Abstract
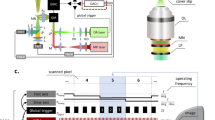
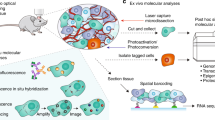
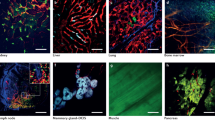
Multiphoton intravital imaging is essential for understanding cellular behavior and function in vivo. The adipose-rich environment of the mammary gland poses a unique challenge to in vivo microscopy due to light scattering that impedes high-resolution imaging. Here we provide a protocol for high-quality, six-color 3D intravital imaging of regions across the entire mouse mammary gland and associated tissues for several hours while maintaining tissue access for microdissection and labeling. An incision at the ventral midline and along the right hind leg creates a skin flap that is then secured to a raised platform skin side down. This allows for fluorescence-guided microdissection of connective tissue to provide unimpeded imaging of mammary ducts. A sealed imaging chamber over the skin flap creates a stable environment while maintaining access to large tissue regions for imaging with an upright microscope. We provide a strategy for imaging single cells and the tissue microenvironment utilizing multicolor Confetti lineage-tracing and additional dyes using custom-designed filters and sequential excitation with dual multiphoton lasers. Furthermore, we describe a strategy for simultaneous imaging and photomanipulation of single cells using the Olympus SIM scanner and provide steps for 3D video processing, visualization and high-dimensional analysis of single-cell behavior. We then provide steps for multiplexing intravital imaging with fixation, immunostaining, tissue clearing and 3D confocal imaging to associate cell behavior with protein expression. The skin-flap surgery and chamber preparation take 1.5 h, followed by up to 12 h of imaging. Applications range from basic filming in 1 d to 5 d for multiplexing and complex analysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data in Fig. 5a,b were published previously12. The source data for Fig. 6e are included in a source data file to this protocol. All other data are too large to include in the paper but are available from the authors upon reasonable request. Source data are provided with this paper.
References
Germain, R. N., Robey, E. A. & Cahalan, M. D. A decade of imaging cellular motility and interaction dynamics in the immune system. Science 336, 1676–1681 (2012).
Zhang, F. et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat. Protoc. 5, 439–456 (2010).
Ritsma, L., Vrisekoop, N. & van Rheenen, J. In vivo imaging and histochemistry are combined in the cryosection labelling and intravital microscopy technique. Nat. Commun. 4, 2366 (2013).
Scheele, C. L. G. J., Maynard, C. & van Rheenen, J. Intravital insights into heterogeneity, metastasis, and therapy responses. Trends Cancer 2, 205–216 (2016).
Condeelis, J. & Segall, J. E. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer 3, 921–930 (2003).
Ellenbroek, S. I. J. & van Rheenen, J. Imaging hallmarks of cancer in living mice. Nat. Rev. Cancer 14, 406–418 (2014).
Masedunskas, A., Chen, Y., Stussman, R., Weigert, R. & Mather, I. H. Kinetics of milk lipid droplet transport, growth, and secretion revealed by intravital imaging: lipid droplet release is intermittently stimulated by oxytocin. Mol. Biol. Cell 28, 935–946 (2017).
Ingman, W. V., Wyckoff, J., Gouon-Evans, V., Condeelis, J. & Pollard, J. W. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev. Dynam. 235, 3222–3229 (2006).
Harper, K. L. et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 540, 588–592 (2016).
Entenberg, D. et al. Time-lapsed, large-volume, high-resolution intravital imaging for tissue-wide analysis of single cell dynamics. Methods 128, 65–77 (2017).
Scheele, C. L. G. J. et al. Identity and dynamics of mammary stem cells during branching morphogenesis. Nature 542, 313-317 (2017).
Dawson, C. A. et al. Tissue-resident ductal macrophages survey the mammary epithelium and facilitate tissue remodelling. Nat. Cell Biol. 22, 546–558 (2020).
Miller, M. J., Wei, S. H., Cahalan, M. D. & Parker, I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc. Natl Acad. Sci. USA 100, 2604–2609 (2003).
Hor, J. L. et al. Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4+ and CD8+ T cell activation to localized viral infection. Immunity 43, 554–565 (2015).
Qi, H., Egen, J. G., Huang, A. Y. C. & Germain, R. N. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science 312, 1672–1676 (2006).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Rios, A. C., Fu, N. Y., Lindeman, G. J. & Visvader, J. E. In situ identification of bipotent stem cells in the mammary gland. Nature 506, 322–327 (2014).
Sahai, E. et al. Simultaneous imaging of GFP, CFP and collagen in tumors in vivousing multiphoton microscopy. BMC Biotechnol. 5, 14 (2005).
