Abstract
Kava is a beverage made from the ground roots of the plant Piper Methysticum. Active compounds of Kava have previously been demonstrated to exert an antiproliferative effect through cell cycle arrest and promotion of apoptosis. Our aim was to investigate the in vitro effects of the main constituents derived from Kava on oral squamous cell carcinoma (OSCC) activity. Gas chromatography mass spectrometry (GCMS) was used to characterise the main constituents of two Kava preparations. Cell proliferation was assessed in two human OSCC cell lines (H400 and BICR56) and in normal oral keratinocytes (OKF6) treated with the identified Kava constituents, namely Flavokawain A (FKA), Flavokawain B (FKB), yangonin, kavain and methysticin using an MTS in vitro assay. Cell migration at 16 h was assessed using a Transwell migration assay. Cell invasion was measured at 22 h using a Matrigel assay. Cell adhesion was assessed at 90 min with a Cytoselect Adhesion assay. The two Kava preparations contained substantially different concentrations of the main chemical constituents. Treatment of malignant and normal oral keratinocyte cell lines with three of the identified constituents, 10 μg/ml FKA, 2.5 μg/ml FKB and 10 μg/ml yangonin, showed a significant reduction in cell proliferation in both H400 and BICR56 cancer cell lines but not in normal OKF6 cells. Remarkably, the same Kava constituents induced a significant reduction of OSCC cell migration and invasion. We have demonstrated, for the first time, that Kava constituents, FKA, FKB and yangonin have potential anticancer effects on OSCC. This highlights an avenue for further research of Kava constituents in the development of future cancer therapies to prevent and treat OSCC.
Similar content being viewed by others
Introduction
Oral cancer is the sixth most common malignancy in the world1 with oral squamous cell carcinoma (OSCC) encompassing about 90% of oral cancers2. Treatment of OSCC includes single modality surgery, radiotherapy or various combinations of these modalities with or without systemic therapy (chemotherapy and/or target agents).
Kava is a beverage made from the ground roots of the plant Piper Methysticum and has long held a significant place within Pacific Island communities (Fig. 1). Based on pottery and language evolution, Kava’s consumption likely dates back more than 2500 years, being used as a medicinal, social, as well as ceremonial beverage3. The beverage is still a popular recreational drink in Pacific Island countries akin to alcohol in western societies, inducing sedative, anaesthetic, and euphoriant effects. Conversely, Kava usage in regions of Australia and New Zealand can be attributed to the migration of Pacific Islander communities4. In 1982 Kava was introduced as an alcohol alternative to Australian Aboriginal communities of Arnhem Island in an attempt to reduce alcohol-related harm5,6,7. Reports of Kava hepatoxicity risk have been related to excessive consumption8. Studies assessing the hepatotoxicity of Kava, have identified a number of potentially contributing factors. These include the type of Kava cultivar used, impurities, and other parts of Kava used (stem and leaves) in addition to the rhizome9. Kava hepatotoxicity has also been associated with metabolic aberration in a few individuals10. Additionally, mouldy and non-mouldy contaminants in Kava extracts, and other impurities, have been suggested as potential causes of toxicity11. While hepatoxicity is a debateable topic and effects are not clear12, here we analyse the effect of single active molecules as opposed to the entire Kava extract. Active compounds extracted from Kava, and secondary metabolites, include kavalactones, chalcones, cinnamic acid derivatives and flavanones.
Cancer cells have well-established characteristics that include dysregulated proliferation, resistance to apoptosis, evasion of growth suppressors and angiogenesis activation, resulting in replicative immortality for sustained metastasis13. The ability of individual Kava compounds to inhibit such mechanisms has been researched. A recent systematic review published by our group8 identified key chalcones, such as Flavokawain B (FKB), possessing the ability to induce apoptosis, inhibit proliferation, and interfere with metastasis within multiple cancer cells, in vitro and in vivo. Flavokawain A (FKA), a separate chalcone, was also shown to induce cell cycle arrest and apoptosis in bladder and breast cancer cells14,15.
Our recent review also identified that research into Kava’s anti-cancer effects on OSCC is limited. The study by Hseu et al.16 was the only one to investigate the anti-cancer components of Kava in OSCC cells, specifically exploring the chemo-preventive effect of FKB on two human tongue OSCC cell lines.
Our current study aimed to investigate the main chemical constituents of two Kava mixtures, of varying origin (Fiji and Vanuatu). The study used gas chromatography mass spectrometry (GCMS) to characterize the commercially available Kava products. In particular, the GCMS testing identified five main Kava constituents, namely FKA, FKB, yangonin, kavain and methysticin. These constituents were subsequently tested in in vitro OSCC models to identify potential anticancer effects.
