Abstract
Nuclear Receptor Subfamily 4 Group A Member 2 (NR4A2) transcripts are elevated in the circulation of individuals whose pregnancies are complicated by preterm fetal growth restriction (FGR). In this paper, we show that the cases with preeclampsia (PE) have increased circulating NR4A2 transcripts compared to those with normotensive FGR. We aimed to establish whether the dysfunctional placenta mirrors the increase in NR4A2 transcripts and further, to uncover the function of placental NR4A2. NR4A2 expression was detected in preterm and term placental tissue; expressed higher at term. NR4A2 mRNA expression and protein were not altered in placentas from preterm FGR or PE pregnancies. Hypoxia (1% O2 compared to 8% O2) significantly reduced cytotrophoblast NR4A2 mRNA expression, but not placental explant NR4A2 expression. Silencing cytotrophoblast NR4A2 expression under hypoxia (via short interfering (si)RNAs) did not alter angiogenic Placental Growth Factor, nor anti-angiogenic sFlt-1 mRNA expression or protein secretion, but increased expression of cellular antioxidant, oxidative stress, inflammatory, and growth genes. NR4A2 expression was also not altered in a model of tumour necrosis factor-α-induced endothelial dysfunction, or with pravastatin treatment. Further studies are required to identify the origin of the circulating transcripts in pathological pregnancies, and investigate the function of placental NR4A2.
Similar content being viewed by others
Introduction
Preeclampsia and fetal growth restriction affect around 10% of pregnancies worldwide and cause significant maternal and perinatal morbidity and mortality1,2,3. Furthermore, exposure of a child to an adverse gestational environment is associated with increased risk of permanent neurodevelopmental, cardiovascular and metabolic impairment that persists into adulthood4, and preeclampsia is associated with compromised long-term maternal health5. Unfortunately, we are still unable to predict these conditions accurately, and have limited treatment options available. Improving methods of prediction, diagnosis, and treatment is paramount to improving patient outcomes.
Placental dysfunction is central to the development of both preeclampsia and fetal growth restriction. A healthy pregnancy largely relies on a well-developed and functional placenta, facilitating exchange of nutrients, oxygen, and waste products between the maternal and fetal systems6. Placental dysfunction is associated with impaired maternal uterine spiral artery remodelling, resulting in a high resistance, pulsatile blood supply. This compromised blood supply restricts nutrient and oxygen delivery to the fetus and causes oxidative stress, fluctuating oxygen tensions, and ischemia7,8, thereby impacting fetal growth. Early identification of placental dysfunction may help detect high-risk pregnancies that require additional monitoring of fetal wellbeing.
Multiple protein biomarkers have been proposed for early detection of fetal growth restriction, but to date, none have sufficient accuracy to be recommended for clinical practice9,10. Alternatively, characterising circulating levels of nucleic acid transcripts released by the placenta during pregnancy may offer a diagnostic option for detecting pregnancies affected by placental dysfunction11,12. In the Fetal OXygenation (FOX) Study, we identified altered mRNA transcript levels in the circulation of women whose pregnancies were complicated by preterm fetal growth restriction, associated with placental insufficiency13. One of the genes identified to be most highly altered was Nuclear Receptor Subfamily 4 Group A Member 2 (NR4A2; also known as NURR1). NR4A2 is a transcription factor and early response gene, and has been implicated in many pathways and diseases including inflammation and cell proliferation14,15,16,17,18,19,20,21, intestinal regeneration after ischemic/reperfusion injury22,23, liver fibrosis24, cancer19,25,26,27, impaired neurodevelopment and autism28,29,30, Parkinson’s disease31,32,33, and cardiovascular disease34,35,36.
Although we identified increased NR4A2 transcripts in the maternal circulation of pregnancies complicated by fetal growth restriction, the previous paper did not identify whether the transcripts originated from the dysfunctional placenta. This paper aims to identify whether the altered NR4A2 transcript levels in the maternal circulation of pregnancies complicated by fetal growth restriction had associations with preeclampsia, and whether the elevated circulating NR4A2 transcripts were reflected in the dysfunctional and/or hypoxic placenta. Furthermore, this paper attempts to identify the role of NR4A2 in the placenta.
Results
Circulating NR4A2 mRNA levels are increased in individuals with coexistent preeclampsia and fetal growth restriction
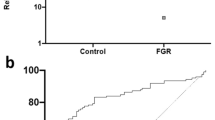
Using next generation sequencing, we previously reported increased NR4A2 transcripts in the circulation of women whose pregnancies were complicated by preterm fetal growth restriction (< 34 weeks gestation)13. In the current study, we performed a sub-analysis, directly comparing cases with preterm preeclampsia and growth restriction to cases of normotensive growth restriction. We found significantly increased circulating NR4A2 transcripts in the growth restricted cases with preeclampsia, compared to normotensive cases (Fig. 1).
Circulating NR4A2 mRNA in cases of normotensive preterm fetal growth restriction and preeclampsia with growth restriction (< 34 weeks gestation). RNA levels were assessed by qPCR. Cases with preeclampsia and growth restriction had significantly higher circulating NR4A2 mRNA compared to normotensive cases of growth restriction. Data presented as relative change from normotensive levels; mean ± SEM. **p < 0.01. Normotensive, n = 45; preeclampsia, n = 71.
Placental NR4A2 expression is increased at term
NR4A2 expression was detected in all placental tissues collected, preterm (24–36 weeks) and term (37–41 weeks). NR4A2 expression was higher in term placental tissue compared to preterm placental tissue (Fig. 2).
NR4A2 expression in preterm and term placental tissue assessed by qPCR. Term placental tissue had significantly higher NR4A2 mRNA expression compared to preterm tissue. Data presented as relative change from preterm levels; mean ± SEM. **p < 0.01. Preterm n = 30, 24–36 weeks; term n = 29, 37–41 weeks.
NR4A2 expression is not altered in preterm pathological placental tissue
To elucidate whether NR4A2 has a role in placental dysfunction, we investigated NR4A2 expression in placenta collected from pregnancies complicated by preterm preeclampsia and fetal growth restriction (≤ 34 weeks gestation), conditions that feature impaired placental development and dysfunction. There was no significant difference in NR4A2 expression in placental tissue from pregnancies complicated by either preterm fetal growth restriction or preeclampsia (≤ 34 weeks gestation) compared to preterm control tissue (Fig. 3a). NR4A2 protein levels were also not altered in the preterm pathological tissue compared to preterm controls (Fig. 3b,c; Supplementary Fig. S1). NR4A2 mRNA expression and protein levels were not significantly altered by fetal sex in preterm pathological placental tissue or controls (data not shown).
