Abstract
A neural reflex mediated by the splanchnic sympathetic nerves regulates systemic inflammation in negative feedback fashion, but its consequences for host responses to live infection are unknown. To test this, conscious instrumented sheep were infected intravenously with live E. coli bacteria and followed for 48 h. A month previously, animals had undergone either bilateral splanchnic nerve section or a sham operation. As established for rodents, sheep with cut splanchnic nerves mounted a stronger systemic inflammatory response: higher blood levels of tumor necrosis factor alpha and interleukin-6 but lower levels of the anti-inflammatory cytokine interleukin-10, compared with sham-operated animals. Sequential blood cultures revealed that most sham-operated sheep maintained high circulating levels of live E. coli throughout the 48-h study period, while all sheep without splanchnic nerves rapidly cleared their bacteraemia and recovered clinically. The sympathetic inflammatory reflex evidently has a profound influence on the clearance of systemic bacterial infection.
Similar content being viewed by others
Introduction
The innate immune system plays a major role in the body’s defense against acute infections1. Initially, this involves recognition of pathogen-associated molecular patterns that trigger acute inflammatory responses2. There then follows a sequence of molecular and cellular events that work towards eliminating the invading pathogen and returning the body to a normal healthy state2.
There is strong evidence that the central nervous system actively modulates inflammatory processes3. It does this by activating at least two anti-inflammatory effector mechanisms: the hypothalamic-pituitary axis and autonomic neural pathways3. Systemic inflammation is most commonly evoked experimentally by intravenous administration of lipopolysaccharide to cause an acute, sterile endotoxaemia. In such a model in rats, the inhibitory influence of autonomic neural pathways is revealed by loss of function when the splanchnic sympathetic nerves are cut: the circulating levels of pro-inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin (IL)-6 and interferon gamma (IFN-γ), are strongly enhanced, while those of the anti-inflammatory cytokine IL-10 are decreased4,5. These changes occur in the absence of any difference in circulating glucocorticoid levels5. This coordinated anti-inflammatory action is activated endogenously as a reflex response to the inflammatory challenge itself, and is mediated by the splanchnic nerves4,5,6,7. In rats, the anti-inflammatory action lasts for at least 6 hours4. What has not yet been established, however, is how the anti-inflammatory action of this reflex affects the body’s ability to combat a bacterial infection. To this end we compared the effect of prior splanchnic nerve section (or a sham operation) on the disease course of a well characterized large animal model of septicaemia: the conscious sheep infused with a bolus of live Escherichia coli (E. coli) bacteria.
Results
E. coli bacteraemia
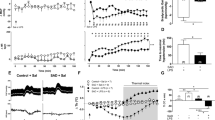
As shown previously8, infusion of live E. coli into conscious sheep caused an initial increase in mean arterial pressure that peaked around 3 h, followed by a slow decrease over the following 24 h (Fig. 1A). The mean arterial pressure in splanchnic-denervated animals tracked ~ 5–10 mmHg lower than in intact animals (Fig. 1A), as would be expected after the removal of vasomotor tone from a major vascular bed. Heart rate increased in both groups (though after an initial bradycardia in sham-operated animals; Fig. 1b). Arterial blood lactate doubled in both groups, though it peaked earlier in the splanchnic-denervated animals before declining (Table 1). All sheep developed a fever, but this was less, and shorter-lasting in the splanchnic-denervated animals (Fig. 1C). As expected from denervation of the adrenal medulla, plasma catecholamine levels, especially adrenaline, remained low after splanchnic nerve sections (Table 1). No animal in either group died before completion of the experiment.
