Abstract
This study aimed to assess the presence of qnrA, qnrB, qnrC, qnrD, qnrS, qepA, and aac(6′)-Ib-cr determinants as well as quinolone resistance pattern of clinical isolates of P. aeruginosa in Ahvaz, southwest Iran. A total of 185 clinical isolates of P. aeruginosa were collected from 5 university-affiliated hospitals in Ahvaz, southwest Iran. The disk diffusion method was applied to assess the quinolone resistance pattern. The presence of qnrA, qnrB, qnrC, qnrD, qnrS, qepA, and aac(6′)-Ib-cr genes was investigated by the polymerase chain reaction (PCR) method. Overall, 120 (64.9%) isolates were non-susceptible to quinolones. The most and the less quinolone resistance rates were observed against ciprofloxacin (59.4%) and ofloxacin (45.9%), respectively. The prevalence rates of qnr genes were as follows: qnrA (25.8%), qnrB (29.2%), and qnrS (20.8%). The qnrB gene was the most common type of qnr genes. The qnr genes were occurred in 37.5% (n = 45/120) of quinolne-resistant isolates, simultaneously. The qnrC, qnrD, qepA, and aac(6′)-Ib-cr genes were not recognized in any isolates. In conclusion, the ofloxacin was the most effective quinolone. This study was the first to shed light on the prevalence of PMQR genes among P. aeruginosa isolates in southwest Iran.
Similar content being viewed by others
Introduction
Pseudomonas aeruginosa as one of the most frequent nosocomial pathogens has become an important leading cause of death in burn, cystic fibrosis (CF), and immunocompromised patients1,2,3. According to the records of the Centers for Disease Control (CDC), approximately 51,000 nosocomial P. aeruginosa infections occur annually in the United States4. Nearly, 13% of them are caused by multidrug-resistant (MDR) P. aeruginosa species that account for about 400 deaths per year4. The widespread use of broad-spectrum antibiotics has resulted in the emergence of MDR bacterial strains as a major problem in health care systems5. It has been remarked that the MDR P. aeruginosa strains are associated with a considerable increase in the length of hospitalization as well as morbidity and mortality6. The most effective antibacterial compounds against P. aeruginosa include β-lactams, aminoglycosides, and fluoroquinolones7.
The use of fluoroquinolone antibiotics has spread widely in the past decade leading to the emergence of resistant bacterial strains8,9. The plasmid-mediated quinolone resistance (PMQR) is considered as a common mechanism contributed to resistance among Gram-negative bacilli10,11. The PMQR was initially reported in 1998 from the clinical isolates of Klebsiella pneumoniae. So far, three PMQR mediated mechanisms were recognized that include qnr genes (coding Qnr proteins), the acetyltransferase aac(6′)-Ib-cr which is a variant of an enzyme involved in aminoglycoside alteration and resistance, and active efflux pumps such as QepA and OqxAB10,11. So far, seven major Qnr families have been identified: QnrA, QnrB, QnrS, QnrC, QnrD, QnrE, and QnrVC12. The qnr genes counteract with the blockage effects of quinolone antibiotics on the microbial enzymes such as topoisomerase II (DNA gyrase) and topoisomerase IV10. Other probable quinolone resistance mechanisms in P. aeruginosa include chromosomal mutations in quinolone resistance determining region (QRDR) of topoisomerase (parC/parE) and DNA gyrase (gyrA/gyrB) encoding genes, and mobile efflux pumps such as OqxAB10,11,12,13,14. Also, mutations of the regulatory genes that affect the permeability or efflux process are among the contributed quinolone resistance mechanisms10,11.
To the best of our knowledge, there are scarce data available on the prevalence of PMQR genes among clinical isolates of P. aeruginosa worldwide, especially in Iran. Thus, considering the importance of P. aeruginosa and the qnr genes, the present study aimed to assess the prevalence of qnrA, qnrB, qnrS, qnrC, qnrD, qepA, and aac(6′)-Ib-cr genes as well as the antibiotic resistance pattern of fluoroquinolone-resistant P. aeruginosa strains isolated from clinical specimens in Ahvaz city, southwest of Iran.
