Abstract
Cerebrospinal fluid (CSF) biomarkers are useful in the diagnosis and the prediction of progression of several neurodegenerative diseases. Among them, CSF neurofilament light (NfL) protein has particular interest, as its levels reflect neuroaxonal degeneration, a common feature in various neurodegenerative diseases. In the present study, we analyzed NfL levels in the CSF of 535 participants of the SPIN (Sant Pau Initiative on Neurodegeneration) cohort including cognitively normal participants, patients with Alzheimer disease (AD), Down syndrome (DS), frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), dementia with Lewy bodies (DLB), progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS). We evaluated the differences in CSF NfL accross groups and its association with other CSF biomarkers and with cognitive scales. All neurogenerative diseases showed increased levels of CSF NfL, with the highest levels in patients with ALS, FTD, CBS and PSP. Furthermore, we found an association of CSF NfL levels with cognitive impairment in patients within the AD and FTD spectrum and with AD pathology in DLB and DS patients. These results have implications for the use of NfL as a marker in neurodegenerative diseases.
Similar content being viewed by others
Introduction
Biomarkers of neurodegenerative diseases are key for the evaluation, differential diagnosis and follow-up of patients with cognitive impairment or dementia. In particular, three cerebrospinal fluid (CSF) biomarkers (β-amyloid 1–42 [Aβ1–42], total Tau [t-Tau] and its phosphorylated form [p-Tau]) have been extensively studied due to their high diagnostic accuracy for the diagnosis of Alzheimer disease (AD)1. Thus, the quantification of these biomarkers in the CSF is currently being implemented in clinical practice either to confirm the biochemical AD signature in the evaluation of a patient with mild cognitive impairment (MCI) or dementia or to exclude it in other dementia syndromes, such as frontotemporal dementia (FTD) or dementia with Lewy Bodies (DLB)2,3. More recently, other CSF biomarkers, such as YKL-40, the soluble β fragment of amyloid precursor protein (sAPPβ), neurogranin, glial fibrillary acidic protein (GFAP) or Neurofilament Light (NfL), have been described to be potentially informative for the discrimination of various neurodegenerative conditions, such as AD, FTD, progressive supranuclear palsy (PSP), corticobasal syndrome (CBS), or DLB4,5,6,7,8.
Neurofilaments provide structural suport to neurons. Different forms of neurofilaments exist, including NfL, which is strongly expressed in myelinated axons and physiologically secreted in small amounts in the CSF. Disruption of neurofilament organisation is one of the key characteristics of many neurological conditions, such as amyotrophic lateral sclerosis (ALS), AD, FTD or vascular dementia among others6,9,10. In addition, recent studies have shown that levels of NfL in CSF are associated with clinical progression and severity in ALS11,12,13 and in other neurodegenerative diseases due to its capacity to reflect the extent of neuro-axonal damage14,15. Recently, a large control-case study that included various neurodegenerative disorders confimed the importance of CSF NfL in the evaluation and follow-up of patients with cognitive impairment16: in particular, CSF NfL levels were increased in patients with a diagnosis of MCI, AD, FTD or ALS compared to controls, thus reflecting the intensity of neurodegenerative processes.
In the present work, we investigated CSF NfL in the SPIN cohort17, which includes a variety of neurodegenerative disorders, such as AD, FTD, ALS, DLB, PSP, CBS and subjects with Down syndrome (DS). This is the first time to our knowledge that such different phenotypes can be compared through a monocentric cohort. We compared CSF NfL levels accross these disorders and studied its association with other CSF biomarkers, with the severity of cognitive impairment and with the presence of AD pathology in DLB (DLB-AD) and DS (DS-AD). This study highlights the potential role of CSF NfL for the early diagnosis (including prodromal stages) and follow-up of DLB patients.
Results
Demographics and core CSF biomarkers
We included a total of 535 participants from the SPIN cohort, comprising 118 cognitively healthy participants and 417 patients with various neurodegenerative disorders (Table 1). Age was different among the groups (F = 79.438, p < 0.001) but there was no significant difference in the male:female ratio. As expected, frequency of APOEε4 allele was significantly higher in AD patients than in the other groups (X2 = 52.7, p = 0.001), and no differences were observed among the other groups. As expected, MMSE scores were lower in all clinical groups compared to control subjects (F = 11.972, p < 0.001).