Entenberg, D. et al. Imaging tumor cell movement in vivo. Curr. Protoc. Cell Biol. 58, 19.7.1–19.7.19 (2013).
Entenberg, D. et al. Setup and use of a two-laser multiphoton microscope for multichannel intravital fluorescence imaging. Nat. Protoc. 6, 1500–1520 (2011).
Ritsma, L. et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 507, 362–365 (2014).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Rios, A. C. et al. Intraclonal plasticity in mammary tumors revealed through large-scale single-cell resolution 3D imaging. Cancer Cell 35, 618–632.e6 (2019).
Ewald, A. J., Werb, Z. & Egeblad, M. Preparation of mice for long-term intravital imaging of the mammary gland. Cold Spring Harb. Protoc. 2011, 168–173 (2011).
Harney, A. S., Wang, Y., Condeelis, J. S. & Entenberg, D. Extended time-lapse intravital imaging of real-time multicellular dynamics in the tumor microenvironment. J. Vis. Exp. 112, e54042 (2016).
Kotsuma, M. et al. Nondestructive, serial in vivo imaging of a tissue-flap using a tissue adhesion barrier. IntraVital 1, 69–76 (2012).
Chtanova, T. et al. Real time interactive two photon photoconversion of recirculating lymphocytes for discontinuous cell tracking in live adult mice. J. Biophotonics 7, 425–433 (2014).
Ritsma, L. et al. Intravital microscopy through an abdominal imaging window reveals a pre-micrometastasis stage during liver metastasis. Sci. Transl. Med. 4, 158ra145–158ra145 (2012).
Uderhardt, S., Martins, A. J., Tsang, J. S., Lämmermann, T. & Germain, R. N. Resident macrophages cloak tissue microlesions to prevent neutrophil-driven inflammatory damage. Cell 177, 541–555 (2019).
Linde, N. et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun. 9, 21 (2018).
Jamieson, P. R. et al. Derivation of a robust mouse mammary organoid system for studying tissue dynamics. Development 144, 1065–1071 (2017).
Gonen, E. et al. Toll-like receptor 4 is needed to restrict the invasion of Escherichia coli P4 into mammary gland epithelial cells in a murine model of acute mastitis: E. coli induces LPS-independent mastitis. Cell Microbiol. 9, 2826–2838 (2007).
Halasa, T., Huijps, K., Østerås, O. & Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Quart. 29, 18–31 (2007).
Bayarmagnai, B., Perrin, L., Pourfarhangi, K. E. & Gligorijevic, B. Intravital imaging of tumor cell motility in the tumor microenvironment context. in Cell Migration: Methods and Protocols (ed. Gautreau, A.) 175–193 (Springer Science+Business Media, 2018).
Masedunskas, A., Porat-Shliom, N., Tora, M., Milberg, O. & Weigert, R. Intravital microscopy for imaging subcellular structures in live mice expressing fluorescent proteins. J. Vis. Exp. 79, e50558 (2013).
Kedrin, D. et al. Intravital imaging of metastatic behavior through a mammary imaging window. Nat. Methods 5, 1019–1021 (2008).
Shan, S., Sorg, B. & Dewhirst, M. W. A novel rodent mammary window of orthotopic breast cancer for intravital microscopy. Microvasc. Res. 65, 109–117 (2003).
Sobolik, T. et al. Development of novel murine mammary imaging windows to examine wound healing effects on leukocyte trafficking in mammary tumors with intravital imaging. IntraVital 5, e1125562 (2016).
Ritsma, L. et al. Surgical implantation of an abdominal imaging window for intravital microscopy. Nat. Protoc. 8, 583 (2013).
Meijer, E. F. J. et al. Murine chronic lymph node window for longitudinal intravital lymph node imaging. Nat. Protoc. 12, 1513–1520 (2017).
Entenberg, D. et al. A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nat. Methods 15, 73–80 (2018).
Ewald, A. J., Werb, Z. & Egeblad, M. Dynamic, long-term in vivo imaging of tumor-stroma interactions in mouse models of breast cancer using spinning-disk confocal microscopy. Cold Spring Harb. Protoc. 2011, 122137 (2011).
Hayashi, K. et al. Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res. 67, 8223–8228 (2007).
Ahmed, F., Wyckoff, J., Lin, E., Wang, W. & Wang, Y. GFP expression in the mammary gland for imaging of mammary tumor cells in transgenic mice. Cancer Res. 62, 7166–7169 (2002).
Egeblad, M. et al. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis. Model. Mech. 1, 155–167 (2008).
Pineda, C. M. et al. Intravital imaging of hair follicle regeneration in the mouse. Nat. Protoc. 10, 1116–1130 (2015).