Systemic hemodynamics in response to Escherichia coli in conscious sheep following bilateral splanchnic denervation or sham surgery. Mean arterial pressure (A), heart rate (B) and core temperature (C) after infusion of live E. coli at time 0. Data are mean ± sem. Time 0 is the mean of the 12th hour of the baseline period, and times 1.5–48 are means of 30-min periods. P values represent experimental group effects (Splanchnic or Sham denervation) from a two-way repeated measures analysis of variance from 0 to 48 h after inoculation with E. coli. *P < 0.05, **P < 0.01 and ***P < 0.001 indicates significant differences between Splanchnic denervated animals and sham-operated sheep using a Bonferroni post hoc comparison.
Plasma cytokines
As in rodents challenged with LPS derived from E. coli4,5, the response of sheep to live E. coli was characterised by a large early rise in plasma TNF-α, followed by a slower, more prolonged rise in plasma IL-6. Levels of the anti-inflammatory cytokine IL-10 followed a similar time course (Fig. 2): all returned to normal by 48 h (Fig. 2). As also established for rodents, splanchnic denervation enhanced plasma levels of TNF-α and IL-6, while reducing plasma IL-10: evidence that a coordinated anti-inflammatory reflex response had been disabled5,9.
Plasma inflammatory and anti-inflammatory cytokine and cortisol responses to Escherichia coli in conscious sheep following bilateral splanchnic denervation or sham surgery. Tumor necrosis factor α (A), interleukin-6 (B), interleukin-10 (C) and cortisol (D) after infusion of live E. coli at time 0. Data are mean ± sem. Time 0 is the mean of the 12th hour of the baseline period, and times 1.5–48 are means of 30-min periods. P values represent experimental group effects (Splanchnic or Sham denervation) from a two-way repeated measures analysis of variance from 0 to 48 h after inoculation with E. coli. *P < 0.05, **P < 0.01 and ***P < 0.001 indicates significant differences between Splanchnic denervated animals and sham-operated sheep using a Bonferroni post hoc comparison.
Bacterial clearance
Remarkably, splanchnic denervation had a profound influence on bacterial clearance (Fig. 3). In sham-operated animals, blood E. coli counts rose rapidly after infusion; peaking after 6–24 h (Fig. 3). Blood levels > 10,000 colony forming units (CFU)/ml were measured in 5 of 8 sham-operated animals. In contrast, all 7 splanchnic-denervated animals showed no, or minimal (only one sheep < 50 CFU/ml), blood E. coli counts from 1.5 h onwards (Fig. 3). This difference could not be explained by plasma cortisol levels, which were actually greater in the splanchnic-denervated animals (Fig. 2).
Blood bacterial counts in response to an infusion of Escherichia coli in conscious sheep following bilateral splanchnic denervation or sham surgery. Gram-negative colony forming units found in arterial blood after infusion of live E. coli at time 0. Data are mean ± sem. Time 0 is the mean of the 12th hour of the baseline period, and times 1.5–48 are means of 30-min periods. P values represent experimental group effects (Splanchnic or Sham denervation) from a two-way repeated measures analysis of variance from 0 to 48 h after inoculation with E. coli. *P < 0.05, **P < 0.01 and ***P < 0.001 indicates significant differences between Splanchnic denervated animals and sham-operated sheep using a Bonferroni post hoc comparison.
Circulating leukocytes
Circulating leukocytes in the two animal groups behaved similarly, except for a pronounced reduction in neutrophils of splanchnic-denervated sheep over the first 3 h (Fig. 4B). Thereafter, neutrophil levels were raised in both sets of animals. Total white cells in both groups showed an acute fall followed by a delayed rise (Fig. 4A). In both groups also, monocytes disappeared from the blood by 1.5 h; then recovered gradually over the next 24-48 h (Fig. 4C), mirroring what is seen in experimental endotoxaemia in humans10.
Total white blood cells and leukocyte subsets in response to Escherichia coli in conscious sheep following bilateral splanchnic denervation or sham surgery. Total white blood cells (A), total neutrophils (B) and total monocytes (C) after infusion of live E. coli at time 0. Data are mean ± sem. Time 0 is the mean of the 12th hour of the baseline period, and times 1.5–48 are means of 30-min periods. P values represent experimental group effects (Splanchnic or Sham denervation) from a two-way repeated measures analysis of variance from 0 to 48 h after inoculation with E. coli. *P < 0.05, **P < 0.01 and ***P < 0.001 indicates significant differences between Splanchnic denervated animals and sham-operated sheep using a Bonferroni post hoc comparison.
