Abstract
Different body weight-supported gait-training strategies are available for improving ambulation in individuals with spinal cord injury (SCI). These include body weight-supported overground training (BWSOGT), body weight-supported treadmill training (BWSTT), and robot-assisted gait training (RAGT). We conducted a network meta-analysis of randomised controlled trials (RCTs) to assess the effect and priority of each training protocol. We searched the PubMed, Cochrane Library, Scopus, and Embase databases from inception to 6 August 2022. The eligibility criteria were as follows: (1) being RCTs, (2) recruiting participants with SCI diagnosis and requiring gait training, (3) comparing different body weight-supported gait training strategies, and (4) involving ambulatory assessments. We conducted a network meta-analysis to compare different training strategies using the standard mean difference and its 95% credible interval. To rank the efficacy of training strategies, we used the P score as an indicator. Inconsistency in network meta-analysis was evaluated using loop-specific heterogeneity. We included 15 RCTs in this analysis. RAGT was had significantly more favourable performance than had the control intervention. The ranking probabilities indicated that the most effective approach was RAGT, followed by BWSOGT, BWSTT, and the control intervention. No significant inconsistency was noted between the results of the direct and indirect comparisons.
Similar content being viewed by others
Introduction
Following the acute phase of spinal cord injury (SCI), patients and their families must take on challenges such as restoring arm and hand function, regaining sexual function, improving bladder and bowel function, and enhancing walking ability1,2,3. Failure to restore ambulation before subjecting the patient to alternative gait training strategies leads to severe disability and psychosocial and economic problems1,4. One main strategy for the rehabilitation of patients with SCI is improving lower limb motor function5,6. Repetitive and intensive exercises can induce plasticity in the involved motor centres7. However, severe motor impairment in patients with SCI leads to fatigue, making it difficult for such patients to perform related exercises for a long period. Fatigue is thus a crucial limiting factor in conventional rehabilitation programmes7.
Body weight-supported training while walking is used in neurological rehabilitation8,9,10. It partially decreases the burden of load bearing and enables those who cannot walk to complete training protocols11. Body weight-supported overground training (BWSOGT) and body weight-supported treadmill training (BWSTT) are alternative training strategies for patients with SCI12,13. Automatic electromechanical devices are being increasingly used in neurorehabilitation14,15. Robot-assisted gait training (RAGT) has many advantages, such as maintenance of a physiological gait pattern and increases in training intensity and overall training duration16,17,18.
Although these train alternative training protocols—BWSOGT, BWSTT and RAGT—have been reported to be more favourable than conventional training, no study has compared them together. Therefore, we used a network meta-analysis approach for comparing the effectiveness of the three strategies for ambulatory improvements in patients with SCI. We conducted a comprehensive literature review to identify randomised controlled trials (RCTs) focusing on gait training for SCI.
Methods
This network meta-analysis was registered prospectively in the International Prospective Register of Systematic Reviews database under the number CRD42021270919 on 29 August 2021. Our protocol adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for network meta-analysis19.
Eligibility criteria
The eligibility criteria for studies were as follows: (1) being RCTs; (2) recruiting participants with an SCI diagnosis; (3) having an intervention group with different body weight-supported gait training strategies (RAGT, BWSTT, and BWSOGT); (4) having a control group with conventional gait training, such as sit to stand, static and dynamic standing balance, weight shifting, walking, turning, and stand to sit; and (5) involving ambulatory assessments. We excluded studies that (1) were not peer reviewed, such as conference papers and letters to the editor; (2) only presented protocols; (3) did not involve ambulatory assessments; and (4) used combination therapy. No language restrictions were applied.
Search strategy
We independently reviewed the literature, extracted data, and performed crosschecks following the PRISMA guidelines (Supplementary Information)20. We searched the PubMed, Cochrane Library, Scopus, and Embase databases for relevant articles from inception to 6 August 2022 by using the following search string: ((spinal cord) OR (SCI) OR (myelopathy) OR (myelitis)) AND (((robot*) OR (RAGT) OR (end effector) OR (exoskeleton) OR (lokomat*) OR (locomat*)) OR (((locomot*) OR (treadmill)) AND (support*))). An additional search with other brand names of different body weight-supported gait training strategies was also conducted to facilitate a more detailed search. The following were used as keywords: Rysen, the Float, ZeroG, Keeogo, Dermoskeleton, ReWalk, Ekso Indego, HAL, WPAL, H2, REX, Ekso, ReWalk, Robin, CUHK-EXO, ITRI, Vanderbilt Exoskeleton, ARKE, Curara, Arazpour2103a, Kim2013, Chang2017, SMA, Kinesis, Lerner2017, Alter G Bionic Leg, Arazpour2013b, Kawasaki2017, Yeung2017, Boes2017, Welwalk, LiteGait, ALEX, LOPES, Gait Trainer, and Haptic Walker. RCTs were identified using the filter function of the databases. Additional articles were identified through a manual search of the reference lists of relevant articles. Two reviewers independently reviewed the full text of all potentially relevant articles to identify those that met the eligibility criteria. Any disagreement was resolved by a third reviewer.