Results
Bacterial isolates
Based on standard bacteriological tests, overall, 185 (22.5%) clinical strains of P. aeruginosa were collected from four main hospitals in Ahvaz, southwest of Iran during a two-year period. All isolates were positive for ecfX gene. The distribution of P. aeruginosa isolates according to hospital, gender, age, wards, and specimens are summarized in Table 1. The cases included 75 (40.5%) females and 110 (59.5%) males. The bacterial isolates were obtained from different clinical specimens. The most isolates were collected from wound, n: 69 (37.3%); followed by urine, n: 40 (21.6%); blood, n: 36 (19.4%); abscess, n: 15 (8.1%); sputum, n: 12 (6.5%); abdominal secretion, n: 8 (4.3%); and cerebrospinal fluid, n: 5 (2.7%). Also, the majority of isolates were collected from Burn, Urology, and Intensive Care Unit (ICU) ward.
Resistance to quinolone compounds
Overall, 120 (64.9%) isolates were non-susceptible to quinolone compounds used in this study. The antibacterial susceptibility testing revealed that ofloxacin (54.1% susceptible) was the most effective drug compared to the other fluoroquinolone antibiotics. The highest resistance rates of isolates were against ciprofloxacin (59.4%) followed by levofloxacin (56.8%), norfloxacin and gatifloxacin (each 48.6%) (Table 2). All 120 quinolone-resistant isolates were simultaneously resistant against 2 or more quinolones (Table 3).
Antibiotic resistance rates of quinolone-susceptible and quinolone-resistant isolates
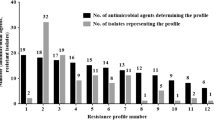
The P. aeruginosa isolates showed the most predominant resistance against third generation cephalosporins: ceftriaxone (78.4%) and ceftazidime (76.2%). Antibacterial resistance rates of quinolone-susceptible and quinolone-resistant isolates are summarized in Table 4. Aztreonam, cefepime, and tobramycin were the most effective antibiotics against quinolone-susceptible and quinolone-resistant isolates. There was no significant differences between the quinolone-susceptible and quinolone-resistant isolates in term of antibiotic resistance rates. The rates of MDR, extensively drug-resistant (XDR), and pandrug-resistant (PDR) isolates were, 78.4% (n = 145), 8.1% (n = 15), and 0.0 % (n = 0), respectively. The MDR isolates showed 10 various resistotypes (Table 5).
Presence of PMQR genes
Out of 120 fluoroquinolone-resistant P. aeruginosa strains, 46 (38.3%) isolates were positive for the qnr genes by PCR screening. The results of molecular assay indicated that qnrB gene was the most predominant 29.2% (n = 35), followed by qnrA 25.8% (n =31), and qnrS gene 20.8% (n = 25), respectively. The qnr genes were occurred in 37.5% (n = 45/120) of isolates, simultaneously. The qnrA gene was found to coexist with qnrB in 20 (16.7%) and coexist with qnrS in 11 (9.2%) isolates, respectively. Also, 10 (8.3%) isolates harbored both qnrB and qnrS, and 4 (3.3%) isolates harbored the qnrA, qnrB, and qnrS genes, simultaneously. The qnrC, qnrD, qepA, and aac(6′)-Ib-cr genes were not detected in studied isolates.
Discussion
In the Iran, few studies on the incidence of quinolone-resistant P. aeruginosa and its plasmid-mediated mechanisms have been reported. In this regard, we designed the present study to the better understanding of molecular and epidemiological aspects of quinolone resistance pattern of clinical P. aeruginosa isolates in Ahvaz, southwest Iran. In this study, 185 clinical P. aeruginosa isolates were investigated. The most strains were isolated from wound (37.3%), urine (21.6%), and blood (19.4%). Also, the majority of them were collected from Burn, Urology, and ICU ward. In agreement with these findings, a previous report from Iran by Izadi Pour Jahromi et al.15 from Iran, isolated the most P. aeruginosa strains from wound and urine. Also, they showed the more incidence of P. aeruginosa in Burn, Pediatric, and ICU wards. Also, Shahraki Zahedani et al.16 reported the most prevalent P. aeruginosa isolates in urine (17.44%), wound (24.41 %), and blood (33.72%) samples.