There were differences in CSF core AD biomarkers (Aβ1–42, t-Tau and p-Tau) among the groups (Table 1). In particular, Aβ1–42 was significantly lower in all groups (F = 30.551, p < 0.001) compared to control subjects, Table 1. T-Tau was significantly increased in all groups except in DS (F = 26.863, p < 0.001) compared to control participants, Table 1. Levels of p-Tau were significantly increased in AD, DS-AD, DLB, prodDLB and CBS groups (F = 24.079, p < 0.001) compared to control participants, Table 1.
Relationship between CSF NfL and age, gender, cognitive scores or core AD biomarkers
CSF NfL levels were positively correlated with age (ρ = 0.490, p < 0.001) and were associated with sex (higher in males, t = 2.592, p = 0.01) in the entire cohort. All group comparisons and correlation analysis were thus subsequently age and sex-adjusted. In addition, as shown in Table 2, CSF NfL levels negatively correlated with Mini-Mental State Examination (MMSE) scores in control participants, AD, prodDLB and FTD groups. CSF NfL levels positively correlated with t-Tau levels in control participants, AD, ALS, DS and DS-AD. NfL and p-Tau levels were positively correlated in control participants, AD, DS and DS-AD. Aβ1–42 levels were negatively correlated with NfL in FTD group.
CSF NfL levels accross clinical groups
CSF NfL levels were elevated in all groups (with exception of DS) compared to control participants (F = 40.809, p < 0.001), Table 1 and Fig. 1.
CSF Neurofilament Light (NfL) protein levels in the SPIN cohort. Box and whisker plots of the median concentrations of CSF NfL in control participants and patients with Alzheimer Disease (AD), Down Syndrome (DS), Dementia with Lewy Bodies (DLB), Amyotrophic Lateral Sclerosis (ALS), Frontotemporal Dementia (FTD), Corticobasal Syndrome (CBS) and Progressive Supranuclear Palsy (PSP). The central black lines show the median values, regions above and below these lines show the upper and lower quartiles, respectively. Outliers (indicated with grey circles) are defined as a value that is larger than the upper quartile plus three times the interquartile range.
FTLD-related clinical syndromes (ALS, FTD, CBS, PSP)
The highest CSF NfL levels were found in the ALS group, followed by patients with CBS, PSP and FTD (Table 1 and Fig. 1). All these groups showed higher CSF NfL levels compared to control participants (p < 0.001), Table 1. We found a gradient in NfL levels in the ALS-FTD spectrum (Fig. 2) with highest levels in patients with ALS without FTD (median = 3093, IQR = [2107–4261] pg/mL) followed by those with ALS-FTD (median = 1386, IQR = [836–2731] pg/mL) p = 0.005, and those with FTD without motor neuron symptoms (median = 1240, IQR = [859–2378] pg/mL). The AUC for CSF NfL for the detection of ALS in patients with FTD was 0.705 (95% CI 0.576–0.874).
CSF Neurofilament light (NfL) protein levels in patients within the FTD-ALS spectrum. Box and whisker plots of the median concentrations of CSF NfL in control participants and patients with dementia: Alzheimer Disease (AD) and Amyotrophic Lateral Sclerosis associated or not with FTD (ALS-FTD and ALS, respectively) and Frontotemporal Dementia (FTD). The central black lines show the median values, regions above and below these lines show the upper and lower quartiles, respectively. Outliers (indicated with grey circles) are defined as a value that is larger than the upper quartile plus three times the interquartile range. **p = 0.005.
DLB patients
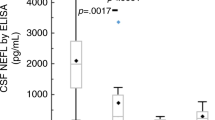
The DLB group showed higher CSF NfL levels compared to control participants (p < 0.001), Table 1 and Fig. 1. Interestingly, when comparing prodDLB and DLB patients, we observed a significant and gradual increase in CSF NfL levels in these subgroups (p = 0.01), Fig. 3A, while the levels of the core AD biomarkers (t-Tau, p-Tau and Aβ1–42) were comparable (data not shown). The AUC for CSF NfL comparing prodDLB and DLB patients was 0.694 (95% CI 0.564–0.805). Compared to control subjects, CSF NfL was increased in prodDLB and DLB groups, Fig. 3A (p < 0.001), and the AUC was 0.875 (95% CI 0.806–0.927) comparing control and prodDLB subjects and 0.944 (95% CI 0.895–0.974), comparing control and DLB patients. CSF NfL levels were elevated in DLB patients with AD pathology (DLB-AD) compared to patients with pure DLB (p = 0.020), Fig. 3B. The AUC for NfL remained lower than the AUC for core AD biomarkers to discriminate these subgroups (data not shown).