Discussion
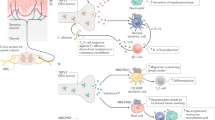
Here we report that reflex neural influences, mediated by the splanchnic sympathetic nerves, have a profound effect on the resolution of sublethal E. coli bacteraemia. Animals with cut splanchnic nerves mounted a stronger acute inflammatory response than animals with intact nerves, characterised by higher circulating levels of the pro-inflammatory cytokines, TNF-α and IL- 6, and lower levels of the anti-inflammatory cytokine IL-10. This coordinated pro-inflammatory shift matched that seen in endotoxaemic rats after acute splanchnic nerve section4,9, indicating a basic biological response that transcends species. The greater pro-inflammatory response in splanchnic-denervated animals most likely contributed to their enhanced ability to clear bacteraemia. In the present study, plasma cortisol levels were not lower in the splanchnic-denervated animals (in fact they were higher), so this factor could not account for their stronger inflammatory response to a systemic E. coli infection. A direct neural anti-inflammatory action is thus implicated, and the present findings show its importance in the context of an acute systemic infection.
Despite the removal within minutes of bacteria from the blood of the splanchnic-denervated animals, their enhanced inflammatory cytokine profile outlasted the presumed stimulus by about a day (Fig. 2). We may therefore expect the consequences of this shift for innate immune function to last for at least that long. Interestingly, the greater inflammatory cytokine response in sheep with cut splanchnic nerves did not result in a higher fever. In fact, fever was lower and briefer. This shows that the fever in these animals was not driven primarily by circulating TNF-α or IL-6. It seems likely that the persistent bacteraemia in sham-operated animals was a major factor driving their enhanced, prolonged fever, but unmeasured cytokines could have contributed. In this context, we may note the finding in rats that lipopolysaccharide-stimulated levels of the major pyrogenic cytokine, IL-1ß11 were actually reduced in animals with cut splanchnic nerves4, (against the pattern for proinflammatory cytokines).
Flow cytometry revealed one important difference between animals with and without splanchnic nerves. In the first 3 h after infection, there was a pronounced fall in circulating neutrophils (reduced percentage of a reduced white cell count) that was deeper in splanchnic-denervated sheep. That fall likely reflects removal of activated neutrophils from the circulating pool as a result of adhesion to endothelial cells12. The stronger inflammatory cytokine response of splanchnic-denervated animals evidently enhanced this process. Additionally, the lack of circulating adrenaline in these animals might have contributed to the difference, by removing beta receptor-mediated inhibition of neutrophils13. Thereafter, circulating neutrophil levels rose as expected in a bacterial infection, and did so with a similar trajectory in the two sets of animals. Monocytes also disappeared rapidly from the circulating pool to peripheral tissues, although their loss and subsequent replenishment were not different between splanchnic-denervated and sham-operated sheep.
The lower blood pressure of splanchnic-denervated animals in the face of vasodilator cytokines was expected, given that a major vascular bed (the splanchnic) had been disconnected from its neural vasomotor drive, preventing compensatory vasoconstriction. The hypotension seems unlikely to be a cause of improved bacterial clearance. The differences in heart rate between the two animal groups were not expected. The early bradycardia seen in the sham-operated sheep was almost certainly a vagal baroreflex response to the early peak in blood pressure. Yet this was absent from the denervated sheep. It seems most likely that some factor(s) (e.g. their stronger inflammatory cytokine profile) suppressed their cardiac vagal tone and vagal baroreflex. This then allowed the increased sympathetic drive to the heart (which we have measured directly in this ovine model8) to dominate. Such suppression has been reported to occur in humans injected with low doses of lipopolysaccharide14, although not in rats15.