This research revealed a total quinolone resistance rate of 64.9% among clinical P. aeruginosa isolates that was higher than the previous reports from Saudi Arabia (42.4%) and Egypt (57.2%)13,17. In this study, the resistance rates against five tested quinolones including gatifloxacin, norfloxacin, ciprofloxacin, ofloxacin, and levofloxacin varied from 45.9% to 59.4%. The antimicrobial susceptibility testing revealed that the most effective quinolone in the present study was ofloxacin, while more of our isolates were resistant to ciprofloxacin. In contrast to the current findings, Rajaei et al.18 from Iran reported the ciprofloxacin as the most effective antibiotic against clinical P. aeruginosa isolates. In another study by Adwan et al.14 from Palestine who investigated 11 clinical P. aeruginosa isolates, 100.0 % of them were resistant against norfloxacin and ciprofloxacin. El-Badawy et al.13 who investigated seven quinolone antibiotics including nalidixic acid, ciprofloxacin, norfloxacin, ofloxacin, levofloxacin, gemifloxacin, and moxifloxacin against P. aeruginosa isolates, disclosed resistance rates ranging from 28.3% to 41.3%. In their study, the gemifloxacin with 28.3% and nalidixic acid with 41.3% resistance rates were the most and the less effective quinolones, respectively that were not evaluated in the current research.
In this study, although the resistance to all tested antibiotics was more than 50.0%, aztreonam, tobramycin, cefepime, and imipenem were among the most effective drugs against both quinolone-resistant and quinolone-susceptible strains. However, our isolates showed a high resistance rates (more than 70.0%) against other aminoglycosides (gentamycin, amikacin), cephalosporins (ceftazidime, ceftriaxone), and penicillins (piperacillin, piperacillin/tazobactam). Previous studies from Iran have approved the high resistance rates of the P. aeruginosa to a wide range of antibiotics, which was similar to current results19,20. In contrast to these findings, Brzozowski et al.21 from Poland reported a lower resistance rates for ciprofloxacin (39.1%), amikacin (30.7%), cefepime (42.6%), ceftazidime (33.2%), gentamycin (37.6%), piperacillin/tazobactam (39.6%), tobramycin (38.1%), and imipenem (67.8%) in clinical P. aeruginosa. These discrepancies could be due to differences in the geographical regions and diversity of antibiotic prescription patterns, as well as the lack of a comprehensive and organized monitoring program for the proper use of antibiotics in several countries.
In recent years, the prevalence of carbapenem-resistant Gram-negative bacteria has increased worldwide. This is a unique clinical problem, as these drugs are long regarded to be the most active and powerful treatment for the infections caused by MDR bacteria. In the current study, 68.1% of P. aeruginosa isolates were resistant to imipenem. In line with our findings, previous reports by Farajzadeh Sheikh et al.19 (90.7%) and Tarafdar et al.20 (95.8%) from Iran, stated a high resistance rate against carbapenems in the clinical P. aeruginosa isolates. This may be due to the presence of metallo-β-lactamase or carbapenemase enzymes and upregulation of different efflux pumps in these strains22.
Another remarkable result of the current study was the high occurrence of MDR P. aeruginosa (78.4%) that was greater than previous indicated statistics from Iran (31.4%), Poland (48%)28, and Egypt (66.6%)23. However, the XDR rate (8.1%) was lower than a report by Shahraki Zahedani et al.16 from Iran (12.3%). No PDR isolate was detected in this study. In another study by Pérez et al.24, who investigated 53 P. aeruginosa isolates from Greece, Italy, and Spain, a total of 30.2% MDR, 35.8% XDR, and 3.8% PDR strains were reported. In our region, incorrect antibiotic prescriptions might be a contributing factor to this higher prevalence of MDR isolates.