Study selection
After studies were retrieved from the databases, duplicate entries were removed using manual screening. Subsequently, the titles and abstracts of the remaining studies were screened so that relevant articles could be independently selected by two reviewers. Disagreements were resolved through mutual discussion or adjudication by a third reviewer. Subsequently, the full texts of the remaining articles were read in detail to determine the eligibility of the articles.
Data items
The following data were obtained from each RCT: RCT type, American Spinal Injury Association Impairment Scale (AIS) grade, number and mean age of participants, protocol used in different groups, treatment duration, and outcome measurements.
Outcome measurements
Ambulatory function impairment may limit daily activities and social performance. Thus, our primary outcome was walking ability. When data on walking ability were unavailable, another outcome measurement associated with ambulatory function was selected. The first priority was given to the 6-min walk test, which is recommended for the assessment of walking in patients with SCI21. The second priority was given to the 10-m walk test, which is also a recommended ambulation assessment method21. We determined the priority of the 6-min walk test prior to the 10-m walk test because although it is much easier to perform the 10-m walk test, the 6-min walk test is longer and therefore provides much more discriminative data on participant’s ambulation ability. The third priority was given to the lower extremity motor score, which is a standard neurologic assessment developed by the American Spinal Injury Association in which the voluntary muscle strength of five key muscle groups (hip flexors, knee extensors, ankle dorsiflexors, long toe extensors, and ankle plantarflexors) of both lower extremities are tested22. The fourth priority was given to the Walking Index for Spinal Cord Injury, which is an ordinal scale that evaluates the extent and nature of assistance (orthoses, supporting equipment such as walkers, and human helpers) that people with SCI require to be able to walk23. Only data on the highest ranking priority of ambulatory measures in each study were extracted for the network meta-analysis. Studies without ambulatory measurements were excluded. Data representing the longest duration of follow-up were pooled in the network meta-analysis.
Risk-of-bias assessment
The risk of bias was assessed using the Cochrane RoB 2 tool, which is widely used for assessing the quality of RCTs24. We considered the overall bias and all five domains of bias: (1) bias arising from the randomisation process, (2) bias due to deviations from intended interventions, (3) bias due to missing outcome data, (4) bias in outcome measurements, and (5) bias in the selection of reported results24. In accordance with the Cochrane Handbook for Systematic Reviews of Interventions, the risk of bias was assessed by two independent reviewers25, and disagreements were resolved through discussion and consultation with a third reviewer.
Statistical analysis
Network meta-analysis is a technique used to compare three or more interventions simultaneously in a single analysis through the combination of both direct and indirect evidence across a network of studies25. Network meta-analysis generates estimates of the relative effects between any pair of interventions in a network, and it usually yields estimates that are more precise than are single direct or indirect estimates25. It also allows the estimation of the ranking and hierarchy of interventions25. The network meta-analysis was performed using the ShinyNMA Version 1.01 website26 (https://jerryljw.shinyapps.io/ShinyNMA_/). This is a free online cloud computing network meta-analysis website for researchers, and it can be used to create charts as per the standards of the latest PRISMA 2020 guidelines20. It synthesises results and provides a rationale for choosing R software (version 4.1.0) and specific packages, namely metafor (version 2.4-0), netmeta (version 1.3-0), or BUGSnet (version 1.0-4).
We extracted continuous data by changing the baseline measurements. In the absence of standard deviation values, data were estimated through the calculation of correlation coefficients in accordance with the instructions in the Cochrane Handbook for Systematic Reviews of Interventions25. The transitivity assumption underlying network meta-analysis was evaluated by comparing the distribution of clinical and methodological variables that could serve as effect modifiers across treatment comparisons25. A random-effects model was used in this network meta-analysis. We conducted a head-to-head comparison of body weight-supported gait training for SCI by estimating the standard mean difference (SMD) and 95% credible interval (CI). Furthermore, we analysed the distribution of probabilities in the ranking of body weight-supported gait training strategies for ambulatory improvement among patients with SCI. For efficacy ranking, we used the P score as an indicator. The P score is used to measure the extent of certainty that a treatment is better than other treatments, averaged over all competing treatments27. It is rated from 0 (worst) to 1 (best). If one treatment is better than the other treatments, its P score is higher than that for other treatments. Moreover, inconsistency in network meta-analysis was evaluated using loop-specific heterogeneity and local incoherence estimates and by comparing differences in effect sizes between standard meta-analyses (direct comparisons) and indirect comparisons25.
