Abstract
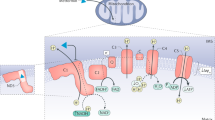
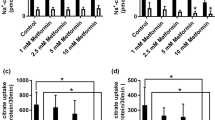
Metformin, the universal first-line treatment for type 2 diabetes, exerts its therapeutic glucose-lowering effects by inhibiting hepatic gluconeogenesis. However, the primary molecular mechanism of this biguanide remains unclear, though it has been suggested to act, at least partially, by mitochondrial complex I inhibition. Here we show that clinically relevant concentrations of plasma metformin achieved by acute intravenous, acute intraportal or chronic oral administration in awake normal and diabetic rats inhibit gluconeogenesis from lactate and glycerol but not from pyruvate and alanine, implicating an increased cytosolic redox state in mediating metformin’s antihyperglycemic effect. All of these effects occurred independently of complex I inhibition, evidenced by unaltered hepatic energy charge and citrate synthase flux. Normalizing the cytosolic redox state by infusion of methylene blue or substrates that contribute to gluconeogenesis independently of the cytosolic redox state abrogated metformin-mediated inhibition of gluconeogenesis in vivo. Additionally, in mice expressing constitutively active acetyl-CoA carboxylase, metformin acutely decreased hepatic glucose production and increased the hepatic cytosolic redox state without altering hepatic triglyceride content or gluconeogenic enzyme expression. These studies demonstrate that metformin, at clinically relevant plasma concentrations, inhibits hepatic gluconeogenesis in a redox-dependent manner independently of reductions in citrate synthase flux, hepatic nucleotide concentrations, acetyl-CoA carboxylase activity, or gluconeogenic enzyme protein expression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
07 February 2019
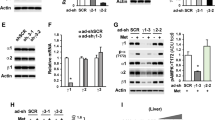
In the version of this article originally published, the VPC and VCS flux data shown in Fig. 6e,f were inadvertently duplicated from Fig. 5j,k. The correct data are now shown in Fig. 6e,f. In these corrected data, VPC flux in response to chronic oral metformin treatment was still significantly decreased (Fig. 6e), and there was still no impact of metformin on VCS flux (Fig. 6f). Therefore, the text describing these data remains the same and this correction does not change the conclusion of this study.
References
Owen, M. R., Doran, E. & Halestrap, A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 (2000).
El-Mir, M.-Y. et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 (2000).
He, L. et al. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 137, 635–646 (2009).
Cao, J. et al. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK). J. Biol. Chem. 289, 20435–20446 (2014).
Fullerton, M. D. et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 19, 1649–1654 (2013).
Cool, B. et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 3, 403–416 (2006).
Guigas, B. & Viollet, B. Targeting AMPK: from ancient drugs to new small-molecule activators. EXS 107, 327–350 (2016).
Rena, G., Hardie, D. G. & Pearson, E. R. The mechanisms of action of metformin. Diabetologia 60, 1577–1585 (2017).
Shaw, R. J. et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005).
Smith, B. K. et al. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am. J. Physiol. Endocrinol. Metab. 311, E730–E740 (2016).
Foretz, M. et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 120, 2355–2369 (2010).
Miller, R. A. et al. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 494, 256–260 (2013).
Johanns, M. et al. AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B. Nat. Commun. 7, 10856 (2016).
Hawley, S. A., Gadalla, A. E., Olsen, G. S. & Hardie, D. G. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51, 2420–2425 (2002).
Madiraju, A. K. et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510, 542–546 (2014).
Cederbaum, A. I., Lieber, C. S., Beattie, D. S. & Rubin, E. Characterization of shuttle mechanisms for the transport of reducing equivalents into mitochondria. Arch. Biochem. Biophys. 158, 763–781 (1973).
Kobayashi, K. et al. The gene mutated in adult-onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nat. Genet. 22, 159–163 (1999).
LaNoue, K. F. & Williamson, J. R. Interrelationships between malate–aspartate shuttle and citric acid cycle in rat heart mitochondria. Metabolism 20, 119–140 (1971).
Jomain-Baum, M. & Hanson, R. W. Regulation of hepatic gluconeogenesis in the guinea pig by fatty acids and ammonia. J. Biol. Chem. 250, 8978–8985 (1975).
Sugano, T. et al. Intracellular redox state and stimulation of gluconeogenesis by glucagon and norepinephrine in the perfused rat liver. J. Biochem. 87, 153–166 (1980).
Williamson, J. R., Scholz, R. & Browning, E. T. Control mechanisms of gluconeogenesis and ketogenesis. II Interactions between fatty acid oxidation and the citric acid cycle in perfused rat liver.J. Biol. Chem 244, 4617–4627 (1969).
Williamson, J. R., Scholz, R., Browning, E. T., Thurman, R. G. & Fukami, M. H. Metabolic effects of ethanol in perfused rat liver. J. Biol. Chem. 244, 5044–5054 (1969).
Perry, R. J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015).
Perry, R. J. et al. Leptin reverses diabetes by suppression of the hypothalamic–pituitary–adrenal axis. Nat. Med. 20, 759–763 (2014).