We previously demonstrated in rats that the splanchnic nerve-mediated anti-inflammatory action is distributed across the abdominal organs innervated by that sympathetic nerve, including the spleen, intestines, stomach, pancreas, liver and adrenal medulla16. While the splanchnic nerves drive the release of catecholamines from the adrenal medulla17, the reflex survives adrenalectomy, indicating that direct release of neurotransmitters from sympathetic nerve terminals suppresses inflammation16. We infer that removal of the braking influence of the splanchnic nerves enhances the capacity to sequester and kill circulating bacteria by phagocytic and cytotoxic cells distributed broadly in the abdominal viscera. In addition, we infer that the stronger circulating pro-inflammatory cytokine profile of denervated animals would have enhanced phagocytosis by cells distant from nerves, such as neutrophils12. Their greater early sequestration from the circulating pool likely reflects this.
Strengths and limitations
In the present study, the disappearance of live bacteria from the circulation in splanchnic-denervated sheep was extremely rapid (within 1.5 h). It should be borne in mind that these animals had all experienced a month without functional splanchnic nerves, and consequently a lack of circulating adrenaline, which might have affected their inflammatory responsiveness. This seems unlikely to be the main explanation, however, because the same pattern of enhanced TNF-α and IL-6 with attenuated IL-10 responses to inflammatory stimulation was seen in rats after acute splanchnic nerve section5,9.
Caution is needed before extrapolating from these findings. The present study used a single bolus infusion of live bacteria, rather than a sustained infection leading to sepsis with end organ damage18,19,20; the optimal balance between pro- and anti-inflammatory host responses may be different between those conditions, and that difference may be time-dependent. Also, this study only investigated systemic infection with one Gram–negative bacterial species, albeit one that is the commonest cause of Gram-negative sepsis in humans; different rules may govern how nerves interact with immune responses to other infectious agents such as viruses, fungi or Gram-positive bacteria21, or when the inflammation is localised rather than systemic22.
Significance
We speculate that this neurally-mediated action is relevant in clinical sepsis, where an initial hyperinflammatory state is typically followed 1–3 days later by a phase of immunosuppression23,24. During the early hyperinflammatory phase, neural inhibition of inflammation should be helpful in reducing the damaging effects of inflammatory mediators on host tissues. But if the neural influence persists for days rather than hours, as may be expected if the infection that drives it has not been cleared, it could impede innate immune defenses against pathogens. This neural reflex may thus be a factor that contributes to the delayed phase of immunosuppression in sepsis23,24. Carefully timed interventions to block the reflex (e.g. giving β2-adrenoreceptor antagonists or local anaesthesia of the splanchnic nerves) might therefore have therapeutic benefit. It may also be worth considering prophylactic enhancement of innate immunity by reducing the neural inhibition of inflammation, for example, before surgical procedures with a high risk of infection.
Materials and methods
Animal preparation
All protocols were approved by the Animal Ethics Committee of the Florey Institute of Neuroscience and Mental Health under guidelines of the National Health and Medical Research Council of Australia. Merino ewes (35–45 kg bodyweight) were housed in individual metabolic cages with free access to 5 L of water and 800 g of oaten chaff daily.