Our study showed that preparations of Kava from different origins may contain substantially different concentrations of lactones and dihydrochalcones. Our results from in vitro models have clearly demonstrated, for the first time, that three Kava constituents, FKA, FKB and yangonin exert anticancer effects on OSCC. Our findings show potential for the translation of these compounds from bench to preclinical animal models.
Results
Composition of Kava constituents in samples from Fiji and Vanuatu
GCMS analysis was able to successfully quantify 9 chemical components belonging to the lactone and dihydrochalcone family from both mixtures. In addition to the 9 investigated constituents 3 relatively larger peaks were identified as presented in Fig. 2B. The two most researched constituents of Kava, FKA and FKB, were present in both samples, with the commercial preparation from Vanuatu showing higher concentrations compared to traditional Fijian Kava (7.68 ± 0.83 vs 0.29 ± 0.21 g/Kg and 15.14 ± 1.05 vs 0.88 ± 0.01 g/Kg, respectively) (Table 1). Interestingly, FKC was not detected in any of the mixtures (Fig. 2). The constituents investigated in this study included 5 promising active compounds namely FKA, FKB, yangonin, methysticin and kavain. Overall, preparations of Kava from different origins contained substantially different concentrations of lactones and dihydrochalcones.
Chromatograms showing the differences in Kava constituents between two Kava samples. (A) Kava Fiji; (B) Kava Vanuatu. Peaks: (1) internal standard, methyl heptadecanoate; (2) dihydrokavain; (3) kavain; (4) desmethoxyyangonin; (5) dihydromethysticin; (6) flavokawain B; (7) yangonin; (8) methysticin; (9) flavokawain A. Flavokawain C not detected in either of the samples.
Treatment with Kava constituents; FKA, FKB and Yangonin, reduces OSCC cell proliferation
An MTS assay was performed to analyse the effect of Kava constituents; FKA, FKB, yangonin, methysticin and kavain on OSCC cells (H400, BICR56), and human oral epithelial cell line OKF6.
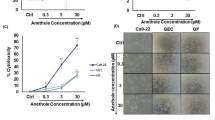
Treatment with FKA, in the H400 cell line, significantly reduced cell growth at a concentration of 10 μg/ml, at timepoint 72 h. Similar results were also noted in BICR56 cells as early as 48 h. FKA did not exert any significant reduction of cell growth in OKF6 cells. Low concentrations of FKA promoted OSCC cell growth. This was evident as early as 48 h in the H400 cell line and at 24 h in BICR56 cell line (Fig. 3).
MTS assay. the effect of 2.5 μg/ml, 5 μg/ml and 10 μg/ml of FKA, FKB, yangonin, methysticin and kavain on OKF6 (A), H400 (B) and BICR56 (C) cell proliferation. Data are represented as mean ± SD. Statistical significance is given as follows: *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001; compared to control group.
OSCC cells treated with a low concentration of FKB, 2.5 μg/ml, confirmed a significant reduction of cell growth in both the H400 and BICR56 cell lines. 2.5 μg/ml of FKB did not present any significant reduction of cell growth in OKF6 cells but did indicate a significant increase at 48 h (Fig. 3). High concentrations of FKB indicated cytotoxicity in all cell lines as early as 48 h at a concentration of 5 μg/ml and as early as 24 h at a concentration of 10 μg/ml (Fig. 3).
Yangonin, at a high concentration of 10 μg/ml significantly reduced cell growth both H400 and BICR56 cell lines as early as 48 h. Cell growth was unaffected in OKF6 cells. The BICR56 cell line treated with 2.5 μg/ml and 5 μg/ml of yangonin, experienced a significant reduction of growth as early as 24 h (Fig. 3). Whereas the H400 cell line experienced a significant reduction of growth only at 72 h when treated with 5 μg/ml. In OKF6 cell line, treatment with yangonin at a concentration of 5 μg/ml induced a significant reduction in cell growth at 72 h (Fig. 3).
Treatment with kavain, in H400 cell line, significantly increased cell growth at concentrations of 2.5 μg/ml at 48 h, 5 μg/ml at 48 h and 10 μg/ml as early as 48 h (Fig. 3). Significant cell growth was only indicated in the BICR56 cell line at a concentration of 5 μg/ml at 72 h. Similarly, 5 μg/ml of kavain in the OKF6 cell line significantly increased cell growth after 72 h (Fig. 3).
Methysticin treated BICR56 cells significantly increased growth at concentrations of 2.5 μg/ml and 10 μg/ml at 24 h. BICR56 cell line treated with 5 μg/ml, significantly increased growth at 24 and 72 h (Fig. 3). Similarly, H400 cell line treated with 5 μg/ml significantly increased growth at 72 h. OKF6 cell line treated with 10 μg/ml of methysticin, significantly increased growth at 24 and 72 h (Fig. 3). Overall, our data show that the anti-proliferative effects of Kava constituents were concentration-specific and had greater effect in OSCC cells compared to normal OKF6 cells.
