Abstract
This study examined whether the use of SRIs is associated with an increased risk of bone loss using a nested case–control design with a nationwide population–based cohort in Korea. Using the Korean National Health Screening Cohort, subjects newly diagnosed with osteoporosis or osteopenia (n = 55,799) were matched with controls (n = 278,995) at a ratio of 1:5. We stratified the participants by their time-dependent use of SRIs and sex and controlled for various confounders, including lifestyle habits, laboratory data, and comorbidities. Conditional logistic regression showed that both recent and former users of SRIs had an increased risk of subsequent bone loss compared with non-users: men [recent users: odds ratio (OR) 1.35, 95% confidential interval (CI) 1.20, 1.53; former-users: OR 1.10, 95% CI 1.01, 1.20]; women (recent users: OR 1.38, 95% CI 1.28–1.48; former-users: OR 1.07, 95% CI 1.02, 1.21). The use of SRIs was associated with an increased risk of bone loss in both men and women. In particular, the association was stronger in recent users. These findings provide population-level evidence for the risk of bone loss associated with SRI exposure and highlight the importance of monitoring the bone health of SRI users.
Similar content being viewed by others
Introduction
The use of antidepressants, especially serotonin reuptake inhibitors (SRIs), has become increasingly common in recent decades all around the world1,2,3,4,5. Reports of low bone mineral density (BMD)6,7,8 and bone fractures9,10,11,12,13,14,15,16,17,18,19 in people taking antidepressants have raised the concern that SRIs could have adverse effects on bone. A meta-analysis of 16 studies reported that users of selective serotonin reuptake inhibitors (SSRIs) are 1.61 times more likely to develop bone fractures than non-users (Relative risk 1.61 95% CI 1.49, 1.74)15. In addition, some studies found that the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) can be associated with an increased risk of bone fractures16,17. Although those fracture-risk findings suggest a possible link between use of SRIs and bone health, any real connection between them remains unclear20, and the risk of bone fractures among people taking antidepressants could be related to other factors, such as falls and depressive status.
Findings about the adverse effects of SRI use on BMD are limited and mixed. Although several studies reported that a history of taking SSRIs predicted decreased BMD in both men and women6,7,8,18, some studies found no significant association between them21,22. A meta‐analysis of 4 studies examining the association between antidepressants and BMD in women showed that antidepressant was not associated with lower or higher BMD23. A cohort study (173 men and 323 women) reported that taking antidepressants was associated with low BMD in women (Coefficient -0.141, 95% CI -0.263, -0.020) but not in men (Coefficient 0.073, 95% CI -0.086, 0.232)22. Moreover, current users of antidepressants were reported to show a significantly higher risk of osteoporotic fractures than former users17. These inconsistent results across studies might be attributable to small sample sizes, different study designs, sample characteristics such as sex differences, different duration of antidepressant use, and sampling bias. More research into the independent effect of SRIs on bone health in a large unbiased clinical sample controlled for various confounders is needed to improve the safety of SRI treatment and the healthcare quality.
In this study, we used a nationwide population-based Korean cohort to examine whether the use of SRIs is associated with subsequent bone loss. We used a nested case–control design with a 1:5 matching ratio of cases to controls and stratified the participants according to their time-dependent use of SRIs and their sex. In addition, we controlled for various potential confounders such as laboratory test results and lifestyle factors that could affect bone health.
Results
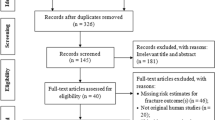
We identified 388,979 individuals who had received a medical check-up between Jan 2004 and Dec 2006. We excluded participants with a previous diagnosis related to bone loss (n = 51,151) or exposure to SRIs during the wash out period (n = 35,508). Using a 1:5 matching ratio of cases to controls, controls without any diagnosis related to bone loss (n = 278,995) were randomly selected for each case in the group with low bone mass [n = 55,799; 43,313 Women (77.6%); mean (SD) age, 58.0 (9.4) years]. A detailed flow chart is provided in Fig. 1.