Sheep underwent two preliminary aseptic surgical procedures under general anesthesia induced with 15 mg/kg sodium thiopental (JUROX, Rutherford, Australia) and, after intubation, maintained on 2.0–2.5% isoflurane (ISOFLO, Zoetis, Rhodes, Australia). The splanchnic sympathetic nerves on each side were exposed retroperitoneally through flank incisions. Animals in the two experimental groups were alternated to receive either bilateral splanchnic denervation (Splanchnic denervation, 9 sheep) or a sham surgical procedure (splanchnic nerves exposed but not injured; sham-operated, 8 sheep). The wounds were sutured in three layers, and animals allowed to recover, during which time no adverse effects were detected in either group. Three to four weeks later, a brief second surgical procedure prepared the animals for experimentation. In this, the left carotid artery was cannulated for measurement of arterial pressure and the left jugular vein was cannulated for infusion of live E. coli and fluids. A thermocouple was inserted into the neck alongside the left carotid artery for measurement of core temperature. Animals were given analgesia (50 mg flunixin meglumine; FLUNIXON, Norbrook, Tullamarine, Australia) and procaine penicillin (900 mg i.m. ILIUM, Troy Laboratories, Glendinning, Australia) during surgery and then 24 h and 48 h postoperatively after the first and second surgical procedures. Animals were allowed 5 days of recovery from the second surgical procedure before experimentation. A pressure transducer attached to the arterial cannula was used to record arterial pressure. Arterial pressure and core temperature (°C) were recorded on a computer using a CED MICRO 1,401 interface and Spike 2 software (Cambridge Electronic Design, Cambridge, UK). Heart rate was derived from the arterial pressure signal.
Experimental protocol
Apart from a single Splanchnic denervation animal (the first), experiments were performed on two sheep at a time: one Splanchnic-denervated paired with one Sham-operated animal. After 12 h of baseline measurements, bacteraemia was induced in conscious sheep by an intravenous bolus infusion of live E. coli (2.8 × 109 colony-forming units over 30 min). E. coli was cultured from stock derived from a human septic patient, as used in previous studies in this laboratory18,19,20,25,26. Following infusion of E. coli, all sheep received an infusion of isotonic sodium chloride (Baxter, Old Toongabbie, NSW, Australia; 1 mL−1 kg−1 h−1) as fluid supplementation. Arterial pressure and core temperature were monitored over the following 48 h. No antibiotics or catecholamines were administered. At the end of the experiments, animals were killed with an intravenous overdose of sodium pentobarbital (LETHABARB, Virbac, Wetherill Park, Australia).
Blood samples
Arterial blood samples were collected at the end of the 12 h baseline period and then at 1.5, 3, 6, 24, and 48 h following the infusion of live E. coli. Measurements were made of plasma cytokines, cortisol, catecholamines, blood gases, blood bacterial cell counts, and total white blood cell counts; flow cytometry analysis was also performed. Plasma TNF-α was measured using a commercially available enzyme-linked immune-sorbent (ELISA) kit (Kingfisher Biotech Inc., MN, USA). Plasma IL-6 and IL-10 levels were assayed using in-house ELISAs, as previously described19,20,26. Plasma cortisol, adrenaline and noradrenaline concentrations were measured using commercially available ELISA kits (Abnova, Taipei City, Taiwan). Arterial blood gases and lactate were determined using a radiometer (ABL systems 625, Copenhagen, Denmark). Total white cell counts in blood were determined using a HEMOCUE (Radiometer Pacific P/L, Mt Waverley, Vic., Australia).
For flow cytometry, 100µL of heparinised whole blood was lysed with 1 mL of red cell lysis buffer for 10 min at room temperature. Cells were washed three times with 2 mL FACS buffer (2% heat inactivated horse serum, 2 mM EDTA in PBS) and centrifuged at 600 xg for 5 min at 4 °C. All samples were prepared for flow cytometry by resuspending 3 × 106 cells in 25 µL FACS buffer and then adding 25µL of surface marker antibody mixes (made up at 2 ×) for 30 min on ice. Following staining, cells were washed twice with 2 mL FACS buffer and centrifuged at 600×g for 5 min at 4 °C and resuspended in 150 µL FACS buffer for acquisition. The antibodies used were anti–MHC class II (MHC II)–Pacific Blue (clone 49.1; locally produced), anti-CD14-A700 (clone TUK4), anti-CD172a (SIRPα) (clone DH59B), anti-CD16 FITC (clone KD1), anti-CD8 FITC (clone 38.65), anti-CD4 A647 (clone 44.38) anti-CD5R (clone 20.96), and anti-mouse IgG1 coupled to phycoerythrin. Propidium iodide was used as a viability dye. Flow cytometry was performed using a BD LSRFortessa X-20 and analysed using FlowJo software v10.6. Monocytes were defined as MHCIIint, SIRPα+, CD14+, Neutrophils were defined as MHCIIlow SIRPα+ SSChigh, and lymphocytes as FSClow, SSClow, SIRPα- cells, based on our previous work and phenotypes reported in the literature for ovine immune cells27,28.
