Abstract
Bacillus circulans (B. circulans) is widely used as an electrogenic bacterium in microbial fuel cell (MFC) technology. This study evaluated whether B. circulans can ferment glucose to generate electricity and mitigate the effects of human skin pathogens. The electricity production of B. circulans was examined by measuring the voltage difference and verified using a ferrozine assay in vitro. To investigate the fermentation effects of B. circulans on inhibition of human skin pathogens, Cutibacterium acnes (C. acnes) was injected intradermally into mice ears to induce an inflammatory response. The results revealed that the glucose–B. circulans co-culture enhanced electricity production and significantly supressed C. acnes growth. The addition of roseoflavin to inhibit flavin production considerably reduced the electrical energy generated by B. circulans through metabolism and, in vivo test, recovered C. acnes count and macrophage inflammatory protein 2 (MIP-2) levels. This suggests that B. circulans can generate electrons that affect the growth of C. acnes through flavin-mediated electron transfer and alleviate the resultant inflammatory response. Our findings demonstrate that probiotics separated from natural substances and antimicrobial methods of generating electrical energy through carbon source fermentation can help in the treatment of bacterial infections.
Similar content being viewed by others
Introduction
Honey is a naturally sweet substance, mainly consisting of glucose and fructose. Many studies have reported honey to have antibacterial activity and to be effective in wound management1. However, some beneficial microorganisms have been found in honey in the form of spores to resist high concentrations of acids and sugar1. Nonpathogenic bacterial strains in honey can also grow when honey is diluted with water2. These nonpathogenic microorganisms include yeast (1%) and gram-positive bacteria (27%) such as Bacillus was one of the predominant genera3,4. Previous study reported that B. circulans are present in the digestive tracts of honey bees and can inhibit the growth of Ascophaera apis, the causative agent of chalkbrood disease in honeybee larvae, possibly through bacteriocins and other antimicrobial molecules4. Additionally, microorganisms such as Bacillus subtilis5, and Clostridium butyricum6 can produce bioelectricity through extracellular electron transfer (EET). B. circulans is an electrogenic bacterium7 with potential for application in MFC technology. In MFCs, Bacillus cereus strain DIF1 and Rhodococcus ruber strain DIF2 actively secrete riboflavin and flavin mononucleotide (FMN), which contribute as electron mediators in EET, mediate electron transfer to extracellular acceptors, and enhance electric current production8. Furthermore, through the addition of exogenetic flavins, Bacillus megaterium (B. megaterium) strain LLD-1 can increase the production of electricity by the fermentation of different carbon sources9. In short, B. circulans can be isolated from honey and generate electricity.
During metabolism and EET, NADH is oxidised to NAD+ through NADH dehydrogenase and delivers electrons to the extracellular space to reduce extracellular electron acceptors10. EET includes direct and indirect modes, such as conductive protein filaments (microbial nanowires11), electron-shuttling mediators (flavin12 or methyl viologen13), and the extracellular polymeric substances of biofilms (redox proteins14). Recently, flavins that mediate EET have received more attention. Flavins are common cofactors that are highly effective as redox enzymes in natural biological systems. In catalytic reactions, flavins oxidise electron donors, such as hydrogen and bacterial fermentation products, and release electrons. These electrons are then used to reduce extracellular electron acceptors such as Fe(III) or Mn(IV)15,16,17.
Acne vulgaris is a skin disease in which the skin commensal C. acnes overcolonises the pilosebaceous unit and secretes lipase. The lipase breaks down triglycerides to release free fatty acids and stimulates the cells to produce proinflammatory cytokines, including interleukin (IL)-8, IL-1218, IL-1β19, and MIP-220, resulting in severe inflammation21. Acne is most commonly treated through antibiotic application, which inhibits C. acnes overgrowth and lipase activity; however, this therapy has several side effects, such as promoting the emergence of antibiotic‐resistant C. acnes strains and nonspecific killing of other skin commensal bacteria22. Alternatively, we reported that short-chain fatty acids (SCFAs), the fermentation metabolite from Staphylococcus epidermidis (S. epidermidis) could inhibit the growth of C. acnes. The injection of S. epidermidis with sucrose in an animal model led to decreases in MIP-2 levels and C. acnes-induced inflammation23. In the present study, we further evaluated whether the honey probiotic B. circulans can ferment glucose to generate electricity, thereby reducing C. acnes lipase-induced MIP-2 levels, in addition to supressing C. acnes growth, through flavin-based EET.
