, J. D. & Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl Acad. Sci. USA 100, 9440–9445 (2003).
Jun, G., Wing, M. K., Abecasis, G. R. & Kang, H. M. An efficient and scalable analysis framework for variant extraction and refinement from population-scale DNA sequence data. Genome Res. 25, 918–925 (2015).
Danecek, P. et al. The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011).
Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015).
Graffelman, J. Exploring diallelic genetic markers: the HardyWeinberg package. J. Stat. Softw. 64, 1–23 (2015).
Anders, S., Pyl, P. T. & Huber, W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2009).
Stegle, O., Parts, L., Durbin, R. & Winn, J. A Bayesian framework to account for complex non-genetic factors in gene expression levels greatly increases power in eQTL studies. PLoS Comput. Biol. 6, 1–11 (2010).
Parts, L., Stegle, O., Winn, J. & Durbin, R. Joint genetic analysis of gene expression data with inferred cellular phenotypes. PLoS Genet. 7, e1001276 (2011).
Wen, X. Molecular QTL discovery incorporating genomic annotations using Bayesian false discovery rate control. Ann. Appl. Stat. 10, 1619–1638 (2016).
Carithers, L. J. et al. A novel approach to high-quality postmortem tissue procurement: the GTEx project. Biopreserv. Biobank. 13, 311–319 (2015).
van der Maaten, L. J. P. Accelerating t-SNE using tree-based algorithms. J. Mach. Learn. Res. 15, 3221–3245 (2014).
Huang, D. W. et al. The DAVID Gene Functional Classification Tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 8, R183 (2007).
Taggart, A. J. et al. Large-scale analysis of branchpoint usage across species and cell lines. Genome Res. 27, 639–649 (2017).
Zerbino, D. R. et al. Ensembl 2018. Nucleic Acids Res. 46, D754–D761 (2018).
Artimo, P. et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 40, W597–W603 (2012).
Jones, P. et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240 (2014).
Mitchell, A. L. et al. InterPro in 2019: improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 47, D351–D360 (2019).
Buchan, D. W. A. & Jones, D. T. The PSIPRED Protein Analysis Workbench: 20 years on. Nucleic Acids Res. 47, W402–W407 (2019).
Zimmermann, L. et al. A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 430, 2237–2243 (2018).
Klausen, M. S. et al. NetSurfP-2.0: improved prediction of protein structural features by integrated deep learning. Proteins 87, 520–527 (2019).
Almagro Armenteros, J. J. et al. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 37, 420–423 (2019).
Emanuelsson, O., Brunak, S., von Heijne, G. & Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2, 953–971 (2007).
Sonnhammer, E. L., von Heijne, G. & Krogh, A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 6, 175–182 (1998).
Tsirigos, K. D., Peters, C., Shu, N., Käll, L. & Elofsson, A. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res. 43, W401–W407 (2015).
Blom, N., Gammeltoft, S. & Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294, 1351–1362 (1999).
Blom, N., Sicheritz-Pontén, T., Gupta, R., Gammeltoft, S. & Brunak, S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4, 1633–1649 (2004).
Lehne, B. et al. A coherent approach for analysis of the Illumina HumanMethylation450 BeadChip improves data quality and performance in epigenome-wide association studies. Genome Biol. 16, 37 (2015).
Schulz, H. et al. Genome-wide mapping of genetic determinants influencing DNA methylation and gene expression in human hippocampus. Nat. Commun. 8, 1511 (2017).
Nikpay, M. et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 47, 1121–1130 (2015).
Canela-Xandri, O., Rawlik, K. & Tenesa, A. An atlas of genetic associations in UK Biobank. Nat. Genet. 50, 1593–1599 (2018).
Malik, R. et al. Low-frequency and common genetic variation in ischemic stroke: the METASTROKE collaboration. Neurology 87, 1306–1306 (2016).
Pattaro, C. et al. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat. Commun. 7, 10023 (2016).
Teumer, A. et al. Genome-wide association studies identify genetic loci associated with albuminuria in diabetes. Diabetes 65, 803–817 (2016).
Bowden, J. et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int. J. Epidemiol. 47, 1264–1278 (2018).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 7, e34408 (2018).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314 (2016).
Bowden, J., Smith, G. D. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Wishart, D. S. et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46, D1074–D1082 (2018).
