Abstract
Renal disease is associated with increased arterial stiffness. The aim was to investigate the effect of renal disease on regional aortic strain and distensibility in children with chronic kidney disease (CKD) by cardiac magnetic resonance imaging (MRI). The study included 30 children with CKD on hemodialysis, and ten healthy control subjects. Using cardiac MRI, maximal and minimal aortic areas were measured in axial cine steady state free precision images at the ascending aorta, proximal descending, and aorta at diaphragm. Regional strain and distensibility were calculated using previously validated formulas. Second reader aortic areas measurements were used to assess inter-observer agreement. Ascending aorta strain was significantly reduced in patients (38.4 ± 17.4%) compared to the control group (56.1 ± 17%), p-value 0.011. Ascending Aorta distensibility was significantly reduced in patients (9.1 ± 4.4 [× 10−3 mm Hg−1]) compared to the control group (13.9 ± 4.9 [× 10−3 mm Hg−1]), p-value 0.006. Strain and distensibility were reduced in proximal descending aorta and aorta at diaphragm but did not reach statistical significance. Only ascending aorta strain and distensibility had significant correlations with clinical and cardiac MRI parameters. Inter-observer agreement for strain and distensibility was almost perfect or strong in the three aortic regions. Aortic strain and distensibility by cardiac MRI are important imaging biomarkers for initial clinical evaluation and follow up of children with CKD.
Similar content being viewed by others
Introduction
The aorta is an elastic conduit that distends in systole and recoils in diastole, transforming pulsatile blood flow ejected from the left ventricle (LV) into continuous peripheral blood flow to maintain blood supply to the organs1,2. During systole, the aorta distends to accommodate almost 60% of the LV stroke volume. Part of the LV contractile energy is stored, and then released during diastole pushing the accommodated blood to the periphery1.
When the aorta becomes less distensible, the stored portion of LV stroke volume in the aorta during systole is decreased, and more blood volume is pushed to the peripheral circulation. Accordingly, systolic blood pressure is increased1. Decreased aortic distensibility is a determinant factor associated with vascular aging3. In addition, it seems to play an important role in the pathogenesis of hypertension and atherosclerosis and is associated with cardiovascular changes in diabetes and obesity1,4.
Chronic kidney disease (CKD) causes premature vascular aging5. Previous studies demonstrated that arterial stiffening is an early sign of cardiovascular disease in patients with CKD, and a promising marker for prediction of cardiovascular risk6.
Magnetic resonance imaging (MRI) is one of the methods for assessment of aortic strain and distensibility based on cross-sectional area measurements2. The advantages of MRI over echocardiography are better visualization of the aorta, and accurate measurements of cross-sectional area changes rather than one-dimensional wall diameter as seen in M-mode echocardiography7. Cardiac MRI is also the gold standard for assessment of LV dimensions and function, and arterial function assessment could be performed in the same investigation.
In children with CKD, previous reports showed that arterial stiffness contributes to cardiovascular morbidity or mortality8. In this context, arterial function is considered an important parameter in clinical evaluation of these patients8.
To our knowledge, the use of cardiac MRI for assessment of aortic function in pediatric CKD is poorly investigated. The aim of this study was to investigate aortic strain and distensibility by cardiac MRI in children with CKD.
Methods
Subjects
This prospective study was performed from January to April 2019. The study was approved by the institutional review board of Mansoura University, Mansoura, Egypt. The study was carried out in accordance to guidelines and regulations of medical research. Participants older than 16 years old, or guardians of younger children gave written informed consent.
Consecutive pediatric patients with stage 5 CKD on hemodialysis attending the pediatric nephrology outpatient clinic at our institution were recruited. The study population is part of a study project on multi-parametric cardiac MRI in children with CKD9.