Methods
Ethics statement
This study was carried out in strict with an approved Institutional Animal Care and Use Committee (IACUC) protocol at National Central University (NCU), Taiwan (NCU-106-016) and in compliance with the Arrive guidelines (https://arriveguidelines.org/). Institute Cancer Research (ICR) mice aged 8–9 weeks females (National Laboratory Animal Centre, Taipei, Taiwan) were sacrificed under CO2 anesthesia in a sealed chamber. All methods were performed in accordance with relevant guidelines and regulations.
Bacterial culture
The wildflower honey (Neu Wang Feng Co., Ltd., Taoyuan, Taiwan) was diluted 1:10 with PBS and incubated at 37 °C on TSB agar plate. After 3 days, bacteria were collected and analyzed by 16S rRNA gene sequencing (Tri-I Biotech Inc., New Taipei, Taiwan). Three bacteria were identified as B. circulans, Lysinibacillus fusiformis (L. fusiformis), and Bacillus asahii (B. asahii). C. acnes (ATCC 6919) and B. circulans were cultured on Reinforced Clostridium Medium (RCM, BD, Sparks, MD, USA) under anaerobic conditions using a Gas-Pak (BD) and Tryptone Soy Broth (TSB, BD) medium. Bacteria were cultured at 37 °C until the logarithmic growth phase. Bacterial pellets were harvested by centrifugation at 5000 × g for 10 min, washed in phosphate-buffered saline (PBS), and then suspended in PBS for further experiments.
Bacteria fermentation
B. circulans (107 colony-forming unit (CFU)/mL) was incubated in 5 mL TSB in the in the presence or absence of 2% glucose at 37 °C. Glucose alone in TSB was included as control. The 0.002% (w/v) phenol red (Sigma, Burlington, MA, USA) in TSB served as fermentation indicator. A colour changes from red–orange to yellow indicated the occurrence of bacterial fermentation, which was detected by optical density at 560 nm (OD560).
Electricity detection by MFC system
MFC compartments was established for detection of bacterial electricity. The carbon cloth (9 × 9 cm) (Homy Tech, Taoyuan, Taiwan) as cathode, carbon filth (2.5 × 5 cm) (Homy Tech, Taoyuan, Taiwan) as anode and proton exchange membrane reated by Nafion membrane N117 (5 × 5 cm) (Homy Tech, Taoyuan, Taiwan). Anode and cathode were linked by copper wires, which in turn were connected to 1000 Ω external resistance. Bacteria (107 CFU) with and without 2% glucose or 0.1 μM roseoflavin (flavin inhibitor) in TSB media was loaded on the surface of anode. The cell voltage was recorded every 30 s by a digital multimeter (DM-9962SD, Lutron, Australia) for 20 min.
Ferrozine assay
Ferrozine assay was performed by suspending B. circulans (107 CFU) in TSB medium with and without 2% glucose and 0.1 μM roseoflavin total 50 μl, an equal volume of ferrozine (8 mM) (Sigma) and 100 μl ferric ammonium citrate (100 mM) (Sigma) were added into each well. The mixture was incubated at 37 °C for 1 h in 96-well. The colour change of media was detected from OD at 562 nm.