Gaulton, A. et al. The ChEMBL database in 2017. Nucleic Acids Res. 45, D945–D954 (2017).
Kuhn, M., Letunic, I., Jensen, L. J. & Bork, P. The SIDER database of drugs and side effects. Nucleic Acids Res. 44, D1075–D1079 (2016).
Cotto, K. C. et al. DGIdb 3.0: a redesign and expansion of the drug–gene interaction database. Nucleic Acids Res. 46, D1068–D1073 (2017).
Giambartolomei, C. et al. A Bayesian framework for multiple trait colocalization from summary association statistics. Bioinformatics 34, 2538–2545 (2018).
Guo, H. et al. Integration of disease association and eQTL data using a Bayesian colocalisation approach highlights six candidate causal genes in immune-mediated diseases. Hum. Mol. Genet. 24, 3305–3313 (2015).
Ernst, J. & Kellis, M. Chromatin-state discovery and genome annotation with ChromHMM. Nat. Protoc. 12, 2478–2492 (2017).
Roadmap Epigenomics Consortium et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–329 (2015).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Smith, G. D. & Ebrahim, S. What can Mendelian randomisation tell us about modifiable behavioural and environmental exposures? Br. Med. J. 330, 1076–1079 (2005).
Burgess, S., Bowden, J., Dudbridge, F. & Thompson, S. G. Robust instrumental variable methods using multiple candidate instruments with application to Mendelian randomization. Preprint at https://arxiv.org/abs/1606.03729 (2016).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698 (2018).
Hemani, G., Tilling, K. & Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 13, e1007081 (2017).
Yavorska, O. O. & Burgess, S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 46, 1734–1739 (2017).
Acknowledgements
This work was supported by British Heart Foundation project grants no. PG/17/35/33001 and no. PG/19/16/34270 and Kidney Research UK grants no. RP_017_20180302 and no. RP_013_20190305 to M.T., National Institutes of Health (NIH) grant no. R01 DK117445-01A1 to A.P.M., NIH (USA) NIDDK grants no. R01 DK108805 and no. R01 DK119380 to M.G.S., British Heart Foundation Personal Chair CH/13/2/30154 and Manchester Academic Health Science Centre: Tissue Bank Grant to B.K., and Medical University of Silesia grants no. KNW-1-152/N/7/K to J.Z. and no. KNW-1-171/N/6/K to W.W. T.J.G. acknowledges support from the European Research Council (ERC-CoG-Inflammatension grant no. 726318) and the European Commission/Narodowe Centrum Badan i Rozwoju, Poland (EraNet-CVD-Plaquefight). P.M. acknowledges support of British Heart Foundation grant no. PG/19/84/34771. D.T. acknowledges support of Medical Research Council New Investigator Research Grant no. MR/R010900/1. B.K. is supported by a British Heart Foundation Personal Chair. G.T. is supported by the Wellcome Trust (grant no. WT206194) and Open Targets. E.C.-G. is supported by a Gates Cambridge Scholarship (no. OPP1144). The Nephrotic Syndrome Study Network Consortium (NEPTUNE), U54-DK-083912, is a part of the NIH Rare Disease Clinical Research Network (RDCRN), supported through a collaboration between the NIH Office of Rare Diseases Research, the National Center for Advancing Translational Sciences and the National Institute of Diabetes, Digestive, and Kidney Diseases. Additional funding and/or programmatic support for this project has also been provided by the University of Michigan, NephCure Kidney International and the Halpin Foundation. Access to TCGA kidney and GTEx data has been granted by the NIH (approvals 50804-2 and 50805-2). The results published here are in part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga. We thank the Oxford Genomics Centre at the Wellcome Centre for Human Genetics funded by the Wellcome Trust (grant reference no. 203141/Z/16/Z) for the generation and initial processing of sequencing data.