CSF Neurofilament light (NfL) protein levels in patients with Dementia with Lewy Bodies (DLB). (A) Box and whisker plots of the median concentrations of CSF NfL in control participants and patients with Dementia with Lewy Bodies (DLB) or prodromal DLB (prodDLB). The central black lines show the median values, regions above and below these lines show the upper and lower quartiles, respectively. Outliers (indicated with grey circles) are defined as a value that is larger than the upper quartile plus three times the interquartile range. (B) Box and whisker plots of the median concentrations of CSF NfL in control participants and patients with Dementia with Lewy Bodies (DLB) and DLB with AD pathology AD (DLB-AD). The central black lines show the median values, regions above and below these lines show the upper and lower quartiles, respectively. Outliers (indicated with grey circles) are defined as a value that is larger than the upper quartile plus three times the interquartile range. *p < 0.05, **p < 0.001.
Down syndrome
As previously described18, CSF NfL levels were increased in the DS-AD group compared to DS group (p < 0.001), Fig. 4 and Table 1. Interestingly, CSF NfL levels were comparable between DS-AD and sporadic AD groups, despite the age difference between groups, Fig. 4.
CSF Neurofilament light (NfL) protein levels in patients with Down Syndrome (DS). Box and whisker plots of the median concentrations of CSF NfL in control participants and patients with dementia (Alzheimer Disease, AD) and Down Syndrome associated or not to AD (DS-AD and DS, respectively). The central black lines show the median values, regions above and below these lines show the upper and lower quartiles, respectively. Outliers (indicated with grey circles) are defined as a value that is larger than the upper quartile plus three times the interquartile range. **p < 0.001.
Discussion
In the present study, we extend previously published results that highlight the importance of CSF NfL in the evaluation of neurodegenerative diseases6,9,10,11,13,14,15,16,18,19. We confirm that ALS and FTD-related syndromes show the highest CSF NfL levels followed by AD and DLB. We also confirm the positive correlation between CSF NfL and age, its association with gender (higher in male)19 and its negative correlation with MMSE in various contexts, including control participants, AD and FTD patients16. Finally, we report that in DLB patients, CSF NfL levels are influenced by the existence of comorbid AD.
Our results confirm that CSF NfL levels are increased in all neurodegenerative conditions studied compared to control participants16,19. In agreement with a recent meta-analysis that included various neurological conditions, our study confirms the overlap of CSF NfL level between various clinical conditions19, which may limit its use as a diagnostic marker in the clinical routine of cognitive impairment. In line with other studies16,20, we found that ALS patients showed the highest CSF NfL levels. As ALS and FTD are associated in a proportion of patients, we evaluated the differential CSF levels of NfL in ALS, ALS-FTD and FTD. Our results show that CSF NfL levels were increased in the three groups compared to controls, with the highest levels for ALS, followed by ALS-FTD and FTD, in agreement with previously published data16. These results are discordant with the recent meta-analysis of Bridel et al. describing the ALS-FTD group to be the clinical group with the highest CSF NfL level19. This discrepancy may be related to the variability of CSF NfL values in these clinical groups or to the differences in sample size. Although our results suggest that high CSF levels of NfL may be indicative of ALS in the context of FTD, the ROC curves showed moderate diagnostic value and its implementation in clinical routine would therefore require further confirmation. Future studies are needed to determine whether longitudinal changes in CSF NfL meaurements are useful to predict the development of motor neuron disease in patients with FTD. We also found increased CSF NfL levels in patients with CBS and PSP compared to controls, which is in agreement with previous studies6,9,16,21. We did not find correlation between MMSE scores and CSF NfL in these two groups, similarly to previously published results16. However, such results may be due to the low number of patients in each group (26 and 12, respectively) or to the lack of sensitivity of MMSE to capture cognitive impairment in these disorders.
We also report high CSF NfL levels in patients with DLB compared to controls, in accordance with a previous study19. Interestingly, patients with prodDLB within this group showed higher levels of CSF NfL compared to controls. Furthermore, patients in the dementia stage had higher levels compared to prodDLB, while levels of t-Tau, p-Tau and Aβ1–42 were similar between both groups. Thus, our results illustrate that CSF NfL levels increase early in DLB, even at prodromal stages, with a further increase in dementia stages. CSF NfL may be of potential value to diagnose prodDLB, as its diagnostic performance appeared higher than CSF core AD biomarkers. These promising results should be further confirmed in a larger cohort. In the present work, we also found higher levels of CSF NfL in DLB patients that had AD copathology compared to DLB patients with negative AD biomarkers. However, the potential added value of CSF NfL for AD pathology in the context of DLB was low (AUC < 0.7, data not shown).