Sistare, F. D. & Haynes, R. C.Jr. The interaction between the cytosolic pyridine nucleotide redox potential and gluconeogenesis from lactate/pyruvate in isolated rat hepatocytes. Implications for investigations of hormone action.J. Biol. Chem. 260, 12748–12753 (1985).
Goodman, L. S. Goodman & Gilman's The Pharmacological Basis of Therapeutics, 12th edn (eds Brunton, L. L., Chabner, B. & Knollmann, B. C.) 1906–2000 (McGraw-Hill, New York, 2011).
Duca, F. A. et al. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 21, 506–511 (2015).
Guigas, B. et al. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside and metformin inhibit hepatic glucose phosphorylation by an AMP-activated protein kinase-independent effect on glucokinase translocation. Diabetes 55, 865–874 (2006).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Hundal, R. S. et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49, 2063–2069 (2000).
Inzucchi, S. E. et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N. Engl. J. Med. 338, 867–872 (1998).
Chu, C. A. et al. The acute effect of metformin on glucose production in the conscious dog is primarily attributable to inhibition of glycogenolysis. Metabolism 49, 1619–1626 (2000).
Mithieux, G., Guignot, L., Bordet, J. C. & Wiernsperger, N. Intrahepatic mechanisms underlying the effect of metformin in decreasing basal glucose production in rats fed a high-fat diet. Diabetes 51, 139–143 (2002).
Lavine, J. E. et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. J. Am. Med. Assoc. 305, 1659–1668 (2011).
Rakoski, M. O., Singal, A. G., Rogers, M. A. & Conjeevaram, H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 32, 1211–1221 (2010).
Shields, W. W., Thompson, K. E., Grice, G. A., Harrison, S. A. & Coyle, W. J. The effect of metformin and standard therapy versus standard therapy alone in nondiabetic patients with insulin resistance and nonalcoholic steatohepatitis (NASH): a pilot trial. Ther. Adv. Gastroenterol. 2, 157–163 (2009).
Gormsen, L. C. et al. Metformin does not affect postabsorptive hepatic free fatty acid uptake, oxidation or resecretion in humans: a 3-month placebo-controlled clinical trial in patients with type 2 diabetes and healthy controls. Diabetes Obes. Metab. https://doi.org/10.1111/dom.13244 (2018).
Ryle, P. R., Chakraborty, J. & Thomson, A. D. The effect of methylene blue on the hepatocellular redox state and liver lipid content during chronic ethanol feeding in the rat. Biochem. J. 232, 877–882 (1985).
Baur, J. A. & Birnbaum, M. J. Control of gluconeogenesis by metformin: does redox trump energy charge? Cell Metab. 20, 197–199 (2014).
He, L. & Wondisford, F. E. Metformin action: concentrations matter. Cell Metab. 21, 159–162 (2015).
Puhakainen, I., Koivisto, V. A. & Yki-Järvinen, H. Lipolysis and gluconeogenesis from glycerol are increased in patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 75, 789–794 (1992).
Nurjhan, N., Consoli, A. & Gerich, J. Increased lipolysis and its consequences on gluconeogenesis in non-insulin-dependent diabetes mellitus. J. Clin. Invest. 89, 169–175 (1992).
Wu, H. et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 23, 850–858 (2017).
Schlienger, J. L., Frick, A., Marbach, J., Freund, H. & Imler, M. Effects of biguanides on the intermediate metabolism of glucose in normal and portal-strictured rats. Diabete Metab. 5, 5–9 (1979).
Gormsen, L. C. et al. In vivo imaging of human 11C-metformin in peripheral organs: dosimetry, biodistribution, and kinetic analyses. J. Nucl. Med. 57, 1920–1926 (2016).
Brønden, A. A. A., et al Single-dose metformin enhances bile acid–induced GLP-1 secretion in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. (in the press) (2017).
Timmins, P., Donahue, S., Meeker, J. & Marathe, P. Steady-state pharmacokinetics of a novel extended-release metformin formulation. Clin. Pharmacokinet. 44, 721–729 (2005).
Larsen, S. et al. Metformin-treated patients with type 2 diabetes have normal mitochondrial complex I respiration. Diabetologia 55, 443–449 (2012).
Said, A. & Akhter, A. Meta-analysis of randomized controlled trials of pharmacologic agents in non-alcoholic steatohepatitis. Ann. Hepatol. 16, 538–547 (2017).
Sawangjit, R. et al. Comparative efficacy of interventions on nonalcoholic fatty liver disease (NAFLD): a PRISMA-compliant systematic review and network meta-analysis. Med. (Baltim.) 95, e4529 (2016).
Kim, C. W. et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 26, 576 (2017).
Konopka, A. R. et al. Hyperglucagonemia mitigates the effect of metformin on glucose production in prediabetes. Cell Rep. 15, 1394–1400 (2016).
Lewis, A. J.et al. Assessment of metformin-induced changes in cardiac and hepatic redox state using hyperpolarized [1-13C] pyruvate. Diabetes 65, 3544–3551 (2016).