NR4A2 expression and protein in preterm control (PT), preeclamptic (PE) and fetal growth restricted (FGR) placental tissue (≤ 34 weeks gestation). (a) mRNA expression assessed by qPCR. (b) Representative western blot. (c) Densitometric analysis of western blot. NR4A2 expression was not altered in the PE and FGR placental tissue compared to respective PT control tissue (PT n = 10, PE n = 49, FGR n = 14). There was no significant difference in NR4A2 protein between the pathological and control tissue (PT n = 15, PE n = 31, FGR n = 17). β-actin acted as the loading control. Data presented as relative change from preterm controls ± SEM.
NR4A2 expression is decreased in cytotrophoblast, but not placental explants under hypoxia
To further elucidate the role of NR4A2 in the placenta, its expression was assessed in placental explants and cytotrophoblast, cells unique to the placenta. These cells and tissues were exposed to hypoxia to simulate a low oxygen environment akin to that in placental insufficiency. NR4A2 expression was detectable in both placental explant tissue and primary cytotrophoblast. There was no difference in NR4A2 expression in placental explants under hypoxia compared to normoxic conditions (Fig. 4a). In cytotrophoblast, NR4A2 expression was significantly reduced under hypoxia (p < 0.0001; Fig. 4b). However, there was no significant change in cytotrophoblast NR4A2 protein levels in hypoxic compared to normoxic conditions (Fig. 4c; Supplementary Fig. S2).
NR4A2 expression in placental explant tissue and primary cytotrophoblast under normoxic (8% O2) and hypoxic (1% O2) conditions. NR4A2 expression was unaltered in placental explant tissue with hypoxia (a). In primary cytotrophoblasts, NR4A2 expression was significantly decreased under hypoxic conditions (b). There was no change in NR4A2 protein production with hypoxia (c). Data presented as fold change from control ± SEM. qPCR: n = 4–5 experimental replicates, each sample from a different patient. Each sample was run in triplicate. Western blot: n = 4 experimental replicates, with each sample from a different patient. Each experiment was run in triplicate and replicate lysates were pooled. β-actin acted as the loading control. ****p < 0.0001.
Knockdown of NR4A2 expression in primary cytotrophoblast
In primary cytotrophoblast, NR4A2 expression was significantly reduced (> 50%) with addition of NR4A2 targeting siRNAs compared to the negative control in both normoxic (p = 0.0028; Supplementary Fig. S3a) and hypoxic conditions (p = 0.0002; Supplementary Fig. S3b). Silencing NR4A2 did not impact cell survival under either normoxic or hypoxic conditions (Supplementary Fig. S3c,d).
Silencing NR4A2 in cytotrophoblasts does not alter expression or secretion of sFLT-1 or PGF
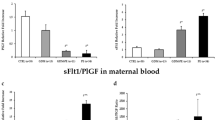
We next assessed the effects of NR4A2 knockdown on sFlt-1, an anti-angiogenic factor increased in preeclampsia, and placental growth factor (PGF), an angiogenic factor decreased in preeclampsia. Silencing NR4A2 in cytotrophoblasts under hypoxia had no significant effect on the expression of the sFlt-1 isoforms, sFlt-1-e15a and sFlt-1-i13 (Fig. 5a,b) or sFlt-1 secretion (Fig. 5c). Silencing NR4A2 under hypoxia had no significant effect on PGF expression (Fig. 5d). Silencing NR4A2 under normoxic conditions did not alter sFlt-1 and PGF expression or sFlt-1 secretion (Supplementary Fig. S4).
Primary cytotrophoblast expression and secretion of anti- and pro-angiogenic factors, sFLT-1 and PGF under hypoxic (1% O2) conditions. Expression assessed by qPCR and secretion by ELISA. There were no significant differences in expression of either sFLT-1 isoform mRNA expression, sFLT-e15a (a) or sFLT-i13 (b), nor sFLT-1 protein secretion (c) or PGF mRNA expression (d) under hypoxia. Data presented as fold change from control ± SEM. n = 3 experimental replicates, with each sample from a different patient. Each experiment was run in triplicate.
Silencing NR4A2 in cytotrophoblasts under hypoxia alters expression of genes associated with oxidative stress, inflammation, and placental insufficiency
To identify pathways that NR4A2 may be working through in the placenta, we assessed expression of genes associated with important placental cellular functions following NR4A2 knockdown. Silencing NR4A2 under hypoxia increased expression of genes involved in oxidative stress pathways including: haemoxygenase-1 (HMOX-1; p = 0.0036; Fig. 6a), NADPH oxidase 4 (NOX4; p = 0.0155; Fig. 6b) and Glutamate-Cysteine Ligase Catalytic Subunit (GCLC; p = 0.0162; Fig. 6c), but did not alter NAD(P)H Quinone Dehydrogenase 1 (NQO1; Supplementary Fig. S5e) or thioredoxin (TXN; Supplementary Fig. S5f.).
Effect of NR4A2 knockdown in primary cytotrophoblasts on expression of genes associated with oxidative stress, inflammation, and placental insufficiency under hypoxic (1% O2) conditions. The expression of HMOX-1 (a), NOX4 (b), GCLC (c), NLRP3 (d) and SPINT1 (e) were significantly increased with NR4A2 knockdown compared to negative control. Data presented as relative change from control ± SEM. *p < 0.05, **p < 0.01. n = 3 experimental replicates, with each sample from a different patient. Each experiment was run in triplicate.
Knockdown of NR4A2 under hypoxia significantly increased expression of NLR family pyrin domain containing 3 (NLRP3), a master regulator of the inflammasome (p = 0.0065; Fig. 6d) and Serine Peptidase Inhibitor, Kunitz Type 1 (SPINT1), associated with placental insufficiency (p = 0.0056; Fig. 6e).
Under hypoxia, silencing NR4A2 did not alter expression of pro-apoptotic gene: BCL2 Associated X (BAX; Supplementary Fig. S5a), pro-survival gene: B-cell lymphoma 2 (BCL2; Supplementary Fig. S5b) or placental growth and proliferation genes: epidermal growth factor receptor (EGFR) and insulin-like growth factor 2 (IGF2) (Supplementary Fig. S5c and S5d).