Treatment with Kava constituents; FKA, FKB and Yangonin, induces a reduction in OSCC cell migration and invasion
The concentrations of Kava constituents which significantly reduced H400 and BICR56 cell growth were selected to assess cell migration and invasion in vitro. These included FKA, FKB and yangonin at concentrations of 10 μg/ml, 2.5 μg/ml and 10 μg/ml, respectively. Serum-free medium in the upper chamber and a tailored timepoint (< 24 h) was used to minimise the effect of cell proliferation. Both the H400 and BICR56 cell lines showed a significant reduction in cell migration at 16 h when treated with either FKA, FKB or yangonin, compared to the control (Fig. 4). Similarly, both the H400 and BICR56 cell lines showed a significant reduction in cell invasion at 22 h when treated with either FKA or yangonin, compared to the control. However, FKB demonstrated a significant reduction in the ability to invade for only the BICR56 cell line (Fig. 5).
Migration assays. migrated cell count of H400 and BICR56 cells incubated with 10 μg/ml FKA, 2.5 μg/ml FKB and 10 μg/mL yangonin. The results were representative of 3 independent experiments. The representative microscopic fields showed (digital white light microscopy, 460 × magnification) are H400 and BICR56 migrated cells at 16 h when treated with no treatment (control), 10 μg/ml FKA, 2.5 μg/ml, FKB and 10 μg/mL yangonin. Data are represented as mean ± SD. Statistical significance is given as follows: *p < 0.05; **p < 0.005; compared to control group.
Invasion assays. invaded cell count of H400 and BICR56 cells incubated with 10 μg/ml FKA, 2.5 μg/ml FKB and 10 μg/mL yangonin. Images were taken after 22 h at × 20 magnification (optical white light microscopy) showing fewer invading cells for treated groups compared to the control (representative fields). Graphs show quantification of invasion assays. The results were representative of 3 independent experiments. Data are represented as mean ± SD. Statistical significance is given as follows: *p < 0.05; compared to control group.
Treatment with Kava constituents; FKA, FKB and Yangonin, does not alter OSCC adhesion
Selective focal adhesion of H400 and BICR56 to ECM molecules was not significantly affected when incubated with Kava constituents; FKA, FKB and yangonin, in comparison to the control. ECM molecules tested were fibronectin, collagen I, collagen IV, laminin I or fibrinogen (Fig. 6).
Discussion
In the present study, we demonstrate that treatment of OSCC cells with Kava constituents; FKA, FKB and yangonin, affects cell proliferation, migration and invasion. Literature has presented constituents of Kava as having chemotherapeutic potential in cancer prevention and treatment. Our results are the first to demonstrate the possible use of Kava constituents in the treatment of OSCC.
Literature indicates FKA to induce apoptosis14,15,17,18,19 and inhibit proliferation14,17,18,19,20. Additional anticancer findings involve the prevention of metastasis, inhibition of angiogenesis, reduction in inflammation and enhancement of immune function14,17,19. In the present study, we show that treatment of FKA affects OSCC cell proliferation. Cells treated with low concentrations of FKA exhibited a promoting effect of cell proliferation in both cancer cell lines. However, at 10 μg/ml FKA exerted an anti-proliferative effect, where the same concentration did not affect human normal oral epithelial OKF6 cells. This finding has salient clinical implications as it suggests that the effects of FKA are specific to oral malignant cells. Further investigation into 10 μg/ml FKA demonstrated a reduction in ability of OSCC migration and invasion and thus a potential to reduce malignant hallmarks of cancer. Our data show that 10 μg/ml of FKA is a targetable concentration for treatment of oral carcinogenesis.
The literature shows FKB to have an antiproliferative ability13,15,16,21,22,23,24,25,26,27 and to induce apoptosis13,16,20,21,22,23,24,25,27,28,29,30,31,32. Similar to FKA, studies have indicated the prevention of metastasis13,28,33, anti‐angiogenic effects13,33, as well as regulation of immune and inflammatory functions28. Our study has demonstrated high concentrations of FKB to be cytotoxic to all cell lines. However, low concentrations of 2.5 μg/ml of FKB were cytotoxic to OSCC cell lines H400 and BICR56 but had no effect on human oral epithelial cell line OKF6. Additional investigation into this concentration of FKB demonstrated a reduction in ability of OSCC migration and cancer cell dependent reduction in invasion. This indicates that 2.5 μg/ml of FKB may be a promising anti-cancer target as it did not affect normal OKF6 cells.