In participants with DS, CSF NfL levels were associated with clinical stages. As previously published18, we found a clear and progressive increase of CSF NfL in DS patients with prodromal AD and DS-AD compared to asymptomatic DS participants. These results indicate that CSF NfL could be informative for the diagnosis of dementia in this population, where clinical assessment might be complex. Interestingly, despite the difference in age between DS-AD and sporadic AD patients, NfL levels were similar in both groups indicating a comparable degree in neuroaxonal damage in both types of AD. These results, together with the good correlation of CSF NfL levels with those in plasma found in previous studies18, highlight the potential of this biomarker in the diagnosis of dementia in the DS population.
Our work also has some limitations. First of all, the study is retrospective and the clinical protocols differed between clinical groups. Second, the work relied on clinical diagnosis and neuropathological confirmation was not available. Third, MMSE was the only cognitive scale included in this study, which may be less sensitive to capture changes in some groups, such as FTD. Finally, some groups were small and results should be validated in larger cohorts.
In summary, the present work confirms the importance of CSF NfL in the evaluation of neurodegenerative diseases. The study highlights the influence of AD co-pathology on the levels of CSF NfL in DS and DLB and shows the potential interest of CSF NfL determination for early detection of DLB, at prodromal stages of the disease. Taken together, our data show that CSF NfL levels could be a useful addition to the core AD biomarkers in the diagnostic evaluation of neurodegenerative conditions.
Material and Methods
Study participants and clinical classification
We included 535 subjects from the SPIN cohort17 evaluated at the Memory Unit at Hospital de Sant Pau between January 2009 and October 2017. We included the following diagnostic groups: Alzheimer’s Disease (AD, n = 116), Down Syndrome, without or with dementia (DS, n = 47 and DSAD, n = 50, respectively)18, dementia with Lewy Bodies (DLB, n = 37)22, prodromal DLB (prodDLB, n = 26)22, Amyotrophic Lateral Sclerosis (ALS, n = 46), Frontotemporal dementia (FTD, n = 56)4,20, corticobasal syndrome (CBS, n = 26), and progressive supranuclear palsy (PSP, n = 12). Cognitively normal control participants (n = 118) were also included in the present study. All controls had normal cognitive scores in the formal neuropsychological evaluation23 and normal core CSF AD biomarkers (see17 for further details of the SPIN cohort).
All AD patients had abnormal core AD biomarkers (low Aβ1–42 and high t-Tau or p-Tau) in the CSF based on previously published cut-offs24. FTD patients with an AD CSF profille (low Aβ1–42 and high t-Tau or p-Tau) were excluded from the present study.
Classification of DLB patients was made according to previously published data22. Briefly, patients with prodromal DLB (prodDLB) met general criteria for mild cognitive impairment25 with at least one sign of α-sinucleinopathy (visual hallucinations, parkinsonism, or REM sleep behaviour disorder (RBD))26,27,28 at the time of evaluation and had to meet criteria of probable DLB during the follow up29. Patients with DLB met consensus criteria for probable DLB29 and were evaluated using a previously reported clinical protocol, as previously described17,22. DLB patients with suspected AD copathology were defined according to the ratio tTau/Aβ1–42 considering values ≥0.52 as indicative of underlying AD pathology30. Patients with ALS fulfilled El Escorial revised criteria31 for probable, probable laboratory-supported or definite ALS, and were classified as ALS-FTD according to Raskovsky32 criteria.
CSF collection and analysis
CSF was obtained by lumbar puncture as previously described, collected and processed in polypropylene tubes following international recommendations33. CSF levels of core AD biomarkers (Aβ1–42, t-Tau, and phosphorylated tau) were measured using commercially available kits from FUJIREBIO-EUROPE (INNOTEST TM, catalog numbers Ref 81583 (Aβ1–42), Ref 81579 (total tau) and Ref 81581 (phosphorylated tau)), as previously described and following provider´s instructions. NfL levels were measured using a commercially available ELISA kit (NF-light, UMAN DIAGNOSTICS, Umea, Sweden,) as previously described4,20.
ApoE genotyping
DNA was extracted using standard procedures and APOE was genotyped accordingly to previously described methods34.