Electrons from B. circulans fermentation with glucose inhibits C. acnes growth in vitro and in the presence of roseoflavin in vivo
In vitro, Co-Culture C. acnes (107 CFU) and B. circulans (107 CFU) in TSB with and without 2% glucose under anaerobic conditions for 3 days at 37 °C. B. circulans and glucose alone mix with C. acnes was included as a control. After 3 days, dilute with PBS for C. acnes bacterial counts. In vivo, the ears of ICR mice were injected intradermally with C. acnes (107 CFU) mix with B. circulans and 2% glucose in the presence or absence of 0.1 μM roseoflavin. B. circulans and glucose mix with C. acnes was included as a control. After 3 days, cut the mice ears and homogenized for C. acnes bacterial counts.
Bacteria counting
The C. acnes loads in in vitro and in vivo sample were enumerated by plating serial dilutions (1:10–1:105) with PBS of selective agar plates containing RCM media and 10 μg/mL of furazolidone (Sigma). The plates were incubated for 5 days at 37 °C in an anaerobic chamber using Gas-Pak.
Cloning of Lipase
Transformation of a plasmid encoding lipase (accession number: YP_056770.1) into Escherichia coli (E. coli) BL21 competent cells (Invitrogen, Carlsbad, CA, USA). The E. coli BL21 transformed with a plasmid encoding GFP was used as a control by following the same procedure. A transformant of E. coli BL21 was inoculated with Luria–Bertani (LB) (Biokar Diagnostics, Beauvais, France) medium containing ampicillin (Sigma) at 37 °C until the OD600 reached 0.6–0.8. 1 mM Isopropyl-B-D-thiogalactoside (IPTG) (Sigma, Burlington, MA, USA) was added into culture for 4 h at 30 °C to induce protein expression. Proteins were purified by ProBond™ Purification System (Invitrogen, Carlsbad, CA, USA).
C. acnes lipase-induced the proinflammatory MIP-2 cytokine and the treatment of electrons from B. circulans fermented by glucose in vivo
The ears of ICR mice were injected intradermally with 5/10 μl lipase or GFP (as a control) to induces the inflammation. After 24 h, cut the mice ears and the level of MIP-2 cytokine was measured by ELISA. In electrons treatment experiment, the ears of ICR mice were injected intradermally with 5/10 μl lipase to induces the inflammation. After 24 h, B. circulans, 2% glucose with 0.1 μM roseoflavin was injected. B. circulans and glucose without roseoflavin was included as a control. After 24 h, cut the mice ears and homogenized for MIP-2 quantified.
ELISA
The proinflammatory MIP-2 cytokines in the supernatants of ear homogenates was quantified by an ELISA kit, as directed by the manufacturer (R&D System. Inc., Minneapolis, MN, USA).
Statistical analyses
Data analysis was performed by unpaired t-test using Prism software (https://www.graphpad.com/; Version 5.01, GraphPad Software, La Jolla, CA, USA). The levels of statistical significance were indicated as the following: *p < 0.05, **p < 0.01, ***p < 0.001 and ns = non-significant. The mean ± standard.
deviation (SD) for at least three independent experiments was calculated. Animal experiments were performed with at least three animals per each treatment group.
Results
Bacteria sequencing from honey
By using 16S rRNA gene analysis, we isolated three bacteria (Supplementary Fig. S1A), B. circulans, L. fusiformis, and B. asahii (Supplementary Table S1) from TSB agar plate with 10% honey. Particularly, one 16S rRNA gene sequence of the isolated three bacteria shares 99% similarity to that of B. circulans strain FDAARGOS_783 (GenBank accession no NZ_CP053989.1).
Electricity production and electron transfer by B. circulans produced through glucose fermentation
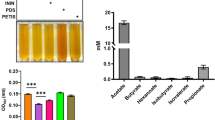
To investigate whether B. circulans can ferment glucose, it was incubated with 2% glucose in TSB media with phenol red for 1 day. With B. circulans alone, phenol red colour changed to orange because of bacterial replication during incubation. However, when B. circulans was incubated along with glucose, phenol red colour changed to yellow with a decrease in pH value, indicating the use of glucose for fermentation (Fig. 1A; upper panel). Furthermore, the quantification of fermentation by measuring the optical density of phenol red at OD560 nm indicated a significant decrease of OD560 values in TSB media containing bacteria plus glucose medium compared with bacteria or glucose alone (Fig. 1A; lower panel).