Yangonin, a minimally researched component of Kava, has demonstrated variable findings in the literature to inhibit growth34,35. Various studies of conflicting results motivated us to investigate the anti-cancer properties of yangonin. The results of our study are the first to identify the cytotoxicity of high concentrations of yangonin (10 μg/ml) in OSCC cells. Low concentrations are cancer cell dependent. Similar to FKA, assessment of 10 μg/ml of yangonin demonstrated a reduction in ability of OSCC migration and invasion and thus a potential to reduce malignant hallmarks of cancer. Our data demonstrates that yangonin is a targetable Kava constituent for treatment of oral carcinogenesis, however, further tests are required on additional cancer cell types.
The limited research on kavain’s anti-cancer properties showed conflicting results. Various studies have shown its ability to reduce cellular growth of cancer cells34,36, while others have demonstrated minimal or lack of ability to effect proliferation or apoptosis20. These contrasting findings were found on bladder cancer cells, where the epithelial lining of the bladder shares similarities to the lining of the oral cavity. Our in vitro model of OSCC, treated with kavain, stimulates cancer cell proliferation in both cell lines at all concentrations. It did not however affect cell proliferation in human oral epithelial cell line OKF6. Kavain is therefore a potential cancer promoting agent.
Methysticin, similar to yangonin, has limited research examining its anticancer properties. Although studies suggest methysticin may have anticancer properties, the concentrations tested and reported potency were variable between studies33,34,37. The variability between studies indicates the need for further research. Our in vitro model of OSCC, treated with methysticin, indicated a stimulation of cancer cell proliferation which was potentially cancer cell specific. Cell proliferation was also observed in human oral epithelial cell line OKF6. Kava constituent methysticin should be tested on additional cancer cell types.
In conclusion, we have shown that preparations of Kava from different origins may contain substantially different concentrations of lactones and dihydrochalcones. Kava constituents; FKA, FKB and yangonin reduce cell proliferation in a time, dose-specific manner in both OSCC cell lines tested but not in normal oral keratinocytes. Additionally, reduction of cell migration of FKA, FKB and yangonin appears to be dose specific but irrespective of OSCC cell line tested. This was also evident in the reduction of cell invasion of FKA and yangonin. The reduction of cell invasion of yangonin appears to be dose and cell line specific. FKA, FKB and yangonin appear to have no effect on cell adhesion to ECM proteins. We have demonstrated, for the first time, that Kava constituents; FKA, FKB and yangonin have potential antiproliferative effects on OSCC with a difference in normal and malignant tissues. These Kava constituents show promising results for the treatment of oral carcinogenesis and a translation into in vivo studies. Further research into the OSCC protein binding and the mechanisms of action are encouraged.
Methods
All experiments were performed in accordance with relevant guidelines and regulations as approved by the Research Ethics Committee of the University of Melbourne (Project 1340716.1).
Gas chromatography mass spectrometry (GCMS)
Kava samples were prepared for lactone and dihydrochalcone analysis based on published protocols with modifications38,39. For each Kava sample, 100 mg of powdered Kava sample was sub-sampled, and extracted three times with 3 ml of acetone by sonication for 30 min. The extracts were combined and adjusted to 10 ml in volumetric flasks and filtered through a 0.45 µm membrane. 0.9 ml of sample was transferred to a 2 ml Gas Chromatography (GC) vial and mixed with 20 µl of methyl heptadecanoate as an internal standard (IS) (1.7 mg/ml in acetone). All samples were prepared in triplicates.
An Agilent Technologies 6850 series II GC system (Agilent Technologies, Santa Clara, CA) connected to an Agilent Technologies 6973 mass selective detector (MSD) and coupled with an Agilent PAL multipurpose autosampler were used for volatile analysis. The instruments were controlled using Agilent G1701EA MSC ChemStation software in conjunction with Agilent PAL autosampler Control software B.01.04 for ChemStation. The GC was fitted with a J&W DB-5 ms column (30 m × 0.25 mm, 0.25 µm film df, with helium as carrier gas (Ultrahigh Purity, BOC Australia, North Ryde, NSW, Australia). The GC inlet was fitted with a borosilicate glass split inlet liner (volume 935 µl, Agilent Technologies) and held at 250 °C. 1 µl of sample was injected into the inlet in split mode at a split ratio of 30:1, and a constant column flow rate of 1 ml/min. The column was held at 50 °C for 6 min before increasing to 280 °C at 5 °C/min, held at 280 °C for 5 min, and post run at 50 °C for 1 min. The MS source, quadruple and transfer line were held at 230 °C, 150 °C, and 280 °C, respectively. The MS was operated in scan mode (35–350 m/z) with positive EI of 70 eV. The standard solution of dihydrokavain, kavain, desmethoxyyangonin, dihydromethysticin, yangonin and methysticin were prepared at 6 scale dilutions from 20 to 1000 mg/L in acetone, while flavokawain A, flavokawain B and flavokawain C were prepared at 7 scale dilutions from 10 to 1000 mg/L in chloroform. All standard solutions were spiked with internal standard and analyzed as sample to generate standard curves (R2 > 0.99). Kavalactones and dihydrochalcones were identified by comparing the mass spectra and retention indices with the NIST library in ChemStation and Webbook database and the standard solutions obtained. All compounds were quantified based on the standard curves using target ions. Blank and internal standards were checked regularly to ensure the sensitivity of GCMSD system.