The inclusion criteria were; age ≤ 18 years old, on chronic maintenance hemodialysis (3 sessions/ week for at least 3 months). Exclusion criteria included children with contraindications to MRI, and children with primary cardiovascular diseases. Ten healthy control children were recruited for pediatric CMR studies at our institution. Normally developed children with no known cardiovascular disease undergoing MRI for other indications were included. Participants older than 16 years old, or guardians of younger children gave written informed consent. Clinical and laboratory data were documented within 1 week of the cardiac MRI, including weight, height, body surface area (BSA), systolic blood pressure index (SBPi), hemoglobin (Hb), parathyroid hormone (PTH) level, serum calcium, phosphorus, alkaline phosphatase and dialysis adequacy (K dialyzer clearance of urea; t dialysis time; V volume of distribution of urea = Kt/V).
Cardiac MRI
Cardiac MRI was performed on a 1.5-T scanner (Philips Ingenia, Best, Netherlands). Using retrospective ECG-gated steady-state free precession (SSFP) sequence, stack of contiguous slices was obtained in the axial, short axis, 3 and 4 chamber planes with the parameters; TR = 3.2–3.65 ms, TE = 1.6–1.83 ms, field of view (FOV) = 270 mm2, slice thickness = 5 mm, no slice gap.
Post processing for ventricular volume and function
Images were transferred to separate workstation (extended MR 130 Workspace 2.6.3.5, Philips medical systems Netherland). Standard LV volumetric and functional analysis was performed using semi- automated method. Results were indexed to BSA.
Image analysis
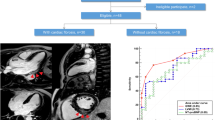
The maximal and minimal aortic areas were obtained by manual tracing on axial cine SSFP images at three regions: the ascending aorta, proximal descending aorta, and aorta at diaphragm, Fig. 1a–d.
Distensibility was calculated according to the following formula: maximal aortic area − minimal aortic area/minimal aortic area × (systolic − diastolic blood pressure)10. Aortic strain was calculated as follows; maximal aortic area − minimal aortic area/minimal aortic area7. Maximal and minimal aortic areas and their absolute difference indexed to BSA were recorded.
Statistical analysis
Statistical analysis was performed using IBM SPSS Version 22.0. Quantitative data were expressed as mean and standard deviation. Student t-test for parametric data and Mann Whitney test for non-parametric data were used to test the difference between patients and control groups. Correlations between variables were examined using Pearson or Spearman’s correlation coefficients. Multi-variable linear regression model was used to assess the effect of different independent variables on strain and distensibility. P values less than 0.05 were considered statistically significant. A posthoc power analysis revealed that a difference of 38% of the mean values of aorta distensibility between patients and control would have been detected with a high test power of more than 0.8.
Results
Participants’ characteristics and LV Cardiac MRI parameters
There were no significant differences between patients and control groups in age, gender, height, weight and BSA, Table 1. In the patients’ group, the duration of dialysis ranged between 2 and 110 months, median 16 months. The mean SBPi ± SD was 0.99 ± 0.13, mean Kt/V was 1.08 ± 1.42 mL/min, mean Hb was 10.1 ± 1.5 g/dl, PTH was 735 ± 465 ng/L, serum calcium was 8.5 ± 1.2 mg/dL, Phosphorus 4.5 ± 1.1 mg/dL, and alkaline phosphatase 727 ± 460 U/L.
Twenty patients received anti-hypertensive drugs (66%), 6 patients received only 1 drug (20%), 8 patients received 2 drugs (27%), 4 patients received 3 drugs (13%) and 2 patients received combination of 4 anti-hypertensive drugs (7%), listed in Table 1.
LV volume and mass were significantly higher and LV ejection fraction (EF) was reduced in patients group compared to control, Table 1.