The baseline characteristics of the study sample are presented in Table 1. The rate of participants who never used SRIs was lower in subjects with a new diagnosis of low bone density than in those without such a diagnosis. At the same time, subjects in the case group were more likely to be classified as former or recent users of SRIs than those in the control group. In both sexes, subjects in the case group were more likely than the controls to take glucocorticoids, GnRH agonists, anticonvulsants, thiazolidinediones, antipsychotics, benzodiazepine, and TCA. Men and women who were newly diagnosed with low bone density had a higher prevalence than controls of using androgen deprivation therapy and aromatase inhibitors, respectively. Subjects with low bone density were more likely to take DMPA and estrogen or a combination of estrogen and progesterone, but those outcomes occurred only in women. The low bone density group was also more likely than the controls to report high CCI, low systolic and diastolic blood pressures, low fasting blood glucose level, and low total cholesterol level; and perform little exercise. The case group presented with lower hemoglobin levels and higher AST than the controls, but this outcome occurred only in men. Participants with osteoporosis had lower urine protein and ALT levels, and those results were driven by women.
The association between the use of antidepressant drugs and the risk of subsequent low bone density is shown in Table 2. The odds ratio (OR) was 1.44 (95% CI 1.38, 1.50) for former users and 1.80 (95% CI 1.69, 1.91) for recent users. Use of SRIs remained an independent predictor of subsequent low bone density after adjusting for potential confounders (glucocorticoids, aromatase inhibitors, androgen deprivation therapy, GnRH agonists, anticonvulsants, thiazolidinediones, DMPA, antipsychotics, benzodiazepine, TCA, Estrogen or Estrogen and progesterone combination, household income, CCI, BMI, systolic and diastolic blood pressures, fasting blood glucose level, total cholesterol level, hemoglobin level, urine occult blood, urine protein, AST, ALT, smoking status, frequency of drinking alcohol, and frequency of exercise). After adjusting for those potential confounders, former use of SRIs was associated with a 1.07-fold higher risk of low bone density than was found among non-users (OR 1.07, 95% 1.03, 1.12), and the risk for recent users was even higher (OR 1.44, 95% CI 1.35, 1.53). The results of the multivariable models showed a similar trend in men and women. In men, the OR was 1.10 (95% CI 1.01, 1.20) for former users and 1.35 (95% CI 1.20, 1.53) for recent users. Among women, former users had a 1.07-fold higher risk of low bone density than non-users (OR 1.07, 95% CI 1.02–1.12), and the risk of low bone density in women increased by 1.38-fold in recent users compared with non-users (OR 1.38, 95% CI 1.28, 1.48). In the sensitivity analysis to examine confounding by frequent healthcare utilization, while the association was not significant for either sex in former users (OR 1.04, 95% CI 0.99 -1.09, Men: OR 1.07, 95% CI 0.98–1.17, Women: OR 1.02, 95% CI 0.97–1.08), the association between the use of SRIs and the risk of bone loss remained statistically significant in recent users (OR 1.34, 95% CI 1.25–1.42, Men: OR 1.29 95% CI 1.14–1.46, Women: OR 1.22 95% CI 1.13–1.31). In the recent SRI users, the mean period between the initiation of SRI treatment and the diagnosis of low bone density was 1.8 years (SD = 2.2 years).
Discussion
This real-world nationwide, longitudinal cohort study investigated the association between the use of SRIs and subsequent low bone density, stratifying participants by their time-dependent use of the medications and their sex. Those exposed to SRIs had an increased risk of newly diagnosed low bone density (osteoporosis or osteopenia) compared with those who did not receive those medications. After adjusting for multiple confounding factors, the association remained significant, suggesting an independent association between the use of SRIs and the risk of osteoporosis. that the use of SRIs independently altered the risk of osteoporosis. After adjusting for confounding factors, the risk of subsequent low bone density was 1.44-fold higher in recent users and 1.07-fold higher in former users compared with the risk in non-users. Given the high prevalence of SRI use, these findings could have significant clinical implications.