Cell lines
Two human malignant oral keratinocyte cell lines derived from different intra-oral sites, H400 and BICR56, and one normal human oral keratinocyte cell line, OKF6 were selected for this study. The OSCC adherent cell lines were established at Bristol Dental School, University of Bristol, UK40, from primary explants of tongue (BICR56) and alveolar process (H400) squamous cell carcinoma. All OSCCs were HPV-negative and were authenticated prior to commencing the experiments.
Culture conditions
The OSCC cell lines were cultured as previously described41. Cell lines were cultured in 100-mm Petri plastic dishes (Corning 430167) and grown to 60%-80% confluence before being further sub-cultured. Cells were cultured using Dulbecco's modified Eagle's medium (DMEM) (D5796) and nutrient mixture F-12 Ham (N6658) in a 1:1 ratio (Sigma-Aldrich, Australia), supplemented with 10% foetal bovine serum (FBS) (SFBS-F, Bovogen, Keilor East, Vic, Australia), 1% penicillin streptomycin mixture (P4333, Sigma-Aldrich, Castle Hill, NSW, Australia) and 0.5 μg/mL hydrocortisone (HC) (H6909, Sigma-Aldrich, Castle Hill, NSW, Australia) in a humidified atmosphere at standard conditions (5% CO2, 37 °C). The normal human oral mucosal epithelial cell line OKF6, was instead cultured in 100-mm Petri plastic dishes (Corning 430167, Corning, NY, USA) and grown to 60–80% confluence before being further sub-cultured. OKF6 cells were cultured using keratinocyte serum-free medium (K-SFM) (#17005‐042, Thermo Fisher Scientific) containing 0.4 μg/ml bovine pituitary extract and 0.2 ng/ml human recombinant epidermal growth factor (as per manufacturer’s instructions), 0.3 mM CaCl2, 1% penicillin streptomycin mixture (P4333, Sigma-Aldrich, Castle Hill, NSW, Australia) and supplemented with 1% Newborn Calf Serum (NCS) (N4637, Sigma-Aldrich, Castle Hill, NSW, Australia). OKF6 cells were incubated at 37 °C, 5% CO2 for 5 to 7 days to reach 80% confluency.
Epithelial cells grown to 80% confluency were subsequently detached via a pre-treatment of 10 mM EDTA for 10 min, followed subsequently with incubation with a 0.25% trypsin in 1 mM EDTA solution (T4049, Sigma-Aldrich, Castle Hill, NSW, Australia) for 5 min. The viability of the keratinocytes was confirmed by trypan blue exclusion (trypan blue dye, 0.4% solution, 1450021, Bio-Rad)41.
Kava constituents
FKA, FKB, yangonin, methysticin and kavain (LKT Laboratories, USA) were all reconstituted in Dimethyl Sulfoxide (DMSO) to respective concentrations of 31.44 mg/mL, 10 mg/mL, 10 mg/mL, 10 mg/mL, and 12.5 mg/mL.
Proliferation assays
Analysis of H400, BICR56 and OKF6 cells was performed by seeding cells in 96-well plates overnight and treating with 2.5 μg/ml, 5 μg/ml and 10 μg/ml of FKA, FKB, yangonin, methysticin or kavain. Cell viability was measured using MTS cell proliferation assay kit (CellTiter 96 AQueous One Solution, G3580, Promega, Madison, USA) as per manufacturer's instructions. Absorbance was measured at 490–500 nm at timepoints 0, 24, 48 and 72 h (Wallac Victor 3, Perkin Elmer). All the experiments were performed in triplicate.
Migration assay
In vitro cell migration of H400 and BICR56 cells was assessed at 16 h using a Transwell migration assay (CLS3422, Sigma-Aldrich) as previously described41. Kava constituents FKA (10 μg/ml), FKB (2.5 μg/ml) and yangonin (10 μg/ml) were used to treat the cells in the upper chambers of the assay. 1.5 × 104 cells/well were seeded in serum-free media. A culture medium with 10% FBS without antibiotics was used in the lower chamber. After incubation in standard conditions (37 °C, 5% CO2), the medium was removed. The cells in the upper chamber were fixed in 4% formalin for 2 min at room temperature (r/t), permeabilised with 100% ice-cold methanol for 20 min at room temperature and stained (UV protected) using 2% crystal violet solution for 20 min. After the removal of the non-migrated cells, the migrated cells (5 random fields/well) were observed under a BH2 Olympus microscope equipped with a PMW-10MD Sony camera. Captured images were analysed to count migrated cells using ImageJ Software (ImageJ v. 1.50i, National Institutes of Health). All the experiments were performed in triplicate.