Aortic strain and distensibility in patients versus control
The maximal aortic and minimal aortic areas, their absolute differences, strain and distensibility of the three aortic regions are listed in Table 2.
a. Ascending aorta
Ascending aorta strain was significantly reduced in patients (38.4 ± 17.4%) compared to the control group (56.1 ± 17%), p-value 0.011. Similarly, aortic distensibility was significantly reduced in patients (9.1 ± 4.4 [× 10−3 mm Hg−1]) compared to the control group (13.9 ± 4.9 [× 10−3 mm Hg−1]), p-value 0.006.
b. Proximal descending aorta
There was no significant difference in strain between patients (27 ± 14.9%) and control group (33 ± 15.6%), p-value 0.29, as well no significant difference in distensibility between patients (6.3 ± 3.9 [× 10−3 mm Hg−1]) and control group (7.9 ± 4.2 [× 10 − 3 mm Hg − 1]), p-value 0.25.
c. Aorta at diaphragm
There was no significant difference in strain between patients (37.9 ± 14.4%) and control group (36.8 ± 14.5%), p-value 0.84, as well as no significant difference in distensibility between patients (8.7 ± 3.9 [× 10−3 mm Hg−1]) and control group (9.2 ± 3.6 [× 10−3 mm Hg−1]), p-value 0.73.
Regional aortic strain and distensibility (Fig. 2a,b)
In the patients’ group, the ascending aorta had significantly higher strain and distensibility than proximal descending aorta (p = 0.016, 0.020). Similarly In the control group, the ascending aorta had higher strain and distensibility than proximal descending aorta (p = 0.013, 0.011).
There was a trend for higher strain and distensibility in the ascending aorta compared to aorta at diaphragm in the patients’ group, (p = 0.99, 0.90). In the control group, aortic strain and distensibility in the ascending aorta were significantly higher than aorta at diaphragm (p = 0.040, 0.048).
The aorta at diaphragm had significantly higher strain and distensibility than proximal descending aorta in the patients’ group (p = 0.023, 0.049). In the control group, there was a trend for higher strain and distensibility in the aorta at diaphragm compared to proximal descending aorta (p = 0.88, 0.80).
Association between aortic function and clinical and LV parameters
Ascending aorta distensibility had significant correlations with LV end diastolic volume indexed (EDVi) (r = −0.37, p = 0.046), LV mass indexed (Mi) (r = −0. 48, p = 0.008), SBPI (r = −0.378, p = 0.04) and Kt/v (r = −0.397, p = 0.03).
Multi-variable regression model showed that only Ktv had still statistically significant effect on distensibility after considering other co-factors (Beta = −0.402, P = 0.026).
Ascending aorta strain had significant correlation with LVEDVi (r = −0.399, p = 0.012), LVMi (r = −0.525, p = 0.001), and Kt/v (r = −0.385, p = 0.035). Multi-variable regression model showed that LVMi and Ktv had still statistically significant effect on strain after considering other co-factors (Beta = −0.488 and −0.416, P = 0.036 and 0.019).
The proximal descending aorta and aorta at diaphragm strain and distensibility were not correlated with cardiac MRI parameters or any other clinical parameter.
Interobserver agreement for regional aortic strain and distensibility
Interobserver agreement analysis revealed almost perfect agreement for ascending aorta strain (ICC = 0.88, 95% CI 0.64–0.96) and distensibility (ICC = 0.91, 95% CI 0.75–0.97), strong agreement for proximal descending aorta strain (ICC = 0.75, 95% CI 0.25–0.92) and distensibility (ICC = 0.76, CI 0.31–0.92), and almost perfect agreement for aorta at diaphragm strain (ICC = 0.81, 95% CI 0.45–0.93) and distensibility (ICC = 0.81, 95% CI: 0.44–0.93).
Discussion
The results of this study demonstrated decreased cardiac MRI-derived strain and distensibility of the thoracic aorta in pediatric patients with CKD compared to healthy controls.
In adult patients with CKD, similar findings were reported, and aortic stiffness worsened with aging5,11,12. Despite lower exposure of children with CKD to traditional cardio-vascular risk factors, and less effect of aging compared to adults; there is evidence that aortic stiffness contributes to increased risk of morbidity and mortality8. To our knowledge, this is the first study to report cardiac MRI-derived aortic strain and distensibility in children with CKD.