Gene expression was also assessed in cytotrophoblasts with silenced NR4A2 under normoxic conditions. In these cells we found significantly decreased expression of BAX (p = 0.0123; Supplementary Fig. S6a), EGFR (p = 0.0301; Supplementary Fig. S6c) and IGF2 (p = 0.0178; Supplementary Fig. S6d). Under normoxic conditions, silencing NR4A2 did not alter levels of BCL2, NOX4, HMOX-1, NQO1, TXN, GCLC, NLRP3, or SPINT1 (Supplementary Fig. S6b, e-k).
NR4A2 is not altered with TNF-α-induced endothelial dysfunction or pravastatin treatment
Given there was no change in NR4A2 expression in the pathological preterm placenta, we assessed whether the increased circulating levels of NR4A2 transcripts in pregnancies complicated by fetal growth restriction and preeclampsia might originate in the vasculature. We assessed this in endothelial cells, which form the inner lining of blood vessels. NR4A2 expression was detectable in Human Umbilical Vein Endothelial cells (HUVECs). Moreover, as preeclampsia is associated with endothelial dysfunction37, we assessed whether dysfunction may alter NR4A2 expression. Tumor necrosis factor (TNF)-α, an inflammatory mediator increased in the circulation of women with preeclampsia38, was used to induce endothelial dysfunction in HUVECs. Our model of endothelial dysfunction revealed that TNF-α-induced endothelial dysfunction did not alter NR4A2 expression (Fig. 7). Furthermore, treatment with 200 µM pravastatin with TNF-α, a candidate drug for prevention of preeclampsia39, did not significantly alter NR4A2 expression (Fig. 7).
NR4A2 expression in human umbilical vein endothelial cells with TNF-α induced dysfunction, and pravastatin treatment. There was no significant change in NR4A2 mRNA expression with the addition of TNF-α. Pravastatin treatment did not alter NR4A2 expression from TNF-α only levels. The control group was not exposed to TNF-α. Data presented as fold change from TNF-α treatment ± SEM. n = 3 experimental replicates, with each sample from a different patient. Each experiment was run in duplicate.
Discussion
Circulating nucleic acid transcripts involved in fetal-maternal signalling40 may provide a means for early identification of conditions involving placental dysfunction, such as preeclampsia and fetal growth restriction12,13. In this study, we extended the findings reported previously13, identifying that preeclampsia further increased circulating NR4A2 transcript levels in pregnancies complicated by preterm fetal growth restriction (< 34 weeks gestation). However, this finding is not mirrored in placental tissue, where we observed no difference in NR4A2 expression in placentas from cases of preterm growth restriction or preeclampsia (≤ 34 weeks gestation). We aimed to elucidate the role of NR4A2 in the placenta, finding that NR4A2 expression is higher in term placenta compared to preterm tissue, and its expression is significantly decreased in cytotrophoblast under hypoxia. Furthermore, we identified that decreased cytotrophoblast expression of NR4A2 is associated with the dysregulation of genes involved in oxidative stress, inflammation, and growth and development.
A key aim of this paper was to identify whether expression of NR4A2 in the pathological placenta mirrored the increased NR4A2 transcripts demonstrated in the maternal circulation of cases of preterm preeclampsia and growth restriction (< 34 weeks gestation)—if so, supporting the concept that they may originate in the dysfunctional placenta. However, we did not find any significant change in NR4A2 expression or protein in placentas from pregnancies complicated by preterm fetal growth restriction or preeclampsia. This contrasts the findings by Enquobahrie et al. who found a significant reduction in NR4A2 expression in preeclamptic placental tissue compared to controls41. However, their study included both preterm and term samples. As we’ve shown that NR4A2 expression is altered between preterm and term gestations, we suggest that this disparity is likely due to gestational differences. Further, early- and late-onset preeclampsia have distinct molecular processes, with the early-onset cases associated with increased severity42,43. Our study overcomes this potential confounder by assessing the harder to source clinical samples exclusively from cases of preterm preeclampsia delivering ≤ 34 weeks. Choosing early-onset cases also allows us to compare levels of placental mRNA to circulating transcripts in the FOX blood samples. Our findings suggest that the growth restricted or preeclamptic placenta is not the origin of the altered circulating mRNA transcripts. Furthermore, we identified that silencing NR4A2 did not alter the expression or secretion of sFlt-1 or PGF, key factors central to the pathogenesis of preeclampsia, suggesting that placental NR4A2 is not involved in driving the release of anti-angiogenic factors from the dysfunctional placenta, a key process in the pathogenesis of preeclampsia. However, we have yet to distinguish whether placental NR4A2 may have a role in late-onset preeclampsia, as we only assessed NR4A2 levels in samples collected from early-onset cases of preeclampsia with preterm delivery (≤ 34 weeks).
Though NR4A2 expression has been previously detected in the placenta20,21,41, alterations in placental expression across gestation had not yet been determined. We found that NR4A2 expression was higher at term compared to preterm gestation. Upregulation in placental NR4A2 has been hypothesised to be associated with inflammatory processes21,44. To further understand these changes across gestation, it would be beneficial to examine NR4A2 in first trimester placental tissue, where, if NR4A2 is involved in inflammation as hypothesised, there may be increased NR4A2 expression given many inflammatory pathways are integral in driving successful extravllous cytotrophoblast invasion and critical arteriole remodelling20.
Although NR4A2 was not significantly altered in the preterm pathological placenta, there is potential it could play a role in the pathophysiology underpinning placental dysfunction and disease. Our in vitro models simulate conditions that drive placental dysfunction, offering insight into whether NR4A2 has a role in placental development, and the development of placental disease. Placental hypoxia is a feature of placental dysfunction, where impaired uteroplacental blood supply can cause periods of abnormally low oxygen tension8. Simulating these conditions, we found that hypoxia significantly reduced NR4A2 expression in cytotrophoblast, but not protein. It is understood that changes in mRNA expression do not always translate to the same changes in protein production. In contrast, the placental explant tissue (presenting whole tissue expression) did not have altered NR4A2 expression under hypoxia. This might be due to the heterogenous contribution and interactions of the multiple cell types in the placenta besides trophoblast cells, including vascular, immune and stromal cells.