Invasion assay
In vitro cell invasion of H400 and BICR56 cells was assessed at 22 h using a Corning BioCoat Matrigel Invasion Chamber assay (Corning 354480) as previously described41. Kava constituents FKA (10 μg/ml), FKB (2.5 μg/ml) and yangonin (10 μg/ml) were used to treat the cells in the upper chambers of the assay. Cells were seeded with a density of 3.0 × 105 cells/well. A culture medium with 10% FBS without antibiotics was used in the lower chamber as chemoattractant. After incubation in standard conditions for 22 h, the medium was removed. The cells in the upper chamber were fixed with 100% ice-cold methanol for 2 min at room temperature and stained (UV protected) using 2% crystal violet solution for 2 min, as per manufacturer's instructions. After the removal of the non-migrated cells, the migrated cells (5 random fields/well) were observed under a BH2 Olympus microscope equipped with a PMW-10MD Sony camera. Captured images were analysed to count migrated cells using ImageJ Software (ImageJ v. 1.50i, National Institutes of Health). All the experiments were performed in triplicate.
Adhesion assay
In vitro cell adhesion assay of H400 and BICR56 cells was assessed at 90 min using a CytoSelect 48-Well Cell Adhesion Assay (Cell Biolabs, Inc., CBA-070) according to the manufacturer’s instruction. Kava constituents FKA (10 μg/ml), FKB (2.5 μg/ml) and yangonin (10 μg/ml) were used to treat the cells in a serum-free culture medium without antibiotics. 5.0 × 104 cells/well were seeded. Absorbance was measured at 540 nm (Wallac Victor 3, Perkin Elmer). All the experiments were performed in triplicate.
Statistical analysis
Quantification of kavalactones and dihydrochalcones by GCMS was compared using ANOVA and post-hoc Tukey’s test with significance determined by p < 0.05 in CoStat Software (version 6.4, CoHort software, Monterey, USA). Differences between groups in the cell proliferation/adhesion assays were analysed using two-way ANOVA test comparing time point, concentration of drug and absorbance using GraphPad Prism 8.0.1 (La Jolla, California, USA). Cell migration and invasion assay data was analysed by variables using t test, ANOVA, and Fisher's exact test and linear models with significance defined as p < 0.05 using IBM SPSS statistical software version 21.0 and R 3.3.3.
References
Kumar, M., Nanavati, R., Modi, T. G. & Dobariya, C. Oral cancer: etiology and risk factors: a review. J. Cancer Res. Therap. 12, 458–463. https://doi.org/10.4103/0973-1482.186696 (2016).
Rhodus, N. L. Oral cancer: leukoplakia and squamous cell carcinoma. Dent. Clin. N. Am. 49, 143–165. https://doi.org/10.1016/j.cden.2004.07.003 (2005).
Lebot, V. M. M. & Lindstrom, L. C. Kava: The Pacific Drug (Yale University Press, Yale, 1992).
WHO, F. A. A. O. O. t. U. N. Kava: a review of the safety of traditional and recreational beverage consumption (2016).
Clough, A. R. et al. Health effects of kava use in an eastern Arnhem Land Aboriginal community. Intern. Med. J. 33, 336–340. https://doi.org/10.1046/j.1444-0903.2003.00405.x (2003).
Prescott, J. Kava use in Australia. Drug Alcohol Rev. 9, 325–328. https://doi.org/10.1080/09595239000185451 (1990).
AaD, F. Kava, <https://adf.org.au/drug-facts/kava/> (2018).
Celentano, A. et al. The protective effects of Kava (Piper Methysticum) constituents in cancers: a systematic review. J. Oral Pathol. Med. 48, 510–529. https://doi.org/10.1111/jop.12900 (2019).
Teschke, R. Kava hepatotoxicity: pathogenetic aspects and prospective considerations. Liv. Int. 30, 1270–1279. https://doi.org/10.1111/j.1478-3231.2010.02308.x (2010).
Olsen, L. R., Grillo, M. P. & Skonberg, C. Constituents in kava extracts potentially involved in hepatotoxicity: a review. Chem. Res. Toxicol. 24, 992–1002. https://doi.org/10.1021/tx100412m (2011).
Teschke, R., Sarris, J. & Lebot, V. Contaminant hepatotoxins as culprits for kava hepatotoxicity–fact or fiction?. Phytotherapy Res. PTR 27, 472–474. https://doi.org/10.1002/ptr.4729 (2013).