Electricity production and electron transfer by B. circulans produced through glucose fermentation. (A) The colour change of phenol red when B. circulans (B) was incubated with/without glucose (G) in TSB medium (M). The OD560 in the media with glucose and B. circulans (BG) was significantly lower than the other groups. (B) Voltage changes detection (mV) for 20 min in medium alone or media containing glucose, B. circulans, or B. circulans plus glucose. (C) The concentration of ferrozine-chelatable iron (mM) in medium or media containing glucose, B. circulans, or B. circulans plus glucose. Data are expressed as the mean ± SD of three separate experiments. *p < 0.05 and ***p < 0.001.
Next, electricity production in the B. circulans with glucose was identified by adding the fermented media to the anode of the MFC system. B. circulans and glucose alone were used as controls and exhibited a low voltage change at 20 min (Fig. 1B; blue and green lines). By contrast, the voltage significantly increased to approximately 4 mV in the B. circulans with 2% glucose group (Fig. 1B; red line). We further verified that B. circulans can produce electrons through glucose fermentation by using the ferrozine assay to identify ferric iron reductase activity. In Fig. 1C, it can be seen that the concentration of ferrozine-chelatable iron (dark brown) in the reaction solution containing a fermentation medium of B. circulans plus glucose was distinctly higher than the medium, glucose, or bacteria alone (Fig. 1C; left panel). Statistical tests further confirmed that the ferric iron reductase activity of B. circulans was significantly increased when with glucose than without glucose (Fig. 1C; right panel). When compared with glucose alone, B. circulans and glucose resulted in significant increase in OD value.
Roseoflavin affects electricity production by B. circulans fermenting glucose
Gram-positive bacteria used flavin-based EET to deliver electrons2. Roseoflavin represses FMN riboswitch and subsequently mediates riboflavin and FMN gene expression24. In Fig. 2A, 0.1 µM roseoflavin was added to the culture of bacteria and glucose, and phenol red colour changed to yellow with a decrease in pH, similar to that in the fermentation experiment illustrated in Fig. 1A, confirming that roseoflavin does not affect the fermentation of B. circulans. Next, to identify electricity production in the B. circulans-glucose-roseoflavin culture, the fermented medium was added to the anode of the MFC system. The voltage change induced by B. circulans in the presence of glucose was completely attenuated by the addition of roseoflavin (Fig. 2B). In Fig. 2C, the concentration of ferrozine-chelatable iron in the reaction solution containing the fermentation medium of B. circulans-glucose-roseoflavin was distinctly decreased compared with medium, glucose, and bacteria. In summary, the findings of similar acidities but significantly decreased voltage production when adding roseoflavin indicated that the voltage change was not due to the pH change during fermentation and the number of B. circulans because 0.1 μM roseoflavin did not influence bacterial growth (Supplementary Fig. S2 for details).
Roseoflavin affects electricity production in the fermentation of B. circulans with glucose. (A) B. circulans with glucose in the presence and absence of roseoflavin (I) in TSB medium with phenol red. The colour change of phenol red in media from red to yellow indicated fermentation still occurred. Furthermore, fermentation was quantified by measuring the optical density of phenol red at OD560. (B) Voltage change detection (mV) for 20 min in the culture of B. circulans with glucose in the absence or presence of roseoflavin. (C) The concentration of ferrozine-chelatable iron (mM) in the media in the absence or presence of roseoflavin. Data are expressed as the mean ± SD of three separate experiments. **p < 0.01 and ns = non-significant.