To the best of our knowledge, this is the largest nationwide cohort study (74,916 men and 259,878 women) to examine the association between the use of SRIs and the risk of bone loss. Our finding that the risk of low bone density was higher in people who took SRIs in a population-based Korean cohort supports the hypothesis that the use of SRIs is associated with an increased risk of bone loss. Although little clinical attention has been paid to the potential adverse effects of SRIs on bones, several reports have linked the use of SRIs to an increased risk of osteoporosis6,7,8,24 (other studies did not show any link between SRI use and bone health10,21). In addition, we found significant associations between SRIs and low bone density in both men and women, whereas mixed and inconsistent results were shown in previous studies with smaller sample sizes8,22. A cross-sectional study of 5995 men reported that SSRI users had 3.7% lower BMD in their hips and 5.9% lower BMD in their lumber spines8, but another study of 141 men did not find any significant difference in BMD among users and non-users of antidepressants22. According to a meta-analysis of 11 observational studies, women taking SSRIs had significantly lower bone mass in their lumbar spine than non-users, but that paper failed to make a comprehensive analysis in men because of an insufficient number of subjects in previous studies24. Our findings are based on a large, well-defined population and demonstrate that the use of SRIs is associated with an increased risk of subsequent bone loss in both sexes. Therefore, people who taking SRIs should be monitored for their bone health.
The mechanism underlying the relationship between bone loss and SRI use remains poorly understood. Although we did not examine the causal mechanism in this study, the role of serotonin receptors in bone is a potential molecular mechanism. Several studies have shown the presence of serotonin receptors and transporters in osteoblasts and osteoclasts, which are the key elements in the bone remodeling cycle, and suggested possible adverse effects, such as reduced osteoblast proliferation, from blockading serotonin reuptake during bone remodeling25,26,27,28,29. An animal study proposed that SSRIs could reduce BMD by altering osteoclast differentiation and increasing sympathetic tone29, and that possibility warrants future research.
In our multivariable models, recent and former SRI users were associated with 1.44-fold and 1.07-fold increased risk of subsequent low bone density, respectively, compared with non-users, indicating that the risk in recent users is higher than that in former users. In addition, the sensitivity analysis examining confounding by frequent healthcare utilization revealed that while the association was no longer significant in former SRI users, a statistically significant association between the use of SRIs and the risk of bone loss was observed in recent users. These results suggest that recent users are at higher risk for bone loss than former users and that discontinuing SRIs might reverse the bone loss related to SRI use. In line with that finding, several studies have reported that although current use of antidepressants is associated with an increased risk of bone fractures compared with non-users, no significant association was found in former users13,17,30. Furthermore, one cohort study that investigated the association between the time since the last use of an SSRI and bone fractures found that the risk of osteoporotic fractures declined rapidly after discontinuing SRI use31. Further research is needed to better understand the reversible or irreversible effects of SRIs on bone health and their underlying mechanisms.
Given the high prevalence of depressive disorders and SRI use, the association between SRI exposure and risk of bone loss could lead to additional public health burdens. Therefore, it is important to pay attention to the issue of the adverse effects of SRIs on bone, especially in individuals who recently took or are currently taking them.
The major strength of our study is the use of a nationwide, longitudinal database, which ensured minimal selection bias. The database included the serial results of healthcare utilization without any losses, and the data were collected uniformly among cases and controls. Furthermore, because the NHIS database includes prescription history and lifestyle variables, we were able to adjust for multiple potential confounding variables, including alcohol use, smoking, and exercise status and the use of other medicines known to induce low BMD.