Statistical analysis
Because biomarker values were non-normally distributed, the nonparametric Kruskal-Wallis test and the post hoc pairwise Mann-Whitney-tests were used to assess differences between groups. Associations of NfL with other biomarkers and with MMSE score were calculated using Spearman rank correlation. All group comparaisons and correlation analysis were age and sex-adjusted. Alpha threshold was set at 0.05 and ccorrection for multiple comparisons was made with the Bonferroni procedure. X2 test was used to assess differences in APOEε4 allele frequency among groups. All tests and area under ROC curve (AUC) analysis were performed using MEDCALC (MEDCALC software ver 15.2.2).
Ethical approval and consent to participate
The study was approved by the Sant Pau Ethics Committee following the standards for medical research in humans recommended by the Declaration of Helsinki and reported to the Minister of Justice according to the Spanish law for research in people with intellectual disabilities. The protocol of the SPIN cohort was approved by the Sant Pau Ethics Committee. All participants and their legally authorised representative gave written informed consent before enrolment; all controls gave written informed consent before enrolment for their medical information to be used for purposes of scientific research in accordance with the guidelines of the local ethics committee.
References
Olsson, B. et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. The Lancet Neurology 15, 673–684 (2016).
Bibl, M. et al. Validation of amyloid-beta peptides in CSF diagnosis of neurodegenerative dementias. Mol. Psychiatry 12, 671–680 (2007).
Skillbäck, T. et al. Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 138, 2716–2731 (2015).
Alcolea, D. et al. CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology 89, 178–188 (2017).
Janelidze, S. et al. Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer’s disease. Ann Clin Transl Neurol 3, 12–20 (2016).
Skillbäck, T. et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 83, 1945–1953 (2014).
Oeckl, P., Steinacker, P., Feneberg, E. & Otto, M. Neurochemical biomarkers in the diagnosis of frontotemporal lobar degeneration: an update. J. Neurochem. 138(Suppl 1), 184–192 (2016).
Ishiki, A. et al. Glial fibrillar acidic protein in the cerebrospinal fluid of Alzheimer’s disease, dementia with Lewy bodies, and frontotemporal lobar degeneration. J. Neurochem. 136, 258–261 (2016).
Skillbäck, T., Mattsson, N., Blennow, K. & Zetterberg, H. Cerebrospinal fluid neurofilament light concentration in motor neuron disease and frontotemporal dementia predicts survival. Amyotroph Lateral Scler Frontotemporal Degener 18, 397–403 (2017).
Abu-Rumeileh, S. et al. Cerebrospinal Fluid Biomarkers in Patients with Frontotemporal Dementia Spectrum: A Single-Center Study. J. Alzheimers Dis. 66, 551–563 (2018).
Gaiani, A. et al. Diagnostic and Prognostic Biomarkers in Amyotrophic Lateral Sclerosis: Neurofilament Light Chain Levels in Definite Subtypes of Disease. JAMA Neurol 74, 525–532 (2017).
Scarafino, A. et al. Diagnostic and prognostic power of CSF Tau in amyotrophic lateral sclerosis. J. Neurol. 265, 2353–2362 (2018).
Lu, C.-H. et al. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology 84, 2247–2257 (2015).
Norgren, N., Rosengren, L. & Stigbrand, T. Elevated neurofilament levels in neurological diseases. Brain Res. 987, 25–31 (2003).
Rosengren, L. E., Karlsson, J. E., Karlsson, J. O., Persson, L. I. & Wikkelsø, C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J. Neurochem. 67, 2013–2018 (1996).
Olsson, B. et al. Association of Cerebrospinal Fluid Neurofilament Light Protein Levels With Cognition in Patients With Dementia, Motor Neuron Disease, and Movement Disorders. JAMA Neurol, https://doi.org/10.1001/jamaneurol.2018.3746 (2018).
Alcolea, D. et al. The Sant Pau Initiative on Neurodegeneration (SPIN) cohort: A data set for biomarker discovery and validation in neurodegenerative disorders. Alzheimers Dement (N Y) 5, 597–609 (2019).
Fortea, J. et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol 17, 860–869 (2018).
Bridel, C. et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurology 76, 1035 (2019).
Illán-Gala, I. et al. CSF sAPPβ, YKL-40, and NfL along the ALS-FTD spectrum. Neurology 91, e1619–e1628 (2018).
Hall, S. et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol. 69, 1445–1452 (2012).