Enumeration of E. coli in the blood was determined using a colony-forming assay29. Blood samples were serially diluted 1 in 10 in sterile PBS. A total volume of 1.0–1.6 ml of undiluted and diluted blood was spotted in 20 μl drops onto Luria–Bertani agar plates incubated in air for up to 48 h at 37 °C. Colonies were counted and expressed as CFU/ml.
Statistical analysis
Data are expressed as mean ± standard error of the mean. The number of observations for each variable (n) is shown in each table or figure, respectively. Mean arterial pressure, heart rate and core temperature are presented as absolute values as 30-min averages over the 12th hour of baseline and at pre-defined time intervals after induction of bacteraemia. After a two-way repeated measures analysis of variance, post hoc comparisons were made by the Bonferroni test. Factors were experimental group and time. Analyses were performed using GraphPad PRISM (GraphPad Software, La Jolla, CA, USA). P values from within-subjects were conservatively adjusted using the Greenhouse–Geisser method. Two-sided P ≤ 0.05 was considered statistically significant.
References
Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007).
Mogensen, T. H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22, 240–273 (2009).
Sternberg, E. M. Neural-immune interactions in health and disease. J. Clin. Invest. 100, 2641–2647 (1997).
Martelli, D., Yao, S. T., Mancera, J., McKinley, M. J. & McAllen, R. M. Reflex control of inflammation by the splanchnic anti-inflammatory pathway is sustained and independent of anesthesia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R1085-1091 (2014).
Martelli, D., Yao, S. T., McKinley, M. J. & McAllen, R. M. Reflex control of inflammation by sympathetic nerves, not the vagus. J. Physiol. 592, 1677–1686 (2014).
Martelli, D., McKinley, M. J. & McAllen, R. M. The cholinergic anti-inflammatory pathway: a critical review. Autonom. Neurosci. Basic Clin. 182, 65–69 (2014).
Martelli, D., Yao, S. T., McKinley, M. J. & McAllen, R. M. Neural control of inflammation by the greater splanchnic nerves. Temperature 1, 14–15 (2014).
Ramchandra, R. et al. Septic shock induces distinct changes in sympathetic nerve activity to the heart and kidney in conscious sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1247–R1253 (2009).
Komegae, E. N. et al. Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain Behav. Immun. 73, 441–449 (2018).
Patel, A. A. et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 214, 1913–1923 (2017).
Dinarello, C. A., Gatti, S. & Bartfai, T. Fever: links with an ancient receptor. Curr. Biol. 9, R143–R146 (1999).
Wright, H. L., Moots, R. J., Bucknall, R. C. & Edwards, S. W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 49, 1618–1631 (2010).
Elenkov, I. J., Wilder, R. L., Chrousos, G. P. & Vizi, E. S. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 52, 595–638 (2000).
Sayk, F. et al. Endotoxemia causes central downregulation of sympathetic vasomotor tone in healthy humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R891-898 (2008).
Rogausch, H., Vo, N. T., Del Rey, A. & Besedovsky, H. O. Increased sensitivity of the baroreceptor reflex after bacterial endotoxin. Ann. N. Y. Acad. Sci. 917, 165–168 (2000).
Martelli, D., Farmer, D. G. S., McKinley, M. J., Yao, S. T. & McAllen, R. M. Anti-inflammatory reflex action of splanchnic sympathetic nerves is distributed across abdominal organs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 316, R235–R242 (2019).