Cardiac MRI has the advantage of combined assessment of cardiac and aortic function in one investigation6. Unlike other methods for assessment of aortic stiffness, which measure the average stiffness of the whole vessel, cardiac MRI enables detection of more subtle regional changes in aortic stiffness, which may be isolated, or contribute to the stiffness of the vessel as a whole6.
The difference in strain and distensibility between patients and control was more evident in the ascending than the descending aorta. Regional variations in aortic distensibility may be explained by discrepancies in mechanical properties of the aorta along its length, which in turn is a function of the ratio between elastin and collagen. This ratio is highest in the proximal aorta and decreases gradually from proximal to distal. Therefore, the effect of different pathologic processes, which eventually cause fragmentation and destruction of elastin fibers; is not uniform along the thoracic aorta, with preferential stiffening more in proximal than distal aorta5,13.
Arteriosclerosis in CKD affects large vessels with reduced elastin and increased collagen in the medial layer, calcification, and hypertrophy of vascular smooth muscle cells14,15. Chronic kidney disease-mineral bone disorder plays an important role in development of vascular calcifications. Other factors that may contribute include anemia, endothelial dysfunction, neuro-hormonal activation and inflammation6,14.
Aortic stiffening exposes the LV to extra load, offsetting the balance between the heart and arterial system, or the ‘arterial-ventricular interaction’6,15. When the ascending aorta capacitance is impaired, the LV generates higher pressure to eject blood into the rigid arterial system, contributing to LV hypertrophy, dilatation, and eventually myocardial fibrosis15,16. Aortic stiffening and loss of its buffering function results in greater transmission of pressure fluctuations caused by ventricular contraction, exposing smaller arteries of the end-organs to higher systolic blood pressure. These changes eventually cause renal microvascular damage, contributing also to the progression of chronic renal disease14,15,17.
Arterial stiffening is an early sign of cardiovascular impairment in CKD, detectable before ventricular systolic dysfunction occurs6,18. Therefore, earlier and accurate quantification of aortic stiffness in renal patients potentially affects risk stratification, and is an interesting imaging biomarker for use in clinical studies and trials of novel treatment options for arterial stiffness6.
The current study, in line with previous studies, demonstrated that cross-sectional-derived strain and distensibility measurements by cardiac MRI have excellent reproducibility and are ideal for serial evaluations2,7. Aortic distensibility is defined as the blood volume change relative to a given pressure change. Because direct measurement of regional changes in blood volume is difficult, cross sectional area changes are alternatively estimated. Given that the axial length of arteries does not change significantly during expansion or recoil, changes in cross-sectional area could be assumed to represent changes in blood volume4.
Limitations of this study include the relatively small number of subjects, which may be the cause of weak correlations of arterial function parameters with clinical and laboratory data. The cross-sectional nature of the study did not allow for evaluating the effect of arterial function on prognosis or risk stratification.
In conclusion, thoracic aortic distensibility and strain are reduced in children with CKD compared to healthy controls. Cardiac MRI-derived aortic function is a potential imaging biomarker that could be used in initial clinical evaluation and follow up of children with CKD. Future studies are recommended to assess the effect of arterial stiffness on cardiovascular morbidity and mortality as well as assessment of response to available treatment options.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BSA:
-
Body surface area
- CKD:
-
Chronic kidney disease
- EDVi:
-
End diastolic volume indexed
- FOV:
-
Field of view
- Hb:
-
Hemoglobin
- Kt/V:
-
K dialyzer clearance of urea; t dialysis time; V volume of distribution of urea
- LV:
-
Left ventricle
- Mi:
-
Mass indexed
- PTH:
-
Parathyroid hormone
- RV:
-
Right ventricle
- SBPi:
-
Systolic blood pressure index
- SSFP:
-
Steady-state free precession
- TE:
-
Time of echo
- TR:
-
Time of repetition
References
Metafratzi, Z. M., Efremidis, S. C., Skopelitou, A. S. & De Roos, A. The clinical significance of aortic compliance and its assessment with magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 4, 481–491 (2002).