Nevertheless, this study had several limitations. First, the newly diagnosed cases of osteoporosis and osteopenia in this study could be potentially susceptible to errors of misclassification. To increase the diagnostic accuracy, we applied a strict operational diagnosis of low bone density with appropriate ICD-10 codes and prescriptions for an osteoporosis-related medication. This operational definition is reliable, because the NHIS allows the prescription of osteoporosis-related medications only if the T score derived from DEXA is lower than -1. However, since the baseline bone mineral density of subjects at inclusion and the extent of bone loss in the form of T or Z scores during the observation period were not available the database, it is possible that a low bone mass at the index date resulted from a baseline bone mass at the lower end of the normal range, and not necessarily due to increased bone loss. Second, because the present database of insurance claims does not provide information about patient adherence to an SRI regime, the possibility that some individuals with poor drug compliance biased the results cannot be completely excluded. Third, frequent healthcare utilization may lead to potential detection bias of low bone density, although our sensitivity analysis revealed that the main results remained statistically significant in the recent SRI users. Lastly, we could not consider depression itself as a covariate, which may be a risk factor for osteoporosis8,32,33. Although the causal link between depression and bone loss is disputed, the physiological alterations associated with depression, such as dysregulation of hypothalamus–pituitary–adrenal axis34 and sympathetic systems and inflammatory immune responses including enhanced pro-inflammatory cytokines35,36, might affect bone mass. In addition, lifestyle patterns in people with depression, such as low physical activities and unhealthy diet, might contribute to osteoporosis37. Further research is needed to clarify the associations between depression and bone loss. Additionally, other confounders that could affect osteoporosis, such as serum vitamin D deficiency38, and menopause status39, were not considered.
In conclusion, this nationwide population-based study showed that the use of SRIs is associated with an increased risk of subsequent bone loss. In particular, recent users were associated with a higher risk of low bone density than former users. These findings provide reliable, population-level evidence for the risk of bone loss associated with SRI exposure, and highlight the importance of carefully monitoring bone loss in individuals taking SRIs.
Method
Study cohort
We used the National Health Insurance Sharing Service-National Health Screening (NHIS-HEALS) cohort, a representative sample cohort database from health screening participants that has been made publicly available and contains both comprehensive health screening data and long-term health outcomes. The cohort includes 514,866 participants, a 10% random sample of all health screening participants aged 40 to 79 years in 2002, and those people were repeatedly followed up until 201540. The cohort provides access to variables such as sociodemographic information, specific health problems (e.g., smoking, alcohol use, medical history, and family history), bio-clinical laboratory results, diagnoses in the form of International Classification of Disease 10th revision [ICD-10] codes, drug codes, date and daily dosage of prescriptions, and date and cause of death.
Within the NHIS-HEALS database, participants who had received a medical check-up between Jan 2004 and Dec 2006 were selected. The date of the medical check-up between 2004 and 2006 was taken as the baseline. Subjects with a preexisting diagnosis related to bone loss (osteoporosis or osteopenia) or exposure to SRIs at least for 2 years before the baseline were excluded (washout period). The date (before 2015) on which participants received both a diagnosis of low bone density and a prescription for an osteoporosis-related medication was regarded as the index date. The period between the baseline and the index date is the observation period. Using a 1:5 matching ratio of cases to controls, controls without any diagnosis related to bone loss were randomly selected based on age, sex, date of medical check-up, and length of the observation period for each case in the group with low bone mass.