Morenas-Rodríguez, E. et al. Different pattern of CSF glial markers between dementia with Lewy bodies and Alzheimer’s disease. Sci Rep 9, 7803 (2019).
Sala, I. et al. Diagnostic and Prognostic Value of the Combination of Two Measures of Verbal Memory in Mild Cognitive Impairment due to Alzheimer’s Disease. J. Alzheimers Dis. 58, 909–918 (2017).
Alcolea, D. et al. Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology 85, 626–633 (2015).
Winblad, B. et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 256, 240–246 (2004).
Thomas, A. J. et al. Diagnostic accuracy of dopaminergic imaging in prodromal dementia with Lewy bodies. Psychol Med 49, 396–402 (2019).
Sadiq, D. et al. Prodromal Dementia with Lewy Bodies and Prodromal Alzheimer’s Disease: A Comparison of the Cognitive and Clinical Profiles. J. Alzheimers Dis. 58, 463–470 (2017).
Génier Marchand, D. et al. How does dementia with Lewy bodies start? prodromal cognitive changes in REM sleep behavior disorder. Ann. Neurol. 83, 1016–1026 (2018).
McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100 (2017).
Alcolea, D. et al. Relationship between β-Secretase, inflammation and core cerebrospinal fluid biomarkers for Alzheimer’s disease. J. Alzheimers Dis. 42, 157–167 (2014).
Brooks, B. R., Miller, R. G., Swash, M. & Munsat, T. L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 1, 293–299 (2000).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477 (2011).
Alcolea, D. et al. Feasibility of lumbar puncture in the study of cerebrospinal fluid biomarkers for Alzheimer’s disease: a multicenter study in Spain. J. Alzheimers Dis. 39, 719–726 (2014).
Carmona-Iragui, M. et al. Cerebral amyloid angiopathy in Down syndrome and sporadic and autosomal-dominant Alzheimer’s disease. Alzheimers Dement 13, 1251–1260 (2017).
Acknowledgements
This work is supported by research grants from the Carlos III Institute of Health, Spain (grants PI11/02526, PI14/01126 and PI17/01019 to Juan Fortea, PI13/01532 and PI16/01825 to Rafael Blesa, PI15/01618 to Ricard Rojas-García, PI18/00435 to Daniel Alcolea, PI14/1561 and PI17/01896 to Alberto Lleó) and the CIBERNED program (Program 1, Alzheimer Disease to Alberto Lleó), partly funded by Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, “Una manera de hacer Europa”. This work has also been supported by a “Marató TV3” grant (20141210 to Juan Fortea, 044412 to Rafael Blesa, 20143710 to Ricard Rojas-García) and by Generalitat de Catalunya (2014SGR-0235 to Alberto Lleó, PERIS SLT006/17/125 to Daniel Alcolea and SLT006/17/00119 to Juan Fortea), BBVA Foundation (grant to A. Lleó) and a grant from the Fundació Bancaria La Caixa to Rafael Blesa. We acknowledge all the participants in this study and all the collaborators of the SPIN cohort. We also acknowledge Soraya Torres for technical assistance.
Author information
Authors and Affiliations
Contributions
D.C., A.D., L.A. designed the study, analyzed the data and wrote the manuscript. D.C., A.D., C.I.M., I.G.I., M.R.E., B.I., A.M., E.T., S.S.M., T.S.J., M.L., R.N.R., S.-M.I., S.S.B., S.A., V.L., B.B., S.S., L.S., B.O., C.J., B.R., P.J., R.G.R., F.J., L.A. contributed to the editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Delaby, C., Alcolea, D., Carmona-Iragui, M. et al. Differential levels of Neurofilament Light protein in cerebrospinal fluid in patients with a wide range of neurodegenerative disorders. Sci Rep 10, 9161 (2020). https://doi.org/10.1038/s41598-020-66090-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66090-x
This article is cited by
-
Proximity extension assay in cerebrospinal fluid identifies neurofilament light chain as biomarker of neurodegeneration in sporadic cerebral amyloid angiopathy
Alzheimer's Research & Therapy (2024)
-
Neuropathological hints from CSF and serum biomarkers in corticobasal syndrome (CBS): a systematic review
Neurological Research and Practice (2024)
-
Neurofilament-light chain quantification by Simoa and Ella in plasma from patients with dementia: a comparative study
Scientific Reports (2023)
-
Biomarkers in amyotrophic lateral sclerosis: current status and future prospects
Nature Reviews Neurology (2023)
-
Roles of the cytoskeleton in human diseases
Molecular Biology Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.