This study was the first report on the presence of the qnr genes in quinolone-resistant clinical P. aeruginosa isolates in patients from southwest Iran. According to our findings, 38.3% of isolates were positive for the qnr genes, from which 29.2%, 25.8%, and 20.8% isolates had the qnrB, qnrA, and qnrS genes, respectively. So far, despite the importance of the subject, few studies have addressed this issue.
In a study by Michalska et al.25 from Poland, the qnrB with a frequency of 20.0% was the only detected gene among clinical P. aeruginosa isolates. In contrast to the current research, they did not find the qnrA and qnrS genes. Also, our findings significantly differed from the results reported by Molapour et al.26 from Iran who did not find any qnr gene in 149 quinolone-resistant P. aeruginosa that were isolated from burn patients. In another study by Cayci et al.27 from Turkey, qnrA, qnrB, and qnrS genes were not detected in P. aeruginosa isolates.
The qnrB gene was the most common qnr type in our region. However, a previous reports by Rajaei et al.18 from Kerman city, Iran indicated the qnrA gene as the predominant PMQR (16.6%). Also, in comparison with our findings, they showed a lower prevalence rates for qnrB (13.3%) and qnrS (11.6%). Moreover, Saleh et al.17 from Egypt, showed a lower prevalence rates for qnr genes in comparison with our findings. In their study, the total prevalence rate for qnr genes was equal to 4.5%. They detected the qnrB and qnrS in 1.8% and 2.7% of P. aeruginosa, respectively. El-Badawy et al.13 from Saudi Arabia reported a much higher occurrence rate for qnrS (79.5%) than our study. Also, in the previous studies from Egypt, Iraq, and China, the qnrS has been recorded as the major quinolone resistant gene among MDR P. aeruginosa strains17,28,29.
In this study, the qnrC, qnrD, qepA, and aac(6′)-Ib-cr genes were not detected in studied isolates. In contrast to our report, El-Badawy et al.13 and Jiang et al.29 reported a prevalence of 79.5% and 0.9% for qnrD, respectively. However, previous studies from Saudi Arabia13, Egypt17, and China29 were not detected the qepA gene that was in line with the current study. The aac(6′)-Ib-cr gene was first identified in 2003, and it has subsequently been found in a variety of regions and sources30. This gene has been reported in P. aeruginosa strains in previous studies from Saudi Arabia (71.8%)13, Iran (8.3%)18, and China (1.9%)29. Also, in a report by Molapour et al.26 from Iran, all 149 P. aeruginosa isolates harbored aac(6′)-Ib-cr gene that was inconsistent with our result.
In the current study, the qnr genes were occurred in 37.5% (n = 45/120) of isolates, simultaneously. In line with these results, the coexistence of PMQR genes has been reported previously in P. aeruginosa isolates from various countries including Saudi Arabia and China13,29.
We found that 74 (61.7%) fluoroquinolone-resistant clinical isolates were negative for the qnrA, qnrB, , qnrC, qnrD, qnrS, qepA, and aac(6′)-Ib-cr genes. The quinolone resistance phenomenon in these isolates may be due to the other qnr genes like qnrE and qnrVC, or recently introduced crpP gene that encodes a ciprofloxacin modifying enzyme CrpP that were not investigated here12,31. After being discovered in a Brazilian Vibrio cholerae strain in 1998, qnrVC gene is now more often linked with bacteria that live in the aquatic environment31,32. This gene has different alleles. In two recent studies by Khan et al.31 and Lin et al.33 the prevalence rates of 12.0% and 2.3% were reported for this gene in clinical P. aeruginosa. Also, Khan et al.31 reported a prevalence of 63.0% for crpP gene in corneal P. aeruginosa isolates. So far, these genes have not been investigated and reported in any bacteria from Iran.
As the majority of quinolone-resistant P. aeruginosa strains in this study lack the PMQR genes, it is recommended to investigate the other aforesaid mechanisms to shed light on the precise molecular epidemiology of these isolates.