Outcome
The primary outcome was a new diagnosis of low bone density (osteoporosis or osteopenia) after exposure to SRIs. To increase the accuracy of diagnosis, the operational definition of low bone density was defined strictly as meeting all of the following requirements: first, a diagnostic code corresponding to osteoporosis or osteopenia (ICD-10 codes: M80, M81, M82, M85.8) during the observational period, second, prescription of osteoporosis-related medication on the same date as diagnosis and third, no diagnosis of osteoporosis or osteopenia or the prescription of SRIs during the washout period. In Korea, prescribing osteoporosis-related medication is allowed only if the T score derived from DEXA is lower than -1, a definition of low bone density that strongly supports the diagnosis of osteoporosis or osteopenia. The qualifying osteoporosis-related medications were vitamin D and its analogues (alfacalcidol, calcitriol, calcifediol), calcium (calcium gluconate, calcium carbonate, calcium citrate, ossopan substance, oyster shell powder), bisphosphonate (etidronic acid, pamidronic acid, alendronic acid, ibandronic acid, risedronic acid, zoledronic acid), calcitonin (salmon synthetic calcitonin, salcatonin, elcatonin), selective estrogen receptor modulators (raloxifene, bazedoxifene), and combination agents (cholecalciferol + ibandronic acid, cholecalciferol + alendronic acid, cholecalciferol + risedronic acid).
Exposure
Because the database includes drug codes and prescription dates, participants who were prescribed SRIs during the observation period were considered to be exposed to SRIs. Furthermore, participants were classified into three time-dependent user groups as follows. Participants who did not receive a prescription for SRIs from the baseline to the index date were considered non-users. Participants exposed to an SRI after the baseline who stopped taking the medication at least 6 months before the index date were considered former users. Participants who were prescribed of SRIs during the last 6 months of the observation period were considered recent users. The SRIs considered in this study were escitalopram, citalopram, sertraline, fluoxetine, fluvoxamine, paroxetine, trazodone, venlafaxine, desvenlafaxine, duloxetine, and milnacipran. The period between the initiation of SRI treatment and the diagnosis of low bone density in the recent SRI users was assessed.
Covariates
The following covariates were measured during the baseline exam: blood pressure (systolic, diastolic)41, body mass index (BMI), fasting blood glucose, total cholesterol, urine occult blood, urine protein, aspartate transaminase (AST), alanine transaminase (ALT), and Gamma-Glutamyltransferase (GTP)42. The analyses were also adjusted for demographic information (age, sex, household income)42,43,44, smoking status (non-smoker, ex-smoker, current smoker), the number of days of alcohol consumption per week, and the number of days of exercise per week42, which were collected through self-reported questionnaires and administration data at baseline. To control for the possible effects of other medications prescribed during the observation period, we made adjustments for several medications based on previous studies of low bone mass42,45: glucocorticoids, aromatase inhibitors, androgen deprivation therapy, gonadotropin-releasing hormone (GnRH) agonists, anticonvulsants, thiazolidinediones, depot-medroxyprogesterone acetate (DMPA), antipsychotics, benzodiazepine, tricyclic antidepressant (TCA : clomipramine, amitriptyline, amoxapine, imipramine, nortriptyline, doxepin), estrogen, and estrogen progesterone combination therapy. To control for medical comorbidities, the Charlson Comorbidity Index (CCI)42 was calculated based on claims collected for two years before the baseline exam. In addition, the number of healthcare visits during the observation period was measured.
Statistical analysis
To compare the baseline characteristics of the study sample, χ2 tests and t-tests were used for categorical variables and continuous data, respectively. A nested case–control analysis, commonly used in cohort studies to eliminate immortal-time bias, was used to determine the association between the use of SRIs and the incidence of a new diagnosis of low bone density. In a nested case–control study design, for each case, a specified number of controls who were free of disease on the diagnosis date of their corresponding cases were matched46. A conditional logistic regression was used to estimate the odds ratios (ORs) and corresponding 95% confidence intervals (CIs). We included the following covariates in the multivariable models: medication (glucocorticoids, aromatase inhibitors, androgen deprivation therapy, GnRH agonists, anticonvulsants, thiazolidinediones, DMPA, antipsychotics, benzodiazepine, TCA, estrogen, and estrogen progesterone combination therapy), demographic information (household income), CCI, physiological measures (systolic blood pressure, diastolic blood pressure, BMI, fasting blood glucose, total cholesterol, urine occult blood, urine protein, AST, ALT, and Gamma- GTP), and lifestyle habits (smoking status, frequency of drinking alcohol, and frequency of exercise). Furthermore, since subjects with poorer health conditions may be more likely to show a higher frequency of healthcare utilization, thus having higher chances of detecting osteoporosis or the prescription of SRIs, we conducted a sensitivity analysis including the number of healthcare visits as an additional covariate. The SAS Enterprise Guide was used for all statistical analyses, and p-values less than 0.05 were considered statistically significant.