This study identified several potential actions that NR4A2 expression may regulate in the placenta specific cytotrophoblast cells under dysfunctional conditions. Silencing NR4A2 under hypoxia increased expression of the cytoprotective antioxidant genes HMOX-1 and GCLC. Indeed, given placental dysfunction is associated with oxidative stress45, this suggested that reducing NR4A2 expression could be beneficial. Intriguingly, silencing NR4A2 under hypoxia also increased the expression of SPINT1. Our team has recently reported the important finding that low expression of SPINT1 in the human placenta and maternal circulation is associated with placental insufficiency and growth restriction9. Again, this result suggests that therapeutic interventions that reduce NR4A2 may be advantageous.
However, we also found that silencing NR4A2 increased the expression of NLRP3, which is a key regulator of the inflammatory response and has been implicated in preeclampsia pathogenesis46. NR4A2 has been previously identified as a NLRP3 inflammasome activation-responsive gene in a human monocyte cell line, with the suggestion that its induction acts in a negative feedback loop to prevent sustained inflammasome activation47. This implies, in contrast to our first suggestion, that loss of NR4A2 may result in persistent inflammation, detrimental to the placenta. Furthermore, silencing NR4A2 also increased expression of NOX4, a marker of oxidative stress. Enhancement of oxidative stress could be harmful to the already stressed placenta. Thus, these findings reveal that a more complex regulation may be at play and further studies are required to gain a better understanding of compensation and causation.
As NR4A2 acts as a transcription factor48, it was not surprising that it was involved in the regulation of many different pathways in cytotrophoblast cells. These results have allowed us to identify the pathways associated with NR4A2, but the conflicting responses mean we cannot clearly conclude whether reduction of NR4A2 in cytotrophoblast cells is a harmful consequence of hypoxic stress or a beneficial adaptation to mediate hypoxic damage in the placenta. Advanced protein assessment could be undertaken in the future to validate these findings.
Although this work predominantly aimed to assess NR4A2 expression and function in the dysfunctional placenta, we were also able to look at the function of NR4A2 at a physiologically normal oxygen tension. We identified several important genes to be differentially expressed in normoxic conditions with NR4A2 suppression, not altered under hypoxia. Silencing NR4A2 beneficially decreased cytotrophoblast levels of BAX, a pro-apoptotic gene, but also adversely decreased expression IGF2 and EGFR, which are markers of cell proliferation and growth. These findings indicate that NR4A2 responds variably under different oxygen tensions. However, once again it is unclear whether reduction of NR4A2 expression may be beneficial.
Given these results suggest that the placenta is unlikely to be the source of increased circulating NR4A2 transcripts in individuals with pregnancies complicated by fetal growth restriction and preeclampsia, we examined whether the vasculature may be the source. Though we could detect NR4A2 expression in endothelial cells, we did not find increased expression under TNF-α-induced dysfunction. This suggests that the endothelium is also unlikely to be the origin of the increased circulating NR4A2 transcripts—but additional studies using additional endothelial cell types beyond HUVECs, and different inducers of endothelial dysfunction are required before this can be confirmed. An important consideration is that the circulating transcripts may also originate from other organs in the maternal system that we did not assess here. Additionally, treatment with the therapeutic pravastatin, a drug currently in trial to prevent preeclampsia, had no effect on NR4A2 expression under endothelial dysfunction, suggesting that pravastatin treatment does not affect regulation of this transcription factor.
Conclusion
The origin of elevated circulating NR4A2 transcripts in patients with preterm fetal growth restriction and preeclampsia remains unknown, but is unlikely to be from the placenta. Although we found that placental NR4A2 expression is not altered with preterm preeclampsia or fetal growth restriction, the gene does regulate several important intracellular pathways associated with oxidative stress, fetal growth, and inflammation under the dysfunctional condition of hypoxia. More research is required to confirm the role of NR4A2 in the placenta, and whether altering the expression and localisation of NR4A2 levels may provide a pathway to enhancing the regulation of protective pathways in placental dysfunction.
Methods
Fetal oxygenation (FOX) study
Maternal blood was collected from women whose pregnancies were complicated by preterm fetal growth restriction across six tertiary hospitals in Australia and New Zealand, as previously described13. The blood was directly collected (after corticosteroid administration, prior to delivery) into PAXgene® Blood RNA tubes (Pre-Analytix, Hombrechtikon, Switzerland) and processed according to manufacturer’s instructions.
Ethical approval was obtained from human research ethics committees at all institutions (Mercy Health Human Research Ethics Committee R11/04, Royal Women’s Hospital + Sunshine Hospital: Royal Women’s Hospital Human Research Ethics Committee 10/41, Mater Mothers’ Hospital: Mater Human Research Ethics Committee 1928 M, Royal Hospital for Women: South Eastern Sydney Local Health District Human Research Ethics Committee 12/240, Royal North Shore Hospital: Northern Sydney Local Health District Human Research Ethics Committee 1305-151 M, National Women’s Health, Auckland City Hospital: Health and Disability Ethics Committee 12/NTA/96) and all women provided informed, written consent. Experiments were performed following the relevant institutional guidelines and regulations.
Preterm fetal growth restriction was defined as a birthweight < 10th centile (www.gestation.net, Australian parameters) requiring iatrogenic delivery prior to 34 weeks’ gestation with uteroplacental insufficiency (asymmetrical growth + abnormal artery Doppler velocimetry +/− oligohydramnios +/− abnormal fetal vessel velocimetry). Fetal growth restriction due to infection, chromosomal or congenital abnormalities, and multiple pregnancy was excluded. For our sub-analysis in the current study, the cases of growth restriction were split into preeclamptic and normotensive groups. Patient clinical characteristics are presented in Table 1.
Placenta and umbilical cord collection
Ethical approval was obtained from the Mercy Health Human Research Ethics Committee (R11/34). Women presenting to the Mercy Hospital for Women (Heidelberg, Victoria) gave informed, written consent for the collection of their placenta and umbilical cord. Experiments were performed following institutional guidelines and regulations.
Placentas were obtained from pregnancies complicated by early-onset preeclampsia (requiring delivery ≤ 34 weeks’ gestation). Preeclampsia was defined according to the American College of Obstetricians and Gynecologists guidelines published in 201349. Placentas were also obtained from cases of preterm fetal growth restriction (requiring delivery ≤ 34 weeks gestation) defined as a birthweight < 10th centile, according to Australian population charts50. Placental tissue from cases associated with congenital infection, chromosomal or congenital abnormalities and multiple pregnancies were excluded.