In conclusion, considering that the antibiotic resistance profiles constantly differ in each area and hospital setting, the periodic surveillance program is very crucial for each country to determine the most appropriate treatment choices. Based on our results, the ofloxacin was the best quinolone for the treatment of P. aeruginosa infections. Also, aztreonam, cefepime, and tobramycin could be suitable alternative treatment when there are restrictions or inhibitions on the use of quinolones. Also, the high frequency of MDR P. aeruginosa justifies the need to develop a monitoring program to reduce this occurrence and control the more spread of these strains in our region. This research was the first work in southwest Iran which adds to our knowledge of how P. aeruginosa withstand quinolones. The qnrB was the most PMQR determinant in clinical P. aeruginosa isolates from southwest Iran, while the qnrC, qnrD, qepA, and aac(6′)-Ib-cr genes were not detected. Other possible mechanisms of resistance should also be studied for better characterization of quinolone-resistant P. aeruginosa isolates. Lack of evaluation of chromosomal mutations in the QRDR region and failure to use whole-genome sequencing to provide more in-depth data on high-risk clones and other resistance genes (such as extended spectrum beta-lactamases and carbapenemases) often associated with PMQR, were some limitations of this study.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee of the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (No: IR.AJUMS.REC.1397.206). All methods were performed in accordance with the relevant guidelines and regulations of the Ethics Committee of the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The informed consent was obtained from all patients.
Samples and patients
In this cross-sectional study during March 2017–April 2019, a total of 185 non-repetitive clinical isolates of P. aeruginosa were obtained from patients who referred to the four university teaching hospitals in Ahvaz, southwest of Iran. The P. aeruginosa isolates were sent to the laboratory of Department of Microbiology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, for further confirmation and molecular assays.
Bacterial isolation and identification
All studied samples were inoculated on 5% sheep blood agar and MacConkey agar plates and incubated aerobically at 37 °C for 24 h. The P. aeruginosa isolates were identified by standard bacteriological analyses such as Gram staining, catalase, oxidase, biochemical reaction on sulfur-indole-motility (SIM) agar, triple sugar iron (TSI) agar, lysine iron agar (LIA), oxidative fermentative (OF) test, growth at 42 °C, and growth on cetrimide agar34. All culture media were purchased from Merck Co., Darmstadt, Germany. The suspected P. aeruginosa isolates were confirmed by polymerase chain reaction (PCR) using specific primers for ecfX gene as described by Sands et al. (Table 6)35.
Antimicrobial susceptibility testing (AST)
The resistance pattern of isolates against 15 antibiotics including 5 fluoroquinolones was determined by disc diffusion method on Mueller-Hinton agar (Merck, Germany) as described by the Clinical Laboratory Standards Institute (CLSI 2017) guidelines36. The antibiotic disks used were as follows: amikacin (30 μg), aztreonam (30 μg), cefepime (30 μg), ceftazidime (30 μg), ceftriaxone (30 μg), ciprofloxacin (5 μg), gatifloxacin (5 μg), norfloxacin (5 μg), gentamycin (10 μg), imipenem (10 μg), levofloxacin (5 μg), ofloxacin (5 μg), piperacillin (100 μg), piperacillin/tazobactam (100 μg/10 μg), and tobramycin (10 μg) (MAST Co., Berkshire, UK). Drug-resistant patterns were defined as follows: MDR isolates (resistant to at least three antibiotics belonging to different chemical classes), XDR strains (resistant to at least one agent in all but two or fewer antimicrobial groups), and PDR strains (resistant to all antimicrobial classes)37. E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as quality control strains.
DNA extraction
DNA was extracted using boiling method as previously described38. Briefly, few colonies were picked up from an overnight growth (24 h) on a Mueller-Hinton agar and suspended into 500 μl of distilled water. The mixture was vortexed for 20 s and then boiled for 10 min. In the end, all samples were centrifuged at 14,000 rpm for 10 min and the supernatants were stored at − 20 °C as DNA template for polymerase chain reaction (PCR) assay. The quality and quantity of DNA (ng/µl) were evaluated by measuring the absorbance of A260 and A280 nm with a NanoDrop spectrophotometer (Thermofisher Scientific, USA). The DNA samples that had a concentration of at least 50 ng/µl, were selected for PCR.