Ricard, C. & Debarbieux, F. C. Six-color intravital two-photon imaging of brain tumors and their dynamic microenvironment. Front. Cell. Neurosci. 8, 57 (2014).
Karreman, M. A. et al. Correlating intravital multi-photon microscopy to 3D electron microscopy of invading tumor cells using anatomical reference points. PLoS ONE 9, e114448 (2014).
Karreman, M. A., Hyenne, V., Schwab, Y. & Goetz, J. G. Intravital correlative microscopy: imaging life at the nanoscale. Trends Cell Biol. 26, 848–863 (2016).
Li, J. L., Goh, C. C. & Ng, L. G. Imaging of inflammatory responses in the mouse ear skin. in Intravital Imaging of Dynamic Bone and Immune Systems: Methods and Protocols (ed. Ishii, M.) 87–107 (Springer, 2018).
Ewald, A. J., Werb, Z. & Egeblad, M. Monitoring of vital signs for long-term survival of mice under anesthesia. Cold Spring Harb. Protoc. 2011, 174–177 (2011).
Thevenaz, P., Ruttimann, U. E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 (1998).
Parslow, A., Cardona, A. & Bryson-Richardson, R. J. Sample drift correction following 4D confocal time-lapse imaging. J. Vis. Exp. 86, e51086 (2014).
Soyal, S. M. et al. Cre‐mediated recombination in cell lineages that express the progesterone receptor. Genesis 41, 58–66 (2005).
Tomura, M. et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc. Natl Acad. Sci. USA 105, 10871–10876 (2008).
Li, J. L. et al. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat. Protoc. 7, 221–234 (2012).
Krull, A., Vicar, T., Prakash, M., Lalit, M. & Jug, F. Probabilistic Noise2Void: unsupervised content-aware denoising. Front. Comput. Sci. 2, 5 (2020).
Visvader, J. E. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 23, 2563–2577 (2009).
Paine, I. et al. A geometrically-constrained mathematical model of mammary gland ductal elongation reveals novel cellular dynamics within the terminal end bud. PLoS Comp. Biol. 12, e1004839 (2016).
Williams, J. M. & Daniel, C. W. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev. Biol. 97, 274–290 (1983).
Sreekumar, A. et al. WNT-mediated regulation of FOXO1 constitutes a critical axis maintaining pubertal mammary stem cell homeostasis. Dev. Cell 43, 436–448.e6 (2017).
Mailleux, A. A. et al. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev. Cell 12, 221–234 (2007).
Acknowledgements
We thank F. Jackling for animal management and S. Devi and R. Yip for imaging assistance. We are grateful to the Walter and Eliza Hall Institute (WEHI) Center for Dynamic Imaging and WEHI Bioservices. This work was supported by the Australian National Health and Medical Research Council (NHMRC) grants #1016701, 1113133; NHMRC IRIISS; the Victorian State Government through VCA funding and Operational Infrastructure Support; and the Australian Cancer Research Foundation. C.A.D was supported by an Australian Government Research Training Program Scholarship; A.C.R. was supported by a National Breast Cancer Foundation (NBCF)/Cure Cancer Australia Fellowship; G.J.L. by NHMRC Fellowship #1078730, #1175960; J.E.V. by NHMRC Fellowships #1037230, 1102742; S.N.M. by NHMRC Fellowship #1136550.
Author information
Authors and Affiliations
Contributions
C.A.D. designed and performed experiments, analyzed data and wrote the manuscript. S.N.M. designed experiments. G.J.L provided general guidance. A.C.R and J.E.V. designed experiments and provided general guidance.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol:
Dawson, C. A. et al. Nat. Cell Biol. 546–558 (2020): https://doi.org/10.1038/s41556-020-0505-0
Supplementary information
Supplementary Information
Supplementary Discussion.
Supplementary Video 1
Six-color IVM of immune cell interactions with mammary terminal end bud cap cells during morphogenesis in puberty. IVM of a TEB in a 5 week-old K5/TetCre/Confetti mouse treated with doxycycline for 3 d at 4 weeks of age and labeled with CD45 APC antibody placed in the imaging chamber (n = 2 mice). CFP, cyan; GFP, pink; YFP, yellow; RFP, magenta; SHG, gray; CD45, orange. Related to Fig. 3c. All experiments were approved by the Walter and Eliza Hall Institute Animal Ethics Committee.
Supplementary Video 2
IVM of resident ductal macrophage behavior in mammary ducts. Animated IVM of mammary ducts in an Elf5-GFP mouse with immunolabeling by MHCII Alexa Fluor 647 antibody in the imaging chamber12. The movie cycles over a 6 h time span with images acquired every 10 min. GFP, magenta; Duct-adjacent MHCII, yellow; stromal MHCII, cyan; collagen SHG, pink. Duct-adjacent MHCII was isolated in Imaris by creating a low resolution GFP surface and masking the MHCII signal. The duct structure is 300 µm across (n = 6 mice). Related to Figure 5a. All experiments were approved by the Walter and Eliza Hall Institute Animal Ethics Committee.