Qi, H. et al. Acute renal metabolic effect of metformin assessed with hyperpolarised MRI in rats. Diabetologia 61, 445–454 (2018).
Nattrass, M., Todd, P. G., Hinks, L., Lloyd, B. & Alberti, K. G. Comparative effects of phenformin, metformin and glibenclamide on metabolic rhythms in maturity-onset diabetics. Diabetologia 13, 145–152 (1977).
Hussey, E. K. et al. Safety, pharmacokinetics and pharmacodynamics of remogliflozin etabonate, a novel SGLT2 inhibitor, and metformin when co-administered in subjects with type 2 diabetes mellitus. BMC Pharmacol. Toxicol. 14, 25 (2013).
Comte, B., Vidal, H., Laville, M. & Riou, J. P. Influence of thyroid hormones on gluconeogenesis from glycerol in rat hepatocytes: a dose-response study. Metabolism 39, 259–263 (1990).
Gregory, R. B., Phillips, J. W. & Berry, M. N. Reducing-equivalent transfer to the mitochondria during gluconeogenesis and ureogenesis in hepatocytes from rats of different thyroid status. Biochim. Biophys. Acta 1137, 34–38 (1992).
Kneer, N. & Lardy, H. Thyroid hormone and dehydroepiandrosterone permit gluconeogenic hormone responses in hepatocytes. Arch. Biochem. Biophys. 375, 145–153 (2000).
Drahota, Z. et al. Biguanides inhibit complex I, II and IV of rat liver mitochondria and modify their functional properties. Physiol. Res. 63, 1–11 (2014).
Reed, M. J. et al. Effect of masoprocol on carbohydrate and lipid metabolism in a rat model of type II diabetes. Diabetologia 42, 102–106 (1999).
Perry, R. J. et al. Non-invasive assessment of hepatic mitochondrial metabolism by positional isotopomer NMR tracer analysis (PINTA). Nat. Commun. 8, 798 (2017).
Perry, R. J. et al. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell Metab. 18, 740–748 (2013).
Alves, T. C. et al. Regulation of hepatic fat and glucose oxidation in rats with lipid-induced hepatic insulin resistance. Hepatology 53, 1175–1181 (2011).
Perry, R. J. et al. Mechanism for leptin’s acute insulin-independent effect to reverse diabetic ketoacidosis. J. Clin. Invest. 127, 657–669 (2017).
Ayala, J. E. et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis. Model. Mech. 3, 525–534 (2010).
Passonneau, J. V. & Lauderdale, V. R. A comparison of three methods of glycogen measurement in tissues. Anal. Biochem. 60, 405–412 (1974).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Acknowledgements
The authors would like to thank J. Dong, M. Kahn, S. Dufour, J. Stack, Y.Kosover, A. Nasiri, X. Ma, W. Zhu, and K. Harry for their technical support and V. Samuel, S. Caprio, and D. Kibbey for helpful discussions. This publication was supported by grants from the US Department of Health and Human Services: R01 DK113984, P30 DK45735, P30 DK034989, K99 CA215315 and R01 DK114793. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or the NIH. G.R.S. is supported by grants and fellowships from a Canada Research Chair in Metabolism and Obesity, the J. Bruce Duncan Endowed Chair in Metabolic Diseases at McMaster University and Diabetes Canada. B.E.K. is supported by grants and a Fellowship (BEK) from the National Health and Medical Research Council (1068813 and 1085460) and the Victorian Government Operational Infrastructure Support Scheme.
Author information
Authors and Affiliations
Contributions
A.K.M., Y.Q., R.J.P., J.-P.G.C., D.F.V., K.F.P., and G.I.S. designed the experimental protocols. A.K.M., Y.Q., Y.R., X.-M.Z., D.Z., G.W.C., G.M.B., G.C., J.-P.G.C., and K.F.P. performed the studies. A.K.M., Y.Q., R.J.P., X-M. Z., G.W.C., G.C., and J.-P.G.C. analyzed the data. B.E.K. and G.R.S. supplied reagents.A.K.M., Y.Q., R.J.P, G.W.C., D.F.V., K.F.P., and G.I.S. wrote the manuscript with contributions from all of the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Madiraju, A.K., Qiu, Y., Perry, R.J. et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med 24, 1384–1394 (2018). https://doi.org/10.1038/s41591-018-0125-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0125-4
This article is cited by
-
Metformin: update on mechanisms of action and repurposing potential
Nature Reviews Endocrinology (2023)
-
Higher mitochondrial DNA copy number is associated with metformin-induced weight loss
Communications Medicine (2023)
-
Anti-cancer Efficacy of Metformin: Recent Updates on Breast and Other Cancers
Current Pharmacology Reports (2023)
-
Molecular mechanisms of action of metformin: latest advances and therapeutic implications
Clinical and Experimental Medicine (2023)
-
Metformin in therapeutic applications in human diseases: its mechanism of action and clinical study
Molecular Biomedicine (2022)