Abstract
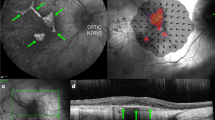
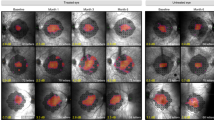
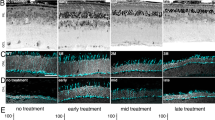
Retinal gene therapy is increasingly recognized as a novel molecular intervention that has huge potential in treating common causes of blindness, the majority of which have a genetic aetiology1,2,3,4,5. Choroideremia is a chronic X-linked retinal degeneration that was first described in 18726. It leads to progressive blindness due to deficiency of Rab-escort protein 1 (REP1). We designed an adeno-associated viral vector to express REP1 and assessed it in a gene therapy clinical trial by subretinal injection in 14 patients with choroideremia. The primary endpoint was vision change in treated eyes 2 years after surgery compared to unoperated fellow eyes. Despite complications in two patients, visual acuity improved in the 14 treated eyes over controls (median 4.5 letter gain, versus 1.5 letter loss, P = 0.04), with 6 treated eyes gaining more than one line of vision (>5 letters). The results suggest that retinal gene therapy can sustain and improve visual acuity in a cohort of predominantly late-stage choroideremia patients in whom rapid visual acuity loss would ordinarily be predicted.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that all of the data supporting the findings of this study are available within the paper and the Supplementary Information and are available from the corresponding author upon reasonable request.
References
Boye, S. E., Boye, S. L., Lewin, A. S. & Hauswirth, W. W. A comprehensive review of retinal gene therapy. Mol. Ther. 21, 509–519 (2013).
Sahel, J. A., Marazova, K. & Audo, I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb. Perspect. Med. 5, a017111 (2014).
Vandenberghe, L. H. What is next for retinal gene therapy? Cold Spring Harb. Perspect. Med. 5, a017442 (2015).
Ghazi, N. G. et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum. Genet. 135, 327–343 (2016).
Bennett, J. Taking stock of retinal gene therapy: looking back and moving forward. Mol. Ther. 25, 1076–1094 (2017).
Mauthner, L. Ein Fall von Choroideremia. Berl. Natur-med. Ver Innsbruck 2, 191 (1872).
Barnard, A. R., Groppe, M. & MacLaren, R. E. Gene therapy for choroideremia using an adeno-associated viral (AAV) vector. Cold Spring Harb. Perspect. Med. 5, a017293 (2014).
Cremers, F. P., van de Pol, D. J., van Kerkhoff, L. P., Wieringa, B. & Ropers, H. H. Cloning of a gene that is rearranged in patients with choroideraemia. Nature 347, 674–677 (1990).
Seabra, M. C., Brown, M. S. & Goldstein, J. L. Retinal degeneration in choroideremia: deficiency of rab geranylgeranyl transferase. Science 259, 377–381 (1993).
Aleman, T. S. et al. Natural history of the central structural abnormalities in choroideremia: a prospective cross-sectional study. Ophthalmology 124, 359–373 (2017).
Edwards, T. L., Groppe, M., Jolly, J. K., Downes, S. M. & MacLaren, R. E. Correlation of retinal structure and function in choroideremia carriers. Ophthalmology 122, 1274–1276 (2015).
Morgan, J. I. et al. High-resolution adaptive optics retinal imaging of cellular structure in choroideremia. Invest. Ophthalmol. Vis. Sci. 55, 6381–6397 (2014).
Jolly, J. K., Xue, K., Edwards, T. L., Groppe, M. & MacLaren, R. E. Characterizing the natural history of visual function in choroideremia using microperimetry and multimodal retinal imaging. Invest. Ophthalmol. Vis. Sci. 58, 5575–5583 (2017).
Vandenberghe, L. H. et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci. Transl. Med. 3, 88ra54 (2011).
Russell, S. et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390, 849–860 (2017).
MacLaren, R. E. et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet 383, 1129–1137 (2014).
Edwards, T. L. et al. Visual acuity after retinal gene therapy for choroideremia. N. Engl. J. Med. 374, 1996–1998 (2016).
Cideciyan, A. V. et al. Vision 1 year after gene therapy for Leber’s congenital amaurosis. N. Engl. J. Med. 361, 725–727 (2009).
Xue, K. et al. Correlation of optical coherence tomography and autofluorescence in the outer retina and choroid of patients with choroideremia. Invest. Ophthalmol. Vis. Sci. 57, 3674–3684 (2016).
Jolly, J. K. et al. A qualitative and quantitative assessment of fundus autofluorescence patterns in patients with choroideremia. Invest. Ophthalmol. Vis. Sci. 57, 4498–4503 (2016).
Aylward, J. W. et al. Retinal degeneration in choroideremia follows an exponential decay function. Ophthalmology 125, 1122–1124 (2018).
Jacobson, S. G. et al. Remodeling of the human retina in choroideremia: rab escort protein 1 (REP-1) mutations. Invest. Ophthalmol. Vis. Sci. 47, 4113–4120 (2006).
Heon, E. et al. Visual function and central retinal structure in choroideremia. Invest. Ophthalmol. Vis. Sci. 57, 377–387 (2016).
Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 372, 1193–1203 (2015).
Simunovic, M. P., Xue, K., Jolly, J. K. & MacLaren, R. E. Structural and functional recovery following limited iatrogenic macular detachment for retinal gene therapy. JAMA Ophthalmol. 135, 234–241 (2017).