Ethics
This study was approved by the Institutional Review Board of Yonsei University Severance Hospital (IRB No. 4-2019-0017) and was conducted in accordance with the principles of the Declaration of Helsinki.
Data availability
The data that support the findings of this study are available from the National Health Insurance Service in South Korea. Restrictions apply to the availability of these data, which were used under license for this study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the National Health Insurance Service.
References
Olfson, M. et al. Antidepressant prescribing practices of outpatient psychiatrists. Arch. Gen. Psychiatry 55, 310–316. https://doi.org/10.1001/archpsyc.55.4.310 (1998).
Hemels, M. E., Koren, G. & Einarson, T. R. Increased use of antidepressants in Canada: 1981–2000. Ann. Pharmacother. 36, 1375–1379. https://doi.org/10.1345/aph.1A331 (2002).
Yu, Z., Zhang, J., Zheng, Y. & Yu, L. Trends in antidepressant use and expenditure in six major cities in China from 2013 to 2018. Front. Psych. 11, 551. https://doi.org/10.3389/fpsyt.2020.00551 (2020).
Abbing-Karahagopian, V. et al. Antidepressant prescribing in five European countries: Application of common definitions to assess the prevalence, clinical observations, and methodological implications. Eur. J. Clin. Pharmacol. 70, 849–857. https://doi.org/10.1007/s00228-014-1676-z (2014).
Lockhart, P. & Guthrie, B. Trends in primary care antidepressant prescribing 1995–2007: A longitudinal population database analysis. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 61, e565-572. https://doi.org/10.3399/bjgp11X593848 (2011).
Ak, E. et al. Evaluation of the effect of selective serotonin reuptake inhibitors on bone mineral density: An observational cross-sectional study. Osteoporos. Int. 26, 273–279. https://doi.org/10.1007/s00198-014-2859-2 (2015).
Cauley, J. A. et al. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos. Int. 16, 1525–1537. https://doi.org/10.1007/s00198-005-1866-8 (2005).
Haney, E. M. et al. Association of low bone mineral density with selective serotonin reuptake inhibitor use by older men. Arch. Intern. Med. 167, 1246–1251. https://doi.org/10.1001/archinte.167.12.1246 (2007).
Ensrud, K. E. et al. Central nervous system active medications and risk for fractures in older women. Arch. Intern. Med. 163, 949–957. https://doi.org/10.1001/archinte.163.8.949 (2003).
Spangler, L. et al. Depressive symptoms, bone loss, and fractures in postmenopausal women. J. Gen. Intern. Med. 23, 567–574. https://doi.org/10.1007/s11606-008-0525-0 (2008).
French, D. D., Campbell, R., Spehar, A., Cunningham, F. & Foulis, P. Outpatient medications and hip fractures in the US: A national veterans study. Drugs Aging 22, 877–885. https://doi.org/10.2165/00002512-200522100-00006 (2005).
Hubbard, R., Farrington, P., Smith, C., Smeeth, L. & Tattersfield, A. Exposure to tricyclic and selective serotonin reuptake inhibitor antidepressants and the risk of hip fracture. Am. J. Epidemiol. 158, 77–84. https://doi.org/10.1093/aje/kwg114 (2003).
Hung, S. C., Lin, C. H., Hung, H. C., Lin, C. L. & Lai, S. W. Use of selective serotonin reuptake inhibitors and risk of hip fracture in the elderly: A case-control study in Taiwan. J. Am. Med. Dir. Assoc. 18, 350–354. https://doi.org/10.1016/j.jamda.2016.12.003 (2017).