Pantano, F. et al. Hepatotoxicity Induced by “the 3Ks”: Kava, Kratom and Khat. Int. J. Mol. Sci. 17, 580. https://doi.org/10.3390/ijms17040580 (2016).
Abu, N. et al. Flavokawain B induced cytotoxicity in two breast cancer cell lines, MCF-7 and MDA-MB231 and inhibited the metastatic potential of MDA-MB231 via the regulation of several tyrosine kinases In vitro. BMC Complem. Altern. Med. 16, 86. https://doi.org/10.1186/s12906-016-1046-8 (2016).
Abu, N. et al. Flavokawain A induces apoptosis in MCF-7 and MDA-MB231 and inhibits the metastatic process in vitro. PLoS ONE 9, e105244. https://doi.org/10.1371/journal.pone.0105244 (2014).
Tang, Y., Simoneau, A. R., Xie, J., Shahandeh, B. & Zi, X. Effects of the kava chalcone flavokawain A differ in bladder cancer cells with wild-type versus mutant p53. Cancer Prevent. Res. (Philadelphia) 1, 439–451. https://doi.org/10.1158/1940-6207.Capr-08-0165 (2008).
Hseu, Y. C. et al. The chalcone flavokawain B induces G2/M cell-cycle arrest and apoptosis in human oral carcinoma HSC-3 cells through the intracellular ROS generation and downregulation of the Akt/p38 MAPK signaling pathway. J Agric Food Chem 60, 2385–2397. https://doi.org/10.1021/jf205053r (2012).
Abu, N. et al. In vivo anti-tumor effects of flavokawain A in 4T1 breast cancer cell-challenged mice. Anticancer Agents Med. Chem. 15, 905–915. https://doi.org/10.2174/187152061507150713111557 (2015).
Jandial, D. D. et al. Induction of G2M Arrest by Flavokawain A, a Kava Chalcone, Increases the Responsiveness of HER2-Overexpressing Breast Cancer Cells to Herceptin. Molecules22 (2017).
Liu, Z. et al. Kava chalcone, flavokawain A, inhibits urothelial tumorigenesis in the UPII-SV40T transgenic mouse model. Cancer Prevent. Res. (Philadelphia) 6, 1365–1375. https://doi.org/10.1158/1940-6207.Capr-13-0219 (2013).
Zi, X. & Simoneau, A. R. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Can. Res. 65, 3479–3486. https://doi.org/10.1158/0008-5472.Can-04-3803 (2005).
An, J. et al. Flavokawain B induces apoptosis of non-small cell lung cancer H460 cells via Bax-initiated mitochondrial and JNK pathway. Biotech. Lett. 34, 1781–1788. https://doi.org/10.1007/s10529-012-0976-6 (2012).
Chang, C. T. et al. Chalcone flavokawain B induces autophagic-cell death via reactive oxygen species-mediated signaling pathways in human gastric carcinoma and suppresses tumor growth in nude mice. Arch. Toxicol. 91, 3341–3364. https://doi.org/10.1007/s00204-017-1967-0 (2017).
Eskander, R. N. et al. Flavokawain B, a novel, naturally occurring chalcone, exhibits robust apoptotic effects and induces G2/M arrest of a uterine leiomyosarcoma cell line. J. Obst. Gynaecol. Res. 38, 1086–1094. https://doi.org/10.1111/j.1447-0756.2011.01841.x (2012).
Ji, T. et al. Flavokawain B, a kava chalcone, inhibits growth of human osteosarcoma cells through G2/M cell cycle arrest and apoptosis. Mol. Cancer 12, 55. https://doi.org/10.1186/1476-4598-12-55 (2013).
Kuo, Y. F. et al. Flavokawain B, a novel chalcone from Alpinia pricei Hayata with potent apoptotic activity: Involvement of ROS and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radical Biol. Med. 49, 214–226. https://doi.org/10.1016/j.freeradbiomed.2010.04.005 (2010).
Yeap, S. K. et al. Gene Expression Analysis Reveals the Concurrent Activation of Proapoptotic and Antioxidant-Defensive Mechanisms in Flavokawain B-Treated Cervical Cancer HeLa Cells. Integr. Cancer Ther. 16, 373–384. https://doi.org/10.1177/1534735416660383 (2017).
Zhao, X. et al. Flavokawain B induces apoptosis of human oral adenoid cystic cancer ACC-2 cells via up-regulation of Bim and down-regulation of Bcl-2 expression. Can. J. Physiol. Pharmacol. 89, 875–883. https://doi.org/10.1139/y11-088 (2011).