Author information
Authors and Affiliations
Contributions
J.M.E., X.J., X.X., S.S. and A. Akbarov performed the main analytical and experimental tasks. E.C.-G., M.T.M., C.F., H.G., H.B.T., S.P., S.C., P.R.P., I.W., E.E., M. Salehi, Y.S., M.E., M.D., F.E., B.G., S.E., C.B., J. Bowes, M.C., M.K., A.S.W., D.T., B.K., P.M., T.J.G., R.T.-O’K., G.T., N.J.S., A.H., M.G.S., A.P.M. and F.J.C. provided additional analyses and data. W.W., M. Szulinska, A. Antczak, M.G., R.K., J. Brown, E.Z.-S., J.Z. and P.B. were involved in the collection of kidney resources. M.D., A.N., P.R.P., I.W. and F.J.C. were involved in additional sample processing and sequencing. J.M.E., X.J., X.X., F.J.C., A.P.M. and M.T. contributed to drafting the manuscript. M.T. performed the overall supervision of the project. All authors reviewed and approved the accepted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.K. reports grants from the NIH and Goldfinch Bio during the conduct of the study, and grants from AstraZeneca, Gilead, Novo Nordisk, Eli Lilly, Janssen, Merck, Elipidera, Certa and Boehringer Ingelheim, outside the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 rs4750358 as BP-GWAS kidney sSNP and its BEND7 (BEN Domain Containing 7) target.
a, The BP-elevating (A) allele of rs4750358 creates a branch point (orange box) for a new downstream acceptor splice site and facilitates excision of isoform I7b. The branch point for intron excision isoform (IEI) I7a is also marked (yellow box). b, Association between rs4750358 and kidney expression of I7a IEI of BEND7, I7b IEI of BEND7 and total renal expression of BEND7 (Wald test, linear regression). Data are standardized expression values stratified on rs4750358 genotype. All sample sizes (n) are biologically independent samples. Outliers (outside 1.5x interquartile range) are denoted as points. Whiskers denote extent of 1.5x interquartile range. Upper, middle and lower box lines denote 75th, 50th and 25th percentiles, respectively. FDR, false discovery rate; NS, non-significant. c, Simplified intron-exon structures of the terminal portions of renal BEND7-002 isoforms. d, Proportional expression of BEND7 mRNA isoforms in the kidney. e, Amino acids and phosphorylation sites in the C-terminus of protein isoforms BEND7-002a and BEND7-002b. Exon 8b in BEND7-002b protein isoform contains a longer coding sequence replacing a pair of valines with a 20-amino acid stretch (with many polar, mostly charged, amino acids and three additional possible phosphorylation sites at the C-terminus). The orange bar above the peptide sequence shows the amino acids encoded by the last exon. Polar amino acids are highlighted in red, whereas hydrophobic amino acids are shown in blue. Asterisks are used to identify possible phosphorylation sites, for each of which a list of possible protein kinases is provided.
Extended Data Fig. 2 rs4932373 as a BP-GWAS kidney mSNP.
a, rs4932373 (orange solid bar) is a BP-GWAS kidney mSNP. Its renal target (cg04510874, blue solid bar) maps onto the CpG island (green segment) and the promoter region of the FES gene. Chromatin states are shown at the bottom (green, Transcribed; yellow, Enhancer; red, TSS). Yellow solid bar, sentinel BP-GWAS SNP (rs2521501); black dotted bar, single nucleotide variants; red solid bar, CpG sites. b, The opposite direction of effect of rs4932373 genotype on renal expression of FES and kidney DNA methylation at cg04510874 (Wald test, linear regression). Outliers (outside 1.5x interquartile range) are denoted as points. Whiskers denote extent of 1.5x interquartile range. Upper, middle and lower box lines denote 75th, 50th and 25th percentiles, respectively. All sample sizes (n) are biologically independent samples. P, level of statistical significance.
Supplementary information
Supplementary Information
Supplementary Note and Figs. 1–8.
Supplementary Tables
Supplementary Tables 1–28.
Rights and permissions
About this article
Cite this article
Eales, J.M., Jiang, X., Xu, X. et al. Uncovering genetic mechanisms of hypertension through multi-omic analysis of the kidney. Nat Genet 53, 630–637 (2021). https://doi.org/10.1038/s41588-021-00835-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-021-00835-w
This article is cited by
-
Immune and inflammatory mechanisms in hypertension
Nature Reviews Cardiology (2024)
-
Unraveling the epigenetic code: human kidney DNA methylation and chromatin dynamics in renal disease development
Nature Communications (2024)
-
A landscape of gene expression regulation for synovium in arthritis
Nature Communications (2024)
-
Genetic imputation of kidney transcriptome, proteome and multi-omics illuminates new blood pressure and hypertension targets
Nature Communications (2024)
-
DNA methylation QTL mapping across diverse human tissues provides molecular links between genetic variation and complex traits
Nature Genetics (2023)