Liu, B. et al. Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet 351, 1303–1307. https://doi.org/10.1016/s0140-6736(97)09528-7 (1998).
Rabenda, V., Nicolet, D., Beaudart, C., Bruyere, O. & Reginster, J. Y. Relationship between use of antidepressants and risk of fractures: A meta-analysis. Osteoporos. Int. 24, 121–137. https://doi.org/10.1007/s00198-012-2015-9 (2013).
Coupland, C. et al. Antidepressant use and risk of adverse outcomes in older people: Population based cohort study. BMJ 343, d4551. https://doi.org/10.1136/bmj.d4551 (2011).
Verdel, B. M. et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone 47, 604–609. https://doi.org/10.1016/j.bone.2010.06.006 (2010).
Diem, S. J. et al. Depressive symptoms and rates of bone loss at the hip in older women. J. Am. Geriatr. Soc. 55, 824–831. https://doi.org/10.1111/j.1532-5415.2007.01194.x (2007).
Bolton, J. M. et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatr. 74, 641–648. https://doi.org/10.1001/jamapsychiatry.2017.0449 (2017).
Sundaresh, V. & Singh, B. Antidepressants and fracture risk: Is there a real connection?. J. Am. Geriatr. Soc. https://doi.org/10.1111/jgs.16729 (2020).
Kinjo, M., Setoguchi, S., Schneeweiss, S. & Solomon, D. H. Bone mineral density in subjects using central nervous system-active medications. Am. J. Med. 118, 1414. https://doi.org/10.1016/j.amjmed.2005.07.033 (2005).
Mezuk, B., Eaton, W. W., Golden, S. H., Wand, G. & Lee, H. B. Depression, antidepressants, and bone mineral density in a population-based cohort. J. Gerontol. A Biol. Sci. Med. Sci. 63, 1410–1415. https://doi.org/10.1093/gerona/63.12.1410 (2008).
Schweiger, J. U. et al. The use of antidepressive agents and bone mineral density in women: A meta-analysis. Int. J. Environ. Res. Public Health 15. https://doi.org/10.3390/ijerph15071373 (2018).
Zhou, C. et al. Effect of selective serotonin reuptake inhibitors on bone mineral density: A systematic review and meta-analysis. Osteoporos. Int. 29, 1243–1251. https://doi.org/10.1007/s00198-018-4413-0 (2018).
Bliziotes, M. M., Eshleman, A. J., Zhang, X. W. & Wiren, K. M. Neurotransmitter action in osteoblasts: Expression of a functional system for serotonin receptor activation and reuptake. Bone 29, 477–486. https://doi.org/10.1016/s8756-3282(01)00593-2 (2001).
Warden, S. J., Robling, A. G., Sanders, M. S., Bliziotes, M. M. & Turner, C. H. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology 146, 685–693. https://doi.org/10.1210/en.2004-1259 (2005).
Battaglino, R. et al. Serotonin regulates osteoclast differentiation through its transporter. J. Bone Miner. Res. 19, 1420–1431. https://doi.org/10.1359/JBMR.040606 (2004).
Battaglino, R. et al. Fluoxetine treatment increases trabecular bone formation in mice. J. Cell Biochem. 100, 1387–1394. https://doi.org/10.1002/jcb.21131 (2007).
Ortuno, M. J. et al. Serotonin-reuptake inhibitors act centrally to cause bone loss in mice by counteracting a local anti-resorptive effect. Nat. Med. 22, 1170–1179. https://doi.org/10.1038/nm.4166 (2016).
Moura, C. et al. Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos. Int. 25, 1473–1481. https://doi.org/10.1007/s00198-014-2649-x (2014).
van den Brand, M. W. et al. Use of anti-depressants and the risk of fracture of the hip or femur. Osteoporos. Int. 20, 1705–1713. https://doi.org/10.1007/s00198-009-0849-6 (2009).