Term (delivery 37–41 weeks’ gestation) and preterm placentas (delivery 24–36 weeks’ gestation) were also collected from normotensive pregnancies where a fetus of normal birthweight percentile (> 10th centile relative to gestation), and no clinical evidence of growth restriction, was delivered. Preterm deliveries in this group were predominantly for iatrogenic reasons (including vasa/placenta previa and suspected placental abruption) or premature rupture of membranes. Cases with hypertensive disease or evidence of chorioamnionitis (confirmed by placental histopathology) were excluded.
Placental tissue was collected within 30 min of delivery. For the groups detailed above, tissue was cut from four sites of the placenta and washed in cold phosphate buffered saline (PBS; 137 mM NaCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, pH 7.4) before being placed in RNAlater™ for 48 h. The tissue was then snap frozen and stored at − 80 °C for subsequent analysis. Patient characteristics are presented in Tables 2, 3 and 4.
Placentas were also obtained from healthy, normotensive term pregnancies (≥ 37 weeks’ gestation) at elective caesarean section for explant dissection and cytotrophoblast isolation. Umbilical cords were collected for the isolation of Human Umbilical Vein Endothelial Cells (HUVECs).
Collection and culture of placental explants and hypoxia treatment
Placental explants were collected with maternal and fetal surfaces removed by careful dissection. Three placental pieces per well were cultured in 24-well plates (10–15 mg per well), in Gibco™ Dulbecco's Modified Eagle Medium (DMEM; ThermoFisher Scientific, Scoresby, Vic) supplemented with 10% fetal calf serum (FCS; Sigma-Aldrich, St Louis, USA) and 1% Anti-Anti (AA; Life Technologies, Carlsbad, California, USA). Explants were cultured under 8% O2, 5% CO2 at 37 °C overnight (16–18 h). After replacement with fresh media (DMEM/10% FCS/1%AA), explant tissue was cultured at 37 °C, 5% CO2 for 48 h under 8% O2 (normoxic conditions) or 1% O2 (hypoxic conditions). This model demonstrates increased sFlt-1 expression and protein51,52, and induced HIF1α protein53 with hypoxia. Following this, explant tissue was snap frozen, and stored at -80 °C for subsequent analysis.
Primary cytotrophoblast isolation and hypoxia treatment
Human primary cytotrophoblast were isolated from healthy, term placentas from elective caesarean section as previously described54. The cells were plated in media (DMEM/10% FCS/1%AA) on fibronectin (10 ug/mL; BD Bioscience, USA) coated culture plates. Viable cells were incubated at 37 °C, 8% O2, 5% CO2 overnight to equilibrate and allow adhesion to cell culture plate. After replacement with fresh media (DMEM/10% FCS/1%AA), cells were incubated under either 8% O2 (normoxic conditions) or 1% O2 (hypoxic conditions) at 37 °C, 5% CO2. After 48 h, the cells were collected for RNA and protein extraction.
Silencing of NR4A2 in primary human cytotrophoblasts
Pre-designed short interfering RNAs (siRNAs) against NR4A2 (M-003427-02-0005; Dharmacon, Lafayette, California, USA) or a pre-tested negative siRNA (Qiagen, Valencia, CA, USA) were combined with lipofectamine (RNAiMax; Invitrogen) in Optimem media (ThermoFisher Scientific) and allowed to complex for 20 min at room temperature. After equilibration of isolated cytotrophoblasts overnight, fresh trophoblast media (DMEM/10% FCS, no AA) was added to each well and 10 nM siRNA solutions added in a dropwise manner. The cells were incubated in either 8% O2 (normoxic conditions) or 1% O2 (hypoxic conditions) at 5% CO2 for 48 h. Media and cell lysates were collected for subsequent analysis.
MTS cell viability assay
Cell viability was assessed after siRNA treatment using an MTS assay. CellTiter 96-AQueous One Solution (Promega, Madison WI) was used according to the manufacturer’s instructions. Optical density was measured using a Bio-Rad X-Mark Microplate Spectrophotometer (Hercules, CA, USA) and Bio-Rad Microplate Manager 6 software.
Endothelial cell isolation and culture
HUVECs were isolated from the umbilical cord of normotensive pregnancies as previously described55. The HUVECs were cultured in M199 media (Life Technologies, California, USA) containing 20% newborn calf serum, 1% endothelial cell growth factor, 1% heparin (Sigma-Aldrich) and 1% AA and used between passages 1–3. Cells were plated in 24-well plates containing M199 media supplemented with 10% FCS, 1% endothelial cell growth factor, 1% heparin and 1% AA.
Endothelial dysfunction and statin treatment
To induce endothelial dysfunction, the HUVECs were pre-treated with 10 ng/mL tumour necrosis factor (TNF)-α, and incubated at 37 °C, 20% O2 and 5% CO2 for 2 h as previous53,56. Following this, 200 µM pravastatin (candidate drug for the treatment of preeclampsia) (Sigma-Aldrich) was added and cells incubated for 24 h. Cell lysates were collected for subsequent analysis.
Real time polymerase chain reaction (RT-PCR)
Total RNA was extracted from whole blood using the PAXgene® Blood miRNA Kit (Pre-Analytix) as described previously13. RNA was extracted from placental tissue, placental explants, cytotrophoblast, and HUVECs using the Qiagen RNeasy Mini Kit following manufacturers’ instructions. The RNA was quantified using a Nanodrop 2000 spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA). Extracted RNA was converted to cDNA using the Applied BiosystemsTM High-Capacity cDNA Reverse Transcription Kit, following manufacturer guidelines on the iCycler iQ5 (Bio-Rad). Quantitative Taqman PCR (Life Technologies) was performed to quantify mRNA expression of NR4A2 (Hs00428691_m1), PGF (Hs00182176_m1), HMOX-1 (Hs01110250_m1), NOX4 (Hs00418356_m1), GCLC (Hs00155249_m1), NLRP3 (Hs00918082_m1), SPINT1 (Hs00173678_m1), BAX (Hs00180269_m1), BCL2 (Hs00608023_m1), EGFR (Hs01076078_m1), IGF2 (Hs04188276_m1), NQO1 (Hs00168547_m1) and TXN (Hs00828652_m1). Stability of reference genes was confirmed for each sample type and used appropriately; for blood YWHAZ (Hs01122454_m1), B2M (Hs00187842_m1) and GUSB (Hs00939627_m1) for cells YWHAZ (Hs01122454_m1) and for explants and placental tissue: TOP1 (Hs00243257_m1) and CYC1 (Hs00357717_m1). Taqman RT-PCR was performed on the CFX384 (BioRad) with the following run conditions: 50 °C for 2 min; 95 °C for 10 min, 95 °C for 15 s, 60 °C for 1 min (40 cycles) or 95 °C for 20 s; 95 °C for 3 s, 60 °C for 30 s (40 cycles; Taqman Fast Advanced Master Mix).