Bornstein, S., Ehrhart-Bornstein, M., Scherbaum, W., Pfeiffer, E. & Holst, J. Effects of splanchnic nerve stimulation on the adrenal cortex may be mediated by chromaffin cells in a paracrine manner. Endocrinology 127, 900–906 (1990).
Di Giantomasso, D., May, C. N. & Bellomo, R. Norepinephrine and vital organ blood flow during experimental hyperdynamic sepsis. Intens. Care Med. 29, 1774–1781 (2003).
Lankadeva, Y. R., Kosaka, J., Evans, R. G., Bellomo, R. & May, C. N. Urinary oxygenation as a surrogate marker of medullary oxygenation during angiotensin II therapy in septic acute kidney injury. Crit. Care. Med. 46, e41-48 (2018).
Lankadeva, Y. R. et al. Intra-renal and urinary oxygenation during norepinephrine resuscitation in ovine septic acute kidney injury. Kidney Int. 90, 100–108 (2016).
Worlicek, M. et al. Splanchnic sympathectomy prevents translocation and spreading of E. col but not S. aureus in liver cirrhosis. Gut 59, 1127–1134 (2010).
Seeley, E. J., Matthay, M. A. & Wolters, P. J. Inflection points in sepsis biology: from local defense to systemic organ injury. Am. J. Physiol. Lung. Cell. Mol. Physiol. 303, L355-363 (2012).
Hotchkiss, R. S., Monneret, G. & Payen, D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13, 862–874 (2013).
Venet, F. & Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 14, 121–137 (2018).
Lankadeva, Y. R., Kosaka, J., Evans, R. G. & May, C. N. An ovine model for studying the pathophysiology of septic acute kidney injury. Methods Mol. Biol. 1717, 207–218 (2018).
Lankadeva, Y. R. et al. Dexmedetomidine reduces norepinephrine requirements and preserves renal oxygenation and function in ovine septic acute kidney injury. Kidney Int. 96, 1150–1161 (2019).
Neeland, M. R., Elhay, M. J., Nathanielsz, J., Meeusen, E. N. T. & de Veer, M. J. Incorporation of CpG into a liposomal vaccine formulation increases the maturation of antigen-loaded dendritic cells and monocytes to improve local and systemic immunity. J. Immunol. 192, 3666–3675 (2014).
Neeland, M. R. et al. The lymphatic immune response induced by the adjuvant AS01: a comparison of intramuscular and subcutaneous immunization routes. J. Immunol. 197, 2704–2714 (2016).
Miles, A. A., Misra, S. S. & Irwin, J. O. The estimation of the bactericidal power of the blood. J. Hyg. 38, 732–749 (1938).
Funding
This study was supported by a grant from the National Health and Medical Research Council of Australia (NHMRC, APP1186382). YRL was supported by a Future Leader Fellowship by the National Heart Foundation of Australia (NHF; 101853).
Author information
Authors and Affiliations
Contributions
The following author contributions were made: Designed study: Y.L., C.M., M.M., D.M., R.M. Performed experiments: Y.L., C.M., S.M. Analysed Bacteria: D.H., R.R.B. Cytometry analyses: M.N. Blood analyses: Y.L., D.F., S.R.B. Interpreted Results: Y.R., C.M., M.M., R.R.B., S.B., D.M., R.M. Wrote the paper: Y.R., M.M., R.M. Edited and modified the paper: Y.R., C.M., M.M., D.H., R.R.B., S.B., D.M., R.M. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lankadeva, Y.R., May, C.N., McKinley, M.J. et al. Sympathetic nerves control bacterial clearance. Sci Rep 10, 15009 (2020). https://doi.org/10.1038/s41598-020-72008-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72008-4
This article is cited by
-
Splanchnic sympathetic nerve denervation improves bacterial clearance and clinical recovery in established ovine Gram-negative bacteremia
Intensive Care Medicine Experimental (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.