Eskandari, F. et al. Low bone mass in premenopausal women with depression. Arch. Intern. Med. 167, 2329–2336. https://doi.org/10.1001/archinte.167.21.2329 (2007).
Cizza, G., Primma, S. & Csako, G. Depression as a risk factor for osteoporosis. Trends Endocrin. Met. 20, 367–373. https://doi.org/10.1016/j.tem.2009.05.003 (2009).
Stetler, C. & Miller, G. E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 73, 114–126. https://doi.org/10.1097/PSY.0b013e31820ad12b (2011).
Marques-Deak, A., Cizza, G. & Sternberg, E. Brain-immune interactions and disease susceptibility (vol 10, pg 23, 2005). Mol. Psychiatr. 10, 972–972. https://doi.org/10.1038/sj.mp.4001732 (2005).
Li, X., Zhou, Z. Y., Zhang, Y. Y. & Yang, H. L. IL-6 contributes to the defective osteogenesis of bone marrow stromal cells from the vertebral body of the glucocorticoid-induced osteoporotic mouse. Plos One 11, e0154677. https://doi.org/10.1371/journal.pone.0154677 (2016)
Bishwajit, G. et al. Physical inactivity and self-reported depression among middle- and older-aged population in South Asia: World health survey. BMC Geriatr. 17, 100. https://doi.org/10.1186/s12877-017-0489-1 (2017).
Holick, M. F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 79, 362–371. https://doi.org/10.1093/ajcn/79.3.362 (2004).
Kanis, J. A. Estrogens, the menopause, and osteoporosis. Bone 19, 185S-190S. https://doi.org/10.1016/s8756-3282(96)90163-5 (1996).
Seong, S. C. et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 7, e016640. https://doi.org/10.1136/bmjopen-2017-016640 (2017).
Tanko, L. B. et al. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J. Bone Miner. Res. 20, 1912–1920. https://doi.org/10.1359/JBMR.050711 (2005).
Walker-Bone, K. Recognizing and treating secondary osteoporosis. Nat. Rev. Rheumatol. 8, 480–492. https://doi.org/10.1038/nrrheum.2012.93 (2012).
Cheng, H. et al. Estimated prevalence and patterns of presumed osteoporosis among older Americans based on Medicare data. Osteoporos. Int. 20, 1507–1515. https://doi.org/10.1007/s00198-009-0835-z (2009).
Hannan, M. T., Felson, D. T. & Anderson, J. J. Bone mineral density in elderly men and women: Results from the Framingham osteoporosis study. J. Bone Miner. Res. 7, 547–553. https://doi.org/10.1002/jbmr.5650070511 (1992).
Xing, D. et al. Association between use of benzodiazepines and risk of fractures: A meta-analysis. Osteoporos. Int. 25, 105–120. https://doi.org/10.1007/s00198-013-2446-y (2014).
Ernster, V. L. Nested case-control studies. Prev. Med. 23, 587–590. https://doi.org/10.1006/pmed.1994.1093 (1994).
Acknowledgements
This study used NHIS-HEALS data made available by the NHIS in South Korea. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2018R1A2B2007714 and NRF-2019R1A2C1084611). The funding source did not give any influences on the study design, data collection, analysis and interpretation of data, the writing of the report, and the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
J.I.K. and S.J.K. conceived and designed the study with input from all authors. M.H. and S.K. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. S.K. drafted the first version of the manuscript. All authors interpreted the data and contributed to the writing of the paper. All authors read and critically revised the whole report. J.I.K. and S.J.K. act as guarantor for the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, S., Han, M., Park, C.I. et al. Use of serotonin reuptake inhibitors and risk of subsequent bone loss in a nationwide population-based cohort study. Sci Rep 11, 13461 (2021). https://doi.org/10.1038/s41598-021-92821-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92821-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.