B. circulans fermentation with glucose affect C. acnes growth in vitro and in the presence of roseoflavin in vivo
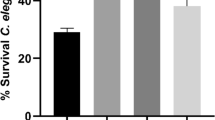
Having established the electricity produced in B. circulans-glucose cultures, we cocultured C. acnes and B. circulans in vitro to test the impact of B. circulans fermentation on the growth of C. acnes. The number of C. acnes was significantly decreased when mixed with B. circulans and glucose (Fig. 3A). Furthermore, the B. circulans-C. acnes-glucose mixture was injected intradermally with or without roseoflavin in ICR mice ears to examine whether glucose fermentation of B. circulans with roseoflavin changed the bacterial growth by electron. The result shows that the C. acnes count was significantly decreased in the absence of roseoflavin but increased in the presence of roseoflavin (Fig. 3B).
B. circulans fermentation with glucose affect C. acnes growth in vitro and in the presence of roseoflavin in vivo. (A) In vitro, the C. acnes (CFU) was assessed by enumerating a plating serial dilution (1:10–1:105) on selective agar containing RCM agar plus furazolidone. (B) The CFU of C. acnes was assessed in B. circulans with glucose in the presence of roseoflavin (BGI) in vivo. C. acnes mix with PBS (C), glucose (G), B. circulans (B), and B. circulans plus glucose (BG) was included. Data are expressed as the mean ± SD of three separate experiments. *p < 0.05.
C. acnes lipase induced proinflammatory MIP-2 cytokine and the treatment of electrons from B. circulans fermented by glucose in vivo
After 24 h induction of lipase injection (with GFP as the control), MIP-2 expression was elevated in the presence of lipase (Fig. 4A). In the electron treatment experiment, the use of roseoflavin dramatically decreased electricity production, attenuated the anti-inflammatory defence of B. circulans fermentation by glucose, and significantly increased the concentrations of lipase-induced MIP-2 (Fig. 4B).
MIP-2 expression through electron treatment in mice ears after lipase injection. (A) MIP-2 expression (ng/mL) after lipase induction. (B) MIP-2 expression (ng/mL) in mouse ears of B. circulans with glucose in the absence (BG) or presence (BGI) of roseoflavin. Data are expressed as the mean ± SD from three separate experiments. *p < 0.05 and ***p < 0.001.
Discussion
Through probiotics’ fermentation of proper carbon sources, pathogen-caused skin diseases can be reduced. For instance, SCFAs, when used as fermentation metabolites, can inhibit bacterial growth to achieve treatment effects25. In addition, it has been shown that B. circulans can convert biomass into electrical energy10. Its halophilic strain BBL03 can ferment 1% chitin and use degraded metabolites as electron donors to generate electricity in seawater; therefore, it can serve as electricity-producing bacteria in MFCs26. In this study, after adding 2% glucose to the medium containing B. circulans, the electricity increased significantly when measured using changes in voltages, indicating that B. circulans can generate a substantial number of electrons through glucose fermentation. Notably, the low electricity was also detected in the medium containing B. circulans without the addition of glucose. The reason for this slight change of electricity production may be due to the presence of a small amount of glucose in TSB (Fig. 1B).
In gram-positive bacteria, EET is involved in iron redox. Fe3+-reducing microorganisms belonging to the Geobacteraceae family can ferment sugars and other organic compounds to produce simple organic acids (such as acetate) that serve as electron donors to the electrodes27. Similarly, by adding B. circulans and glucose to the solution containing ferric (Fe3+) ammonium citrate, we observed that the concentration of ferrozine-chelated Fe2+ was higher than that in the groups without glucose (Fig. 1C). The addition of glucose allowed more electrons to be generated, leading to increased ferric reduction, which rendered the culture medium-dark brown.