The sFlt-1 splice variants sFlt-1-i13 and sFlt-1-e15a were measured in a SYBR PCR with SYBR Green Master mix (Applied Biosystems) using primers specific for each variant. The primers for i13 were 5′-ACAATCAGAGGTGAGCACTGCAA-3′ (forward) 5′-TCCGAGCCTGAAAGTTAGCAA-3′ (reverse), for e15a 5-CTCCTGCGAAACCTCAGTG-3′ (forward) 5′-GACGATGGTGACGTTGATGT-3′ (reverse) and for YWHAZ (reference gene) 5′-GAGTCATACAAAGACAGCACGCTA-3′ (forward) 5′-TTCGTCTCCTTGGGTATCCGATGT-3′ (reverse). The SYBR PCR was run on the CFX384 (Bio-Rad), with 40 cycles of 95 °C for 21 s, then 60 °C for 20 min. All data were normalized to the appropriate reference gene as an internal control and calibrated against the average Ct of the control samples. All cDNA samples were run in duplicate.
Western blot analysis
Protein lysates were collected from human primary cytotrophoblasts and placental tissue collected from pregnancies complicated by preeclampsia, fetal growth restriction and preterm controls (≤ 34 weeks) using RIPA lysis buffer containing proteinase and phosphatase inhibitors (Sigma Aldrich). Protein concentration was assessed with Pierce™ BCA Protein Assay Kit (ThermoFisher Scientific, Massachusetts, USA). Placental lysates (20 µg) were separated on 4–20% Mini-PROTEAN® TGX™ Precast Protein Gels (Bio-Rad) and PVDF membranes (Millipore; Billerica, MA, United States). The membranes were blocked with 1% bovine serum albumin (BSA; Sigma-Aldrich), prior to overnight incubation with the primary antibody, (diluted 1:500 in 1%BSA/TBS-T; GTX133225, Sapphire Bioscience, NSW, Australia). Blots were incubated with secondary anti-rabbit antibody (W401, Promega, Madison WI, USA) at 1:2500 dilution in 5% skim milk for 1 h. Bands were visualized using a chemiluminescence detection system (GE Healthcare Life Sciences) and ChemiDoc XRS (Bio-Rad). β-actin acted as the loading control, (diluted 1:20,000 in 5% skim milk; Santa Cruz, Texas, USA). Relative densitometry was measured using Image Lab software (Bio-Rad).
Enzyme linked immunosorbent assay (ELISA)
Soluble fms-like tyrosine kinase-1 (sFlt-1) secretion was measured in cytotrophoblast conditioned culture media using the DuoSet Human VEGF R1/FLT-1 kit (R&D systems by Bioscience, Waterloo, Australia) according to manufacturer’s instructions. Optical density was measured using a Bio-Rad X-Mark microplate spectrophotometer and Bio-Rad Microplate Manager 6 software.
Statistical analysis
All in vitro experiments were performed with technical duplicates or triplicates and repeated with a minimum of three different patient samples. Data were tested for normal distribution and statistically tested as appropriate. Either an unpaired t test or Mann–Whitney test was used. All data are expressed as mean ± SEM. P values < 0.05 were considered significant. Statistical analysis was performed using GraphPad Prism software 8 (GraphPad Software, Inc.; San Diego, CA, USA).
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Malhotra, A. et al. Neonatal morbidities of fetal growth restriction: Pathophysiology and impact. Front. Endocrinol. 10(55), 15. https://doi.org/10.3389/fendo.2019.00055 (2019).
Kesavan, K. & Devaskar, S. U. Intrauterine growth restriction: Postnatal monitoring and outcomes. Pediatr. Clin. N. Am. 66(2), 403–423. https://doi.org/10.1016/j.pcl.2018.12.009 (2019).
Abalos, E., Cuesta, C., Grosso, A. L., Chou, D. & Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 170(1), 1–7. https://doi.org/10.1016/j.ejogrb.2013.05.005 (2013).
Barker, D. J. P. et al. Fetal nutrition and cardiovascular disease in adult life. The Lancet. 341(8850), 938–941. https://doi.org/10.1016/0140-6736(93)91224-A (1993).
Bokslag, A., van Weissenbruch, M., Mol, B. W. & de Groot, C. J. M. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum. Dev. 102, 47–50. https://doi.org/10.1016/j.earlhumdev.2016.09.007 (2016).
Gude, N. M., Roberts, C. T., Kalionis, B. & King, R. G. Growth and function of the normal human placenta. Thromb. Res. 114(5), 397–407. https://doi.org/10.1016/j.thromres.2004.06.038 (2004).
Burton, G. J. & Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 218(2), S745–S761. https://doi.org/10.1016/j.ajog.2017.11.577 (2018).
Kingdom, J. C. & Kaufmann, P. Oxygen and placental villous development: Origins of fetal hypoxia. Placenta 18(8), 613–621 (1997).
Kaitu’u-Lino, Tu. J. et al. Circulating SPINT1 is a biomarker of pregnancies with poor placental function and fetal growth restriction. Nat. Commun. 11(1), 2411. https://doi.org/10.1038/s41467-020-16346-x (2020).
Bakalis, S., Gallo, D. M., Mendez, O., Poon, L. C. & Nicolaides, K. H. Prediction of small-for-gestational-age neonates: Screening by maternal biochemical markers at 30–34 weeks. Ultrasound Obstet. Gynecol. 46(2), 208–215. https://doi.org/10.1002/uog.14861 (2015).
Whitehead, C. L. & Tong, S. Measuring hypoxia-induced RNA in maternal blood: A new way to identify critically hypoxic fetuses in utero?. Expert Rev. Mol. Diagn. 14(5), 509–511. https://doi.org/10.1586/14737159.2014.915749 (2014).