PCR assay for PMQR genes
The previously published PCR primer pairs were used for the detection of qnrA, qnrB, , qnrC, qnrD, qnrS, qepA, and aac(6′)-Ib-cr genes and the specificity of each primer was proved using the NCBI Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/)39,40. The sequences of all used primers are presented in Table 6. The PCR assay was performed under the following conditions: initial denaturation step for 4 min at 94 °C, 35 cycles of denaturation at 94 °C for 45 s, annealing for 40 s (temperature was depending on the sequence of primers), extension at 72 °C for 50 s, and a final extension step of 72 °C for 5 min. The final products were detected by electrophoresis on 1% agarose gel (70 V, 1 h) containing ethidium bromide (0.5 μg/ml) in the Tris-EDTA buffer, and the gel was seen under ultraviolet illuminator (Proteinsimple, San Jose, CA, USA). The size of PCR amplicons was estimated by the migration pattern of a 100-bp DNA ladder (Sinaclon, Tehran, Iran). For confirmation, some of PCR products were sent to Bioneer Corporation (Korea) for gene sequencing (ABI PRISM 7700 Sequence Detection System, Applied Biosystems, Foster City, CA). These products were used as control positive. Also, in each PCR run, the distilled water was used as negative control.
Statistical analysis
Statistical data analysis was performed by the use of SPSS software version 22.0 (IBM Corporation, Armonk, NY, USA). The results were described as descriptive statistics in terms of relative frequency. The Fisher’s test was used, with a P-value < 0.05 deemed statistically significant.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
03 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-07352-8
References
Chegini, Z. et al. Bacteriophage therapy against Pseudomonas aeruginosa biofilms: A review. Ann. Clin. Microbiol. Antimicrob. 19, 1–7 (2020).
Vaitekenas, A., Tai, A. S., Ramsay, J. P., Stick, S. M. & Kicic, A. Pseudomonas aeruginosa resistance to bacteriophages and its prevention by strategic therapeutic cocktail formulation. Antibiotics. 10, 145 (2021).
Sadredinamin, M. et al. Detection of ISPa1328 and ISPpu21, two novel Insertion sequences in the OprD porin and blaIMP-1 gene among metallo-beta-lactamase-producing Pseudomonas aeruginosa isolated from burn patients. Arch. Trauma. Res. 6, e36239 (2017).
Chatterjee, M. et al. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int. J. Med. Microbiol. 306, 48–58 (2016).
Abadi, A. T., Rizvanov, A. A., Haertlé, T. & Blatt, N. L. World Health Organization report: Current crisis of antibiotic resistance. BioNanoScience. 9, 778–788 (2019).
Mirzaei, B. et al. Prevalence of multi-drug resistant (MDR) and extensively drug-resistant (XDR) phenotypes of Pseudomonas aeruginosa and Acinetobacter baumannii isolated in clinical samples from Northeast of Iran. BMC. Res. Notes. 13, 1–6 (2020).
Pachori, P., Gothalwal, R. & Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes. Dis. 6, 109–119 (2019).
Pham, T. D. M., Ziora, Z. M. & Blaskovich, M. A. T. Quinolone antibiotics. Medchemcomm. 10, 1719–1739 (2019).
Araujo, B. F. et al. Clinical and molecular epidemiology of multidrug-resistant P. aeruginosa carrying aac (6′)-Ib-cr, qnrS1 and blaSPM genes in Brazil. PLoS ONE 11, e0155914 (2016).
Rodríguez-Martínez, J. M. et al. Plasmid-mediated quinolone resistance: Two decades on. Drug. Resist. Updat. 29, 13–29 (2016).
Taha, S. A. & Omar, H. H. Characterization of plasmid-mediated qnrA and qnrB genes among Enterobacteriaceae strains: Quinolone resistance and ESBL production in Ismailia, Egypt. Egypt. J. Med. Hum. Genet. 20, 1–7 (2019).
Hamed, S. M. et al. Plasmid-mediated quinolone resistance in Gram-negative pathogens isolated from cancer patients in Egypt. Microb. Drug. Resist. 24, 1316–1325 (2018).