The flavin-based EET mechanism has been confirmed in various gram-positive bacteria. Flavin in the suspension culture of the B. megaterium LLD-1 strain acted as an electron shuttle, enhancing electron transfer from LLD-1 to the electrode8. Roseoflavin is a natural antibacterial compound. When combined with FMN riboswitch, it can inhibit Rli96 transcription, control the expression of downstream genes, and regulate the in vivo synthesis of flavin, thus impairing the bacterial metabolism and achieving bacteriostasis28. Roseoflavin can also be converted into roseoflavin mononucleotide and roseoflavin adenine dinucleotide, both of which cause defects in cellular physiological functions29. Therefore, FMN riboswitch may serve as a novel target for inhibiting pathogens. We used phenol red to monitor the degree of acid production by bacterial fermentation, and the addition of roseoflavin as flavin inhibitor to the culture medium did not alter the production of organic acids (Fig. 2A). This suggests that the reduction of electrons was not caused by organic acids but by the roseoflavin-induced inhibition of flavin generation. The ferrozine assay indicated that the decrease of iron concentration represented a decrease in electrons produced by fermentation (Fig. 2C).
We previously reported that S. epidermidis can ferment glycerol and PEG-8 laurate to generate potential electron donors for electricity generation to combat ultraviolet damage30 or suppress acne vulgaris31. In this study, we further demonstrated that adding glucose to the culture medium of B. circulans in vitro can inhibit the growth of C. acnes; specifically, this suppression was significantly reversed when roseoflavin was added to the mix. Taken together, B. circulan affected the growth of C. acnes through the electrons generated by glucose fermentation and flavin-mediated EET. These results also reveal an efficient EET mechanism to target pathogenic microorganisms by using electrons. Importantly, the intradermal application of B. circulans plus glucose to mice ears can suppress the C. acnes count while the use of nonselective anti-inflammatory drugs for treatment may lead to epidermal dysbiosis and the spread of resistant strains.
In acne pathogenesis, the increased activity of the virulence factor lipase caused by C. acnes overcolonisation led to an inflammatory response that resulted in the release of proinflammatory cytokines and TNF-α, which modulated host immune response2,20. In line with previous reports, lipase-induced immune responses were confirmed by an elevated MIP-2 content measured using ELISA in this study. Adding glucose to B. circulans can inhibit lipase-induced MIP-2 expression, but this inhibition was reversed when roseoflavin was also added. Therefore, B. circulans can generate electrons through glucose fermentation to affect the growth of C. acnes through flavin-mediated electron transfer, thereby reducing the resultant inflammatory response. In short, weak currents inhibit bacterial growth. The underlying mechanisms for the current-related lysis may be because of electron-induced electrolysis, the generation of free radicals, pH, and changes in biofilm structure32. It was reported that exposure of gram-positive bacteria to pulsed electric fields can induce permeabilization of the plasma membrane, destabilising the cell wall and causing osmotic shock33. In other words, electric current generated with conductivity electrodes can directly inhibit bacterial growth, but the transition of platinum complexes and metal ions generated during electrolysis can harm human cells34. By contrast, the weak current produced by B. circulans through glucose fermentation, as demonstrated in this study, can efficiently and safely supress pathogenic bacterial growth.
Overall, this study revealed the molecular mechanism by which the probiotic B. circulans in honey can generate electrical energy by using glucose as a prebiotic. B. circulans reduced the inflammatory response by disrupting C. acnes growth through FMN riboswitch and flavin-mediated electron transfer. Therefore, generating electrical energy from biomass through the metabolic activities of microorganisms may be a potential antimicrobial therapy. These results are beneficial for the future clinical treatment of acne-prone skin disorders and to development of skincare products.
Data availability
The datasets generated and analyzed during the current study available from the corresponding author on reasonable request.
References
Olaitan, P. B., Adeleke, O. E. & Iyabo, O. O. Honey a reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 7, 159–165 (2007).
Light, S. H. et al. A flavin-based extracellular electron transfer mechanism in diverse gram-positive bacteria. Nature 562, 140–144. https://doi.org/10.1038/s41586-018-0498-z (2018).
Sackett, W. G. Honey as a carrier of intestinal diseases. Bull. CExp. Stad 252, 1–18 (1919).
Reynaldi, F. J., De Giusti, M. R. & Alippi, A. M. Inhibition of the growth of Ascophaera apis by selected strains of Bacillus and Paenibacillus species isolated from honey. Rev. Argent. De Microbiol. 36, 52–55 (2004).