Whitehead, C. L. et al. Original research: Identifying late-onset fetal growth restriction by measuring circulating placental RNA in the maternal blood at 28 weeks’ gestation. Am. J. Obstet. Gynecol. 214, 521.e521-521.e528. https://doi.org/10.1016/j.ajog.2016.01.191 (2016).
Hannan, N. J. et al. Circulating mRNAs are differentially expressed in pregnancies with severe placental insufficiency and at high risk of stillbirth. BMC Med. 18(1), 145. https://doi.org/10.1186/s12916-020-01605-x (2020).
Bonta, P. I. et al. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler. Thrombo. Vasc. Biol. 26(10), 2288–2294 (2006).
Nagata, K. et al. CRTH2, an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor(s). FEBS Lett. 459(2), 195–199. https://doi.org/10.1016/S0014-5793(99)01251-X (1999).
Raveney, B. J. E., Oki, S. & Yamamura, T. Nuclear receptor NR4A2 orchestrates Th17 cell-mediated autoimmune inflammation via IL-21 signalling. PLoS ONE 8(2), 1–10. https://doi.org/10.1371/journal.pone.0056595 (2013).
Kane, M. O., Murphy, E. P. & Kirby, B. The role of corticotropin-releasing hormone in immune-mediated cutaneous inflammatory disease. Exp. Dermatol. 15(3), 143–153. https://doi.org/10.1111/j.1600-0625.2006.00382.x (2006).
Herring, J. A., Elison, W. S. & Tessem, J. S. Function of Nr4a orphan nuclear receptors in proliferation, apoptosis and fuel utilization across tissues. Cells 8(11), 1373 (2019).
Han, Y.-F. & Cao, G.-W. Role of nuclear receptor NR4A2 in gastrointestinal inflammation and cancers. World J Gastroenterol. 18(47), 6865–6873. https://doi.org/10.3748/wjg.v18.i47.6865 (2012).
Suryawanshi, H. et al. A single-cell survey of the human first-trimester placenta and decidua. Sci. Adv. 4(10), eaau4788. https://doi.org/10.1126/sciadv.aau4788 (2018).
Lappas, M. The NR4A receptors Nurr1 and Nur77 are increased in human placenta from women with gestational diabetes. Placenta 35(11), 866–875. https://doi.org/10.1016/j.placenta.2014.08.089 (2014).
Zu, G. et al. Nurr1 promotes intestinal regeneration after ischemia/reperfusion injury by inhibiting the expression of p21 (Waf1/Cip1). J. Mol. Med. 95(1), 83–95 (2017).
Liu, L. et al. miR-381-3p knockdown improves intestinal epithelial proliferation and barrier function after intestinal ischemia/reperfusion injury by targeting nurr1. Cell Death Dis. 9(3), 411–411. https://doi.org/10.1038/s41419-018-0450-z (2018).
Chen, P. et al. Adenovirus-mediated expression of orphan nuclear receptor NR4A2 targeting hepatic stellate cell attenuates liver fibrosis in rats. Sci. Rep. 6, 33593–33593. https://doi.org/10.1038/srep33593 (2016).
Llopis, S. et al. Dichotomous roles for the orphan nuclear receptor NURR1 in breast cancer. BMC Cancer 13, 139–139. https://doi.org/10.1186/1471-2407-13-139 (2013).
Yin, K. et al. NR4A2 promotes DNA double-strand break repair upon exposure to UVR. Mol. Cancer Res. 15(9), 1184–1196 (2017).
Munnur, D. et al. NR4A nuclear receptors target poly-ADP-ribosylated DNA-PKcs protein to promote DNA repair. Cell Rep. 26(8), 2028-2036.e2026. https://doi.org/10.1016/j.celrep.2019.01.083 (2019).
Lévy, J. et al. NR4A2 haploinsufficiency is associated with intellectual disability and autism spectrum disorder. Clin. Gen. 94(2), 264–268. https://doi.org/10.1111/cge.13383 (2018).
Leppa, V. M. et al. Rare inherited and de novo CNVs reveal complex contributions to ASD risk in multiplex families. Am. J. Hum. Genet. 99(3), 540–554. https://doi.org/10.1016/j.ajhg.2016.06.036 (2016).
Reuter, M. S. et al. Haploinsufficiency of NR4A2 is associated with a neurodevelopmental phenotype with prominent language impairment. Am. J. Med. Genet. A 173(8), 2231–2234. https://doi.org/10.1002/ajmg.a.38288 (2017).
Paliga, D., Raudzus, F., Leppla, S. H., Heumann, R. & Neumann, S. Lethal factor domain-mediated delivery of Nurr1 transcription factor enhances tyrosine hydroxylase activity and protects from neurotoxin-induced degeneration of dopaminergic cells. Mol. Neurobiol. 56(5), 3393–3403. https://doi.org/10.1007/s12035-018-1311-6 (2019).
Ruiz-Sánchez, E. et al. Association of polymorphisms and reduced expression levels of the NR4A2 gene with Parkinson’s disease in a Mexican population. J. Neurol. Sci. 379, 58–63 (2017).
Lou, X. & Liao, W. Association of Nurr1 gene mutations with Parkinson’s disease in the Han population living in the Hubei province of China. Neural Regen Res. 7(23), 1791–1796. https://doi.org/10.3969/j.issn.1673-5374.2012.23.005 (2012).
Medzikovic, L., de Vries, C. J. M. & de Waard, V. NR4A nuclear receptors in cardiac remodeling and neurohormonal regulation. Trends Cardiovasc. Med. 29(8), 429–437. https://doi.org/10.1016/j.tcm.2018.11.015 (2019).
van Tiel, C. M. & de Vries, C. J. M. NR4All in the vessel wall. J. Steroid Biochem. Mol. Biol. 130(3), 186–193. https://doi.org/10.1016/j.jsbmb.2011.01.010 (2012).
Kardys, I. et al. Haplotypes of the NR4A2/NURR1 gene and cardiovascular disease: The Rotterdam Study. Hum. Mutat. 30(3), 417–423. https://doi.org/10.1002/humu.20902 (2009).
Brennan, L. J., Morton, J. S. & Davidge, S. T. Vascular Dysfunction in Preeclampsia. Microcirculation 21(1), 4–14. https://doi.org/10.1111/micc.12079 (2014).