Hrabak-Paar, M. et al. Variability of MRI aortic stiffness measurements in a multicenter clinical trial setting: Intraobserver, interobserver, and intracenter variability of pulse wave velocity and aortic strain measurement. Radiol. Cardiothorac. Imaging 2, e190090 (2020).
Redheuil, A. et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension 55, 319–326 (2010).
Kuecherer, H. F., Just, A. & Kirchheim, H. Evaluation of aortic compliance in humans. Am. J. Physiol.-Heart Circ. Physiol. 278, 1411–1413 (2000).
Chue, C. D., Edwards, N. C., Ferro, C. J., Townend, J. N. & Steeds, R. P. Effects of age and chronic kidney disease on regional aortic distensibility: A cardiovascular magnetic resonance study. Int. J. Cardiol. 168, 4249–4254 (2013).
Adenwalla, S. F., Graham-Brown, M. P., Leone, F. M., Burton, J. O. & McCann, G. P. The importance of accurate measurement of aortic stiffness in patients with chronic kidney disease and end-stage renal disease. Clin. Kidney J. 10, 503–515 (2017).
Noda, C. et al. Reproducibility of functional aortic analysis using magnetic resonance imaging: The MESA. Eur. Heart J.-Cardiovasc. Imaging 17, 909–917 (2016).
Voroneanu, L. & Covic, A. Vascular damage in children with chronic kidney disease: The fog is dispersing. Hypertension 69, 791–794 (2017).
Sobh, D. M. et al. Left ventricular strain analysis by tissue tracking-cardiac magnetic resonance for early detection of cardiac dysfunction in children with end-stage renal disease. J. Magn. Reason. Imaging 54, 1476–1485 (2021).
Voges, I. et al. Normal values of aortic dimensions, distensibility, and pulse wave velocity in children and young adults: A cross-sectional study. J. Cardiovasc. Magn. Reson. 14, 1–3 (2012).
Zimmerli, L. U. et al. Vascular function in patients with end-stage renal disease and/or coronary artery disease: A cardiac magnetic resonance imaging study. Kidney Int. 71, 68–73 (2007).
Doyle, A. et al. Aortic stiffness and diastolic flow abnormalities in end-stage renal disease assessed by magnetic resonance imaging. Nephron Clin. Pract. 109, c1-8 (2008).
Nelson, A. J. et al. Cardiovascular magnetic resonance-derived aortic distensibility: Validation and observed regional differences in the elderly. J. Hypertens. 27, 535–542 (2009).
Taal, M. W. et al. Markers of arterial stiffness are risk factors for progression to end-stage renal disease among patients with chronic kidney disease stages 4 and 5. Nephron Clin. Pract. 107, c177–c181 (2007).
Moody, W. E., Edwards, N. C., Chue, C. D., Ferro, C. J. & Townend, J. N. Arterial disease in chronic kidney disease. Heart 99, 365–372 (2013).
Nitta, K. et al. Left ventricular hypertrophy is associated with arterial stiffness and vascular calcification in hemodialysis patients. Hypertens. Res. 27, 47–52 (2004).
Georgianos, P. I., Sarafidis, P. A. & Liakopoulos, V. Arterial stiffness: A novel risk factor for kidney injury progression?. Am. J. Hypertens. 28, 958–965 (2015).
Edwards, N. C. et al. Aortic distensibility and arterial-ventricular coupling in early chronic kidney disease: A pattern resembling heart failure with preserved ejection fraction. Heart 94, 1038–1043 (2008).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
D.S., A.T., N.B., B.G. wrote the main manuscript text. B.G., A.T. performed statistics. D.S., N.B. prepared the figures. H.S., A.B., N.H., R.E., M.H. provided clinical data. A.B., N.H., R.E., M.H. recruited patients. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sobh, D.M., Tawfik, A.M., Batouty, N.M. et al. Impaired aortic strain and distensibility by cardiac MRI in children with chronic kidney disease. Sci Rep 12, 11079 (2022). https://doi.org/10.1038/s41598-022-15017-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15017-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.