Yoganathan, K. G. P. electrogenicity assessment of Bacillus subtilis and Bacillus megaterium using microbial fuel cell technology. Int. J. Appl. Res. 1, 435–438 (2015).
Niessen, J., Schroder, U. & Scholz, F. Exploiting complex carbohydrates for microbial electricity generation ? A bacterial fuel cell operating on starch. Electrochem. Commun. 6, 955–958. https://doi.org/10.1016/j.elecom.2004.07.010 (2004).
Gilliam, M. Microbiology of pollen and bee bread: The Genus Bacillus. Apidologie Springer Verlag 10, 269–274 (1979).
Tian, T. et al. Flavin-mediated extracellular electron transfer in gram-positive bacteria Bacillus cereus DIF1 and Rhodococcus ruber DIF2. RSC Adv. 9, 40903–40909. https://doi.org/10.1039/c9ra08045g (2019).
You, L. X. et al. Flavins mediate extracellular electron transfer in gram-positive Bacillus megaterium strain LLD-1. Bioelectrochemistry 119, 196–202. https://doi.org/10.1016/j.bioelechem.2017.10.005 (2018).
Shaikh, J., Patil, N. P., Shinde, V. & Gaikwad, V. B. Simultaneous decolorization of methyl red and generation of electricity in microbial fuel cell by Bacillus circulans NPP1. J. Microb. Biochem. Technol. 08, 428–432. https://doi.org/10.4172/1948-5948.1000320 (2016).
Liu, X. et al. Biological synthesis of high-conductive pili in aerobic bacterium Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 103, 1535–1544. https://doi.org/10.1007/s00253-018-9484-5 (2019).
Enrico Marsili, D. B. B., Shikhare, I. D., Coursolle, D., Gralnick, J. A. & Bond, D. R. Shewanella secretes flavins that mediate extracellular electron transfer. PNAS 105, 3968–3973 (2008).
Choi, O., Um, Y. & Sang, B. I. Butyrate production enhancement by Clostridium tyrobutyricum using electron mediators and a cathodic electron donor. Biotechnol. Bioeng. 109, 2494–2502. https://doi.org/10.1002/bit.24520 (2012).
Cao, B. et al. Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: Characterization by infrared spectroscopy and proteomics. Environ. Microbiol. 13, 1018–1031. https://doi.org/10.1111/j.1462-2920.2010.02407.x (2011).
Palfey, A., & Fagan, R. L. In: Comprehensive Natural Products II Vol. 7 37–113 (2010).
F. Scott Mathews, L. C., and Rosemary C. E. Durley. In: Subcellular Biochemistry Vol. 35 29–72 (2020).
Bleam, W. F. In: Soil and Environmental Chemistry 321–370 (2012).
Kurokawa, I. et al. New developments in our understanding of acne pathogenesis and treatment. Exp. Dermatol. 18, 821–832. https://doi.org/10.1111/j.1600-0625.2009.00890.x (2009).
Contassot, E. & French, L. E. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J. Invest. Dermatol. 134, 310–313. https://doi.org/10.1038/jid.2013.505 (2014).
Nakatsuji, T. et al. Bioengineering a humanized acne microenvironment model: Proteomics analysis of host responses to Propionibacterium acnes infection in vivo. Proteomics 8, 3406–3415. https://doi.org/10.1002/pmic.200800044 (2008).
Higaki, S. Lipase inhibitors for the treatment of acne. J. Mol. Catal. B Enzym. 22, 377–384. https://doi.org/10.1016/s1381-1177(03)00053-5 (2003).
Wang, Y. et al. The anti-inflammatory activities of Propionibacterium acnes CAMP factor-targeted acne vaccines. J. Invest. Dermatol. 138, 2355–2364. https://doi.org/10.1016/j.jid.2018.05.032 (2018).