El-Badawy, M. F., Alrobaian, M. M., Shohayeb, M. M. & Abdelwahab, S. F. Investigation of six plasmid-mediated quinolone resistance genes among clinical isolates of Pseudomonas: A genotypic study in Saudi Arabia. Infect. Drug. Resist. 12, 915–923 (2019).
Adwan, G. & Omar, G. Phenotypic and molecular characterization of fluoroquinolone resistant Pseudomonas aeruginosa isolates in Palestine. Braz. J. Biol. 82, e239868 (2021).
Izadi Pour Jahromi, S. et al. Occurrence of a multidrug resistant Pseudomonas aeruginosa strains in hospitalized patients in southwest of Iran: Characterization of resistance trends and virulence determinants. Jundishapur. J. Microbiol. 11, e57341 (2018).
Shahraki Zahedani, S., Tahmasebi, H. & Jahantigh, M. Coexistence of virulence factors and efflux pump genes in clinical isolates of Pseudomonas aeruginosa: Analysis of biofilm-forming strains from Iran. Int. J. Microbiol. 2021, 5557361 (2021).
Saleh, M. A. & Balboula, M. M. Plasmid mediated quinolone resistance determinants among nosocomial clinical Pseudomonas aeruginosa isolates. Int. J. Curr. Microbiol. App. Sci. 6, 42–50 (2017).
Rajaei, S., Kazemi-Pour, N. & Rokhbakhsh-Zamin, F. Frequency of plasmid-mediated quinolone resistance genes among clinical isolates of Pseudomonas aeruginosa in Kerman, Iran. Iran. J. Microbiol. 11, 10–18 (2017).
Farajzadeh Sheikh, A. et al. Molecular epidemiology of colistin-resistant Pseudomonas aeruginosa producing NDM-1 from hospitalized patients in Iran. Iran. J. Basic. Med. Sci. 22, 38–42 (2019).
Tarafdar, F., Jafari, B. & Azimi, T. Evaluating the antimicrobial resistance patterns and molecular frequency of blaoxa-48 and blaGES-2 genes in Pseudomonas aeruginosa and Acinetobacter baumannii strains isolated from burn wound infection in Tehran, Iran. New. Microb. New. Infect. 37, 100686 (2020).
Brzozowski, M., Krukowska, Ż, Galant, K., Jursa-Kulesza, J. & Kosik-Bogacka, D. Genotypic characterisation and antimicrobial resistance of Pseudomonas aeruginosa strains isolated from patients of different hospitals and medical centres in Poland. BMC. Infect. Dis. 20, 1–9 (2020).
Doi, Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin. Infect. Dis. 69, S565–S575 (2019).
Farhan, S. M., Ibrahim, R. A., Mahran, K. M., Hetta, H. F. & Abd El-Baky, R. M. Antimicrobial resistance pattern and molecular genetic distribution of metallo-β-lactamases producing Pseudomonas aeruginosa isolated from hospitals in Minia, Egypt. Infect. Drug. Resist. 12, 2125–2133 (2019).
Pérez, A. et al. High incidence of MDR and XDR Pseudomonas aeruginosa isolates obtained from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J. Antimicrob. Chemother. 74, 1244–1252 (2019).
Michalska, A. D., Sacha, P. T., Ojdana, D., Wieczorek, A. & Tryniszewska, E. Prevalence of resistance to aminoglycosides and fluoroquinolones among Pseudomonas aeruginosa strains in a University Hospital in Northeastern Poland. Braz. J. Microbiol. 45, 1455–1458 (2015).
Molapour, A., Peymani, A., Saffarain, P., Habibollah-Pourzereshki, N. & Rashvand, P. Plasmid-mediated quinolone resistance in Pseudomonas aeruginosa isolated from burn patients in Tehran, Iran. Infect. Disord. Drug. Targets 20, 49–55 (2020).
Cayci, Y. T., Coban, A. Y. & Gunaydin, M. Investigation of plasmid-mediated quinolone resistance in Pseudomonas aeruginosa clinical isolates. Indian. J. Med. Microbiol. 32, 285–289 (2014).