Vitoratos, N., Economou, E., Iavazzo, C., Panoulis, K. & Creatsas, G. Maternal serum levels of TNF-alpha and IL-6 long after delivery in preeclamptic and normotensive pregnant women. Mediators Inflamm. 2010, 908649–908649. https://doi.org/10.1155/2010/908649 (2010).
Kumasawa, K., Iriyama, T., Nagamatsu, T., Osuga, Y. & Fujii, T. Pravastatin for preeclampsia: From animal to human. J. Obstet. Gynaecol. Res. 46(8), 1255–1262. https://doi.org/10.1111/jog.14295 (2020).
Wei, J. et al. Placental trophoblast debris mediated feto-maternal signalling via small RNA delivery: implications for preeclampsia. Sci. Rep. 7(1), 14681. https://doi.org/10.1038/s41598-017-14180-8 (2017).
Enquobahrie, D. A. et al. Differential placental gene expression in preeclampsia. Am. J. Obstet. Gynecol. 199(5), 566.e561-566.e511. https://doi.org/10.1016/j.ajog.2008.04.020 (2008).
Ren, Z. et al. Distinct placental molecular processes associated with early-onset and late-onset preeclampsia. Theranostics 11(10), 5028–5044. https://doi.org/10.7150/thno.56141 (2021).
Lisonkova, S. & Joseph, K. S. Incidence of preeclampsia: Risk factors and outcomes associated with early-versus late-onset disease. Am. J. Obstet. Gynecol. 209(6), 544.e541–544.e512. https://doi.org/10.1016/j.ajog.2013.08.019 (2013).
Bordoni, L., Petracci, I., Calleja-Agius, J., Lalor, J. G. & Gabbianelli, R. NURR1 alterations in perinatal stress: A first step towards late-onset diseases? A narrative review. . Biomedicines https://doi.org/10.3390/biomedicines8120584 (2020).
Schoots, M. H., Gordijn, S. J., Scherjon, S. A., van Goor, H. & Hillebrands, J.-L. Oxidative stress in placental pathology. Placenta 69, 153–161. https://doi.org/10.1016/j.placenta.2018.03.003 (2018).
Shirasuna, K., Karasawa, T. & Takahashi, M. Role of the NLRP3 inflammasome in preeclampsia. Front. Endocrinol. https://doi.org/10.3389/fendo.2020.00080 (2020).
Kawana, N., Yamamoto, Y., Kino, Y. & Satoh, J. Molecular network of NLRP3 inflammasome activation-responsive genes in a human monocyte cell line. Austin J. Clin. Immunol. 1(4), 1017 (2014).
Safe, S. et al. Nuclear receptor 4A (NR4A) family—orphans no more. J. Steroid Biochem. Mol. Biol. 157, 48–60. https://doi.org/10.1016/j.jsbmb.2015.04.016 (2016).
Roberts, J. M. et al. Hypertension in pregnancy: Executive summary. Obstetrics & Gynecol. 122(5), 1122–1131 https://doi.org/10.1097/01.Aog.0000437382.03963.88. (2013).
Dobbins, T. A., Sullivan, E. A., Roberts, C. L. & Simpson, J. M. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med. J. Aust. 197(5), 291–294. https://doi.org/10.5694/mja11.11331 (2012).
Kaitu’u-Lino, T. J. et al. Characterization of protocols for primary trophoblast purification, optimized for functional investigation of sFlt-1 and soluble endoglin. Pregnancy Hypertens. 4(4), 287–295. https://doi.org/10.1016/j.preghy.2014.09.003 (2014).
Palmer, K. R. et al. Jumonji domain containing protein 6 is decreased in human preeclamptic placentas and regulates sFLT-1 splice variant production 1. Biol. Reprod. https://doi.org/10.1095/biolreprod.115.134460 (2016).
Onda, K. et al. Proton pump inhibitors decrease soluble fms-Like tyrosine kinase-1 and soluble endoglin secretion, decrease hypertension, and rescue endothelial dysfunction. Hypertension 69(3), 457–468. https://doi.org/10.1161/HYPERTENSIONAHA.116.08408 (2017).
Kaituu-Lino, Tu. J. et al. Original Article: Characterization of protocols for primary trophoblast purification, optimized for functional investigation of sFlt-1 and soluble endoglin. Pregnancy Hypertens. Int. J. Women’s Cardiovasc Health 4, 287–295. https://doi.org/10.1016/j.preghy.2014.09.003 (2014).
de Alwis, N. et al. Pravastatin as the statin of choice for reducing pre-eclampsia-associated endothelial dysfunction. Pregnancy Hypertens. 20, 83–91. https://doi.org/10.1016/j.preghy.2020.03.004 (2020).
Onda, K. et al. Sofalcone upregulates the nuclear factor (erythroid-derived 2)–like 2/heme oxygenase-1 pathway, reduces soluble fms–like tyrosine kinase-1, and quenches endothelial dysfunction: Potential therapeutic for preeclampsia. Hypertension 65(4), 855–862 (2015).
Acknowledgements
The authors acknowledge clinical research midwives Gabrielle Pell, Rachel Murdoch, Genevieve Christophers, the obstetric clinical and midwifery staff and patients at the Mercy Hospital for Women (Heidelberg) for provision of tissues.
Funding
The National Health and Medical Research Council provided salary support (#1146128 to NJH, #1159261 to TKL, and #1136418 to ST). The funders had no role in study design, data collection, analysis, decision to publish or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
N.D.A.—Data generation, literature search, data analysis, interpretation, writing. S.B., N.K.B. —Assistance with data collection. N.P. —Characterisation of clinical cohorts. T.K.L. —Intellectual input and interpretation. O.S., K.G., S.P., A.H., J.M.S., S.S., S.C.K., S.W. —Sample collection and characterisation of clinical cohorts. S.T. —Intellectual input, sample collection and characterisation of clinical cohorts. N.J.H. —Study design, data analysis and interpretation, intellectual input, writing, attained funding support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Alwis, N., Beard, S., Binder, N.K. et al. NR4A2 expression is not altered in placentas from cases of growth restriction or preeclampsia, but is reduced in hypoxic cytotrophoblast. Sci Rep 11, 20670 (2021). https://doi.org/10.1038/s41598-021-00192-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-00192-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.