Supplementary Video 3
IVM of the resident macrophage response to damage of the mammary epithelium. An animation of IVM of a mammary duct in a Cx3cr1GFP/+ mouse12. The movie cycles through time points prior to damage showing the arrangement of GFP-high ductal macrophages (yellow) around a duct, then views an optical section through ductal macrophages before and after precise photoablation at 4 h (bolt symbol). Images were acquired every 3 min (n = 3 mice). Related to Figure 5b. All experiments were approved by the Walter and Eliza Hall Institute Animal Ethics Committee.
Supplementary Video 4
IVM of single photoactivated mammary terminal end bud cells. IVM of a TEB in a 4-week-old Kaede mouse after photoconversion of single cells using the Olympus SIM scanner (n = 2 mice). Related to Figure 5c. All experiments were approved by the Walter and Eliza Hall Institute Animal Ethics Committee.
Supplementary Video 5
IVM of mammary duct basal cell division. IVM of a duct in an 8-week-old K5/TetCre/Confetti mouse after treatment with doxycycline at 4 weeks of age showing a myoepithelial cell dividing longitudinally (n = 5 mice). The duct was imaged every 10 min for 8 h and 40 min. Related to Fig. 6a. All experiments were approved by the Walter and Eliza Hall Institute Animal Ethics Committee.
Supplementary Video 6
IVM of mammary duct basal cells, example 2. IVM of a duct in a 6-week-old K5/TetCre/Confetti mouse after treatment with doxycycline at 4 weeks of age (n = 5 mice). The duct was imaged every 10 min for 4 h. All experiments were approved by the Walter and Eliza Hall Institute Animal Ethics Committee.
Supplementary Video 7
IVM of mammary terminal end bud cap cell dynamics. IVM of a TEB in a 5-week-old K5/TetCre/Confetti mouse treated with doxycycline for 3 d at 4 weeks of age. An optical slice through the outer cap layer is shown to reveal cap cell dynamics (n = 4 mice). Related to Fig. 6c. The TEB is pointing upward. All experiments were approved by the Walter and Eliza Hall Institute Animal Ethics Committee.
Supplementary Video 8
IVM of mammary terminal end bud cap cells in stereo 3D. IVM of a TEB in a 5-week-old K5/TetCre/Confetti mouse treated with doxycycline for 3 d at 4 weeks of age. The full image volume is displayed for viewing with cyan/red 3D glasses. The TEB is pointing to the left (n = 4 mice). Related to Fig. 6c-d. All experiments were approved by the Walter and Eliza Hall Institute Animal Ethics Committee.
Supplementary Video 9
IVM of mammary cap cell migration into the terminal end bud body. IVM of a TEB in a 5 week-old K5/TetCre/Confetti mouse treated with doxycycline for 3 d at 4 weeks of age. An optical slice through the center of the TEB (which is pointing to the left), showing cap cells that migrate from the cap into the TEB body. RFP, magenta (n = 4 mice). Related to Fig. 6d. All experiments were approved by the Walter and Eliza Hall Institute Animal Ethics Committee.
Supplementary Video 10
IVM of mammary terminal end bud cap cells, example 2. IVM of a TEB in a 5 week-old K5/TetCre/Confetti mouse treated with doxycycline for 3 d at 4 weeks of age (n = 4 mice). The TEB is pointing to the right. All experiments were approved by the Walter and Eliza Hall Institute Animal Ethics Committee.
Source data
Source Data Fig. 6
Statistical source data.
Rights and permissions
About this article
Cite this article
Dawson, C.A., Mueller, S.N., Lindeman, G.J. et al. Intravital microscopy of dynamic single-cell behavior in mouse mammary tissue. Nat Protoc 16, 1907–1935 (2021). https://doi.org/10.1038/s41596-020-00473-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00473-2
This article is cited by
-
Cellular heterogeneity in TNF/TNFR1 signalling: live cell imaging of cell fate decisions in single cells
Cell Death & Disease (2024)
-
Noninvasive in vivo microscopy of single neutrophils in the mouse brain via NIR-II fluorescent nanomaterials
Nature Protocols (2024)
-
Intravital imaging to study cancer progression and metastasis
Nature Reviews Cancer (2023)
-
Multiphoton intravital microscopy of rodents
Nature Reviews Methods Primers (2022)
-
Growth factor dependency in mammary organoids regulates ductal morphogenesis during organ regeneration
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.