Abu, N. et al. In vivo antitumor and antimetastatic effects of flavokawain B in 4T1 breast cancer cell-challenged mice. Drug Des. Dev. Ther. 9, 1401–1417. https://doi.org/10.2147/dddt.S67976 (2015).
Lin, E. et al. Flavokawain B inhibits growth of human squamous carcinoma cells: Involvement of apoptosis and cell cycle dysregulation in vitro and in vivo. J. Nutr. Biochem. 23, 368–378. https://doi.org/10.1016/j.jnutbio.2011.01.002 (2012).
Malami, I. et al. Crude extracts, Flavokawain B and Alpinetin compounds from the rhizome of Alpinia mutica induce cell death via UCK2 enzyme inhibition and in turn reduce 18S rRNA biosynthesis in HT-29 Cells. PLoS ONE 12, e0170233. https://doi.org/10.1371/journal.pone.0170233 (2017).
Tang, Y. L. et al. Flavokawain B inhibits the growth of acute lymphoblastic leukemia cells via p53 and caspase-dependent mechanisms. Leukemia Lymphoma 56, 2398–2407. https://doi.org/10.3109/10428194.2014.976819 (2015).
Tang, Y. et al. Flavokawain B, a kava chalcone, induces apoptosis via up-regulation of death-receptor 5 and Bim expression in androgen receptor negative, hormonal refractory prostate cancer cell lines and reduces tumor growth. Int. J. Cancer 127, 1758–1768. https://doi.org/10.1002/ijc.25210 (2010).
Rossette, M. C. et al. The in vitro and in vivo antiangiogenic effects of flavokawain B. Phytotherapy Res. PTR 31, 1607–1613. https://doi.org/10.1002/ptr.5891 (2017).
Li, X. et al. Kava components down-regulate expression of AR and AR splice variants and reduce growth in patient-derived prostate cancer xenografts in mice. PLoS ONE 7, e31213. https://doi.org/10.1371/journal.pone.0031213 (2012).
Liu, Z. et al. Kavalactone yangonin induces autophagy and sensitizes bladder cancer cells to flavokawain A and docetaxel via inhibition of the mTOR pathway. J. Biomed. Res. 31, 408–418. https://doi.org/10.7555/jbr.31.20160160 (2017).
Shaik, A. A., Hermanson, D. L. & Xing, C. Identification of methysticin as a potent and non-toxic NF-kappaB inhibitor from kava, potentially responsible for kava’s chemopreventive activity. Bioorg. Med. Chem. Lett. 19, 5732–5736. https://doi.org/10.1016/j.bmcl.2009.08.003 (2009).
Folmer, F. et al. Inhibition of TNFalpha-induced activation of nuclear factor kappaB by kava (Piper methysticum) derivatives. Biochem. Pharmacol. 71, 1206–1218. https://doi.org/10.1016/j.bcp.2005.12.032 (2006).
Bilia, A. R., Scalise, L., Bergonzi, M. C. & Vincieri, F. F. Analysis of kavalactones from Piper methysticum (kava-kava). J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 812, 203–214. https://doi.org/10.1016/j.jchromb.2004.07.038 (2004).
Ganzera, M. & Khan, I. A. Analytical techniques for the determination of lactones inPiper methysticum forst. Chromatographia 50, 649–653. https://doi.org/10.1007/BF02497298 (1999).
Prime, S. S. et al. The behaviour of human oral squamous cell carcinoma in cell culture. J. Pathol. 160, 259–269. https://doi.org/10.1002/path.1711600313 (1990).
Celentano, A. et al. Inhibition of matrix metalloproteinase-2 modulates malignant behaviour of oral squamous cell carcinoma cells. J. Oral Pathol. Med. https://doi.org/10.1111/jop.12992 (2020).
Acknowledgements
This work was supported by the University of Melbourne—F.A. Kernot Research Grant. The authors would like to thank the staff at the National Herbarium of Victoria (MEL), including the digitizing officer Angharad Johnson and the manager of the herbarium specimen collection Pina Milne, who provided the image included in Figure 1.
Author information
Authors and Affiliations
Contributions
A.C.: Conceptualization, experiments completion, data collection and evaluation, manuscript writing, critical manuscript review. C.Y.: experiments completion, data collection and evaluation, manuscript writing. R.P.: experiments completion, critical manuscript review. P.Z.: experiments completion, data collection and evaluation, manuscript writing, critical manuscript review. C.S.F.: critical manuscript review. N.C.: critical manuscript review. T.Y.: critical manuscript review. M.M.C.: critical manuscript review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Celentano, A., Yiannis, C., Paolini, R. et al. Kava constituents exert selective anticancer effects in oral squamous cell carcinoma cells in vitro. Sci Rep 10, 15904 (2020). https://doi.org/10.1038/s41598-020-73058-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73058-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.