Al-Marjani, M. F. Presence of qnr gene in environmental and clinical Pseudomonas aeruginosa isolates in Baghdad. Int. J. Curr. Microbiol. App. Sci. 3, 853–857 (2014).
Jiang, X. et al. Emergence of plasmid-mediated quinolone resistance genes in clinical isolates of Acinetobacter baumannii and Pseudomonas aeruginosa in Henan, China. Diagn. Microbiol. Infect. Dis. 79, 381–383 (2014).
Pereira, R. V. et al. Genotypic antimicrobial resistance characterization of E. coli from dairy calves at high risk of respiratory disease administered enrofloxacin or tulathromycin. Sci. Rep. 10, 19327 (2020).
Khan, M. et al. Acquired fluoroquinolone resistance genes in corneal isolates of Pseudomonas aeruginosa. Infect. Genet. Evol. 85, 104574 (2020).
Liu, J. et al. Complete sequence of pBM413, a novel multidrug resistance megaplasmid carrying qnrVC6 and blaIMP-45 from Pseudomonas aeruginosa. Int. J. Antimicrob. Agents. 51, 145–150 (2018).
Lin, J., Chen, D. Q., Hong, J., Huang, H. & Xu, X. Prevalence of qnrVC genes in Pseudomonas aeruginosa clinical isolates from Guangdong, China. Curr. Microbiol. 77, 1532–1539 (2020).
Mahon, C. R., Lehman, D. C. & Manuselis, G. Textbook of Diagnostic Microbiology-E-Book (Elsevier Health Sciences, 2014).
Sands, K. M. et al. Microbial profiling of dental plaque from mechanically ventilated patients. J. Med. Microbiol. 65, 147 (2016).
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-sixth informational supplement. CLSI document M100-S26. Wayne, PA: Clinical and Laboratory Standards Institute; (2017).
Magiorakos, A. P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18, 268–281 (2012).
Sheikh, A. F., Bandbal, M. M. & Saki, M. Emergence of multidrug-resistant Shigella species harboring extended-spectrum beta-lactamase genes in pediatric patients with diarrhea from southwest of Iran. Mol. Biol. Rep. 47, 7097–7106 (2020).
Chen, C. M. et al. High diversity of antimicrobial resistance genes, class 1 Integrons, and genotypes of multidrug-resistant Escherichia coli in beef carcasses. Microb. Drug. Resist. 23, 915–924 (2017).
Li, P. et al. Characterization of plasmid-mediated quinolone resistance in gram-negative bacterial strains from animals and humans in China. Microb. Drug. Resist. 25, 1050–1056 (2019).
Acknowledgements
This research was approved in Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Our appreciation goes to Deputy of Vice of Chancellor for Research Affairs, and Tropical and Infectious Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, for their financial and executive support (Grant No. OG-9717).
Author information
Authors and Affiliations
Contributions
M.S., A.F.S., and S.S.M. designed and supervised the study. A.A.Z.D., M.S., H.V., R.K., and P.K. acquired the data. All authors analyzed the results. M.T. and M.S. wrote the draft of main manuscript. All authors reviewed and approved final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors in the Affiliations, the Results section, the Discussion section and the grant number in the metadata. Full information regarding the corrections made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saki, M., Farajzadeh Sheikh, A., Seyed-Mohammadi, S. et al. Occurrence of plasmid-mediated quinolone resistance genes in Pseudomonas aeruginosa strains isolated from clinical specimens in southwest Iran: a multicentral study. Sci Rep 12, 2296 (2022). https://doi.org/10.1038/s41598-022-06128-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06128-4
This article is cited by
-
Ciprofloxacin- and levofloxacin-loaded nanoparticles efficiently suppressed fluoroquinolone resistance and biofilm formation in Acinetobacter baumannii
Scientific Reports (2024)
-
Recent advances in therapeutic targets identification and development of treatment strategies towards Pseudomonas aeruginosa infections
BMC Microbiology (2023)
-
Review of hospital effluents: special emphasis on characterization, impact, and treatment of pollutants and antibiotic resistance
Environmental Monitoring and Assessment (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.