Wang, Y. et al. A precision microbiome approach using sucrose for selective augmentation of Staphylococcus epidermidis fermentation against Propionibacterium acnes. Int. J. Mol. Sci. 17, 1870. https://doi.org/10.3390/ijms17111870 (2016).
Wang, H. et al. Dual-targeting small-molecule inhibitors of the Staphylococcus aureus FMN riboswitch disrupt riboflavin homeostasis in an infectious setting. Cell Chem. Biol. 24, 576-588 e576. https://doi.org/10.1016/j.chembiol.2017.03.014 (2017).
Shu, M. et al. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 8, e55380. https://doi.org/10.1371/journal.pone.0055380 (2013).
Gurav, R. et al. Chitin biomass powered microbial fuel cell for electricity production using halophilic Bacillus circulans BBL03 isolated from sea salt harvesting area. Bioelectrochemistry 130, 107329. https://doi.org/10.1016/j.bioelechem.2019.107329 (2019).
Chaudhuri, S. K. & Lovley, D. R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21, 1229–1232. https://doi.org/10.1038/nbt867 (2003).
Jia Dong-fang, L. J.-s., Diao Yong. Novel targets for antibiotics discovery: Riboswitches. Acta Pharm. Sin. 48, 1361–1368 (2013).
Sheng, S. Y., Chen, Y., Zhang, X. M. & Shi, Y. S. Research progresses on riboswitches and their applications in antimicrobials. Biotechnol. Bull. 33, 114–119. https://doi.org/10.13560/j.cnki.biotech.bull.1985.2017.01.012 (2017).
Balasubramaniam, A. et al. Skin bacteria mediate glycerol fermentation to produce electricity and resist UV-B. Microorganisms 8, 1092. https://doi.org/10.3390/microorganisms8071092 (2020).
Marito, S. et al. Electricity-producing Staphylococcus epidermidis counteracts Cutibacterium acnes. Sci. Rep. 11, 12001. https://doi.org/10.1038/s41598-021-91398-7 (2021).
Giladi, M. et al. Microbial growth inhibition by alternating electric fields. Antimicrob. Agents Chemother. 52, 3517–3522. https://doi.org/10.1128/AAC.00673-08 (2008).
Pillet, F., Formosa-Dague, C., Baaziz, H., Dague, E. & Rols, M. P. Cell wall as a target for bacteria inactivation by pulsed electric fields. Sci. Rep. 6, 19778. https://doi.org/10.1038/srep19778 (2016).
Barentt Rosenberg, L. V., Trosko, J. E. & Virginia, H. Mansour platinum compounds: A new class of potent antitumour agents. Nature 222, 385–386 (1969).
Acknowledgements
The authors thank to Dr. Hsin Chen (National Tsing Hua University) for establishment of a power supply using ELITE.
Funding
This work was supported by 106/107/108-Landseed Hospital-NCU joint grants and Ministry of Science and Technology (MOST) Grants 108-2622-B-008-001-CC1; 108-2314-B-008-003-MY3, and 107-2923-B-008-001-MY3.
Author information
Authors and Affiliations
Contributions
H.-J.K., and A.B.: methodology and formal analysis; A.B.: validation; H.-J.K.: investigation; H.-J.K., and C.-M.H.: data curation; H.-J.K.: writing-original draft preparation; C.-C.C., and C.-M.H.: writing-review and editing; C.-M.H.: Conceptualization, resources, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kao, HJ., Balasubramaniam, A., Chen, CC. et al. Extracellular electrons transferred from honey probiotic Bacillus circulans inhibits inflammatory acne vulgaris. Sci Rep 12, 19217 (2022). https://doi.org/10.1038/s41598-022-23848-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23848-9
This article is cited by
-
Neutrophil extracellular trap-related mechanisms in acne vulgaris inspire a novel treatment strategy with adipose-derived stem cells
Scientific Reports (2024)
-
Modeling and experimental investigation of the effect of carbon source on the performance of tubular microbial fuel cell
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.