Hariri, A. H. et al. Measurement and reproducibility of preserved ellipsoid zone area and preserved retinal pigment epithelium area in eyes with choroideremia. Am. J. Ophthalmol. 179, 110–117 (2017).
Maguire, A. M. et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 (2008).
Patrício, M. I., Barnard, A. R., Orlans, H. O., McClements, M. E. & MacLaren, R. E. Inclusion of the Woodchuck hepatitis virus posttranscriptional regulatory element enhances AAV2-driven transduction of mouse and human retina. Mol. Ther. Nucleic Acids 6, 198–208 (2017).
Duncan, J. L. et al. Macular pigment and lutein supplementation in choroideremia. Exp. Eye Res. 74, 371–381 (2002).
Richter-Mueksch, S., Sacu, S., Weingessel, B., Vécsei-Marlovits, V. P. & Schmidt-Erfurth, U. The influence of cortical, nuclear, subcortical posterior, and mixed cataract on the results of microperimetry. Eye 25, 1317–1321 (2011).
Dimopoulos, I. S. et al. Two-year results after AAV2-mediated gene therapy for choroideremia: the Alberta experience. Am. J. Ophthalmol. 193, 130–142 (2018).
Edwards, T. L. et al. First-in-human study of the safety and viability of intraocular robotic surgery. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-018-0248-4 (2018).
Patrício, M. I., Barnard, A. R., Cox, C. I., Blue, C. & MacLaren, R. E. Biological activity of AAV vectors for choroideremia gene therapy can be measured by in vitro prenylation of RAB6A. Mol. Ther. Methods Clin. Dev. 9, 288–295 (2018).
Fischer, M. D., Hickey, D. G., Singh, M. S. & MacLaren, R. E. Evaluation of an optimized injection system for retinal gene therapy in human patients. Hum. Gene Ther. Methods 27, 150–158 (2016).
Xue, K., Groppe, M., Salvetti, A. P. & MacLaren, R. E. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye 31, 1308–1316 (2017).
Shapiro, S. S. & Wilk, M. B. Analysis of variance test for normality (complete samples). Biometrika 52, 591–611 (1965).
Acknowledgements
We thank all trial participants for their commitment to attending extensive follow-up visits, K. M. Jasani for helping with collection of the OCT and adverse events data, M. Hassall for helping with analysis of the AF data, and staff members of the Eye Research Group Oxford (ERGO) for their support throughout the study. This work was supported primarily by a grant (HICF-1009-006) from the Health Innovation Challenge Fund, a funding partnership between the UK Department of Health and the Wellcome Trust. Additional funding support was from the Health Foundation; Fight for Sight; the Lanvern Foundation; the Special Trustees of Moorfields Eye Hospital; the Royal College of Surgeons of Edinburgh and the National Institute for Health Research (NIHR) Biomedical Research Centres (BRC) at the Oxford University Hospitals NHS Foundation Trust (which includes the University of Oxford) and Moorfields Eye Hospital NHS Foundation Trust (which includes the University College London Institute of Ophthalmology).
Author information
Authors and Affiliations
Contributions
K.X., J.K.J., A.R.B., A.R., A.P.S., M.I.P. and T.L.E. collected the data and performed data analysis. K.X. and M.G. assisted with surgery. T.T., A.R.B., M.I.P. and H.O.O. tested the vector. G.C.B., A.R.W., A.J.L., S.M.D. and R.E.M. were clinical trial investigators, who designed the trial protocol, managed patient recruitment and interpreted the data. G.E.H. performed electrophysiology, data analysis and helped with trial design. R.E.M. and M.C.S. obtained funding and designed the study. R.E.M. and K.X. wrote the manuscript. All authors provided scientific input and read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.E.M.: scientific cofounder of Nightstar Therapeutics Inc.—a gene therapy company established by the University of Oxford and originally funded by the Wellcome Trust through Syncona Partners Ltd. A.R.B., G.C.B., A.J.L., G.C.B. and M.C.S.: consulting or on advisory board for Nightstar Therapeutics Inc. M.I.P., M.C.S. and R.E.M.: named inventors on patents relating to choroideremia gene therapy owned by the University of Oxford and Nightstar Therapeutics Inc. R.E.M., A.J.L. and G.C.B.: scientific advisory board to Spark Therapeutics Inc. The companies had no role in the conduct of this university-sponsored clinical trial, nor in the interpretation of the data, nor in the writing of the results. The views expressed are those of the authors and not necessarily those of the Wellcome Trust, the National Health Service, the NIHR or the UK Department of Health.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Tables 1–7 and Supplementary Methods
Rights and permissions
About this article
Cite this article
Xue, K., Jolly, J.K., Barnard, A.R. et al. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat Med 24, 1507–1512 (2018). https://doi.org/10.1038/s41591-018-0185-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0185-5
This article is cited by
-
Parafoveal cone function in choroideremia assessed with adaptive optics optoretinography
Scientific Reports (2024)
-
Subretinal timrepigene emparvovec in adult men with choroideremia: a randomized phase 3 trial
Nature Medicine (2023)
-
iOCT-guided simulated subretinal injections: a comparison between manual and robot-assisted techniques in an ex-vivo porcine model
Journal of Robotic Surgery (2023)
-
Bilateral visual acuity decline in males with choroideremia: a pooled, cross-sectional meta-analysis
BMC Ophthalmology (2022)
-
Review of gene therapies for age-related macular degeneration
Eye (2022)