Results
Study selection
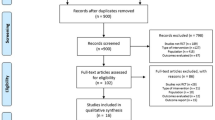
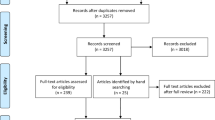
We initially retrieved 1199 RCTs and excluded 412 duplicates. After title and abstract screening, 698 studies were excluded. The full texts of the remaining 89 papers were screened; among them, 1 study did not focus on patients with SCI, 9 did not involve body weight-supported ambulation training, 3 compared only pharmacological interventions, 4 included additional stimulation in the study group, 15 did not include ambulation or functional assessment, 2 were cost-effectiveness studies, 6 were study protocols, 6 were not RCTs, 6 had the same study group, 9 were dose-effectiveness studies, 7 were not peer-reviewed articles, 5 did not provide standard deviations, and 1 was an animal study. Finally, 15 articles were selected for this network meta-analysis7,28,29,30,31,32,33,34,35,36,37,38,39,40,41 (Fig. 1).
Characteristics of the included studies
In the 15 selected RCTs,three body weight-supported gait training protocols were used, namely RAGT7,28,30,31,33,34,36,37,38,39,40,41, BWSTT29,32,35,37, and BWSOGT29,35. Conventional gait training was prespecified as a control intervention. Alexeeva et al. conducted a three-arm study comparing BWSTT, BWSOGT, and a control intervention29. Labruyère et al. conducted a crossover RCT33. According to the Cochrane Handbook for Systematic Reviews of Interventions, the inclusion of crossover studies in a network meta-analysis is acceptable25. In addition, including the final outcome data is more appropriate than including only the outcome data from the first period (before the crossover). We followed these guidelines in our network meta-analysis. Table 1 summarises the main characteristics of the 15 RCTs.
Risk-of-bias assessment
Two reviewers assessed the quality of the selected RCTs by using the RoB 2 tool24. All studies had a low risk of bias in terms of the randomisation process7,28,29,30,31,32,33,34,35,36,37,38,39,40,41. Some concerns were noted for all studies in terms of deviation from the intended intervention7,28,29,30,31,32,33,34,35,36,37,38,39,40,41. Five studies had some concerns regarding missing outcome data7,28,36,39,41, and a low risk of bias was noted for 10 studies29,30,31,32,33,34,35,37,38,40. All studies exhibited a low risk of bias in outcome measurement7,28,29,30,31,32,33,34,35,36,37,38,39,40,41. All studies exhibited a low risk of bias in the selection of reported results7,28,29,30,31,32,33,34,35,36,37,38,39,40,41. The overall risk of bias was uncertain for all studies7,28,29,30,31,32,33,34,35,36,37,38,39,40,41 (Fig. 2).
Synthesis of results: network meta-analysis
Our network meta-analysis included 497 participants across 15 RCTs. Figure 3 presents a network diagram of the included body weight-supported gait training therapies. At least one placebo-controlled trial was included for each therapy. The pooled SMDs of functional scores in the network meta-analysis revealed that RAGT was significantly more favourable than the control intervention, whereas BWSTT and BWSOGT did not result in significant differences compared with the control intervention. The SMDs and 95% CIs from comparisons between the control intervention and other body weight-supported gait training therapies were as follows: RAGT = 0.30 (0.11, 0.50), BWSTT = 0.09 (− 0.40, 0.58), and BWSOGT = 0.09 (− 0.55, 0.73; Fig. 4). Moreover, we synthesised head-to-head studies separately to assess differences among body weight-supported gait training strategies. Table 2 presents the results of the pairwise meta-analysis and network meta-analysis of walking ability with overall training. Furthermore, the distribution of probabilities in the ranking of each training strategy was analysed. The ranking probabilities indicated that RAGT was the most effective, followed by BWSOGT, BWSTT, and the control intervention (Fig. 5).
Network plot of all studies. The nodes, which represent the interventions in the network, and the lines, which highlight the available direct comparisons between pairs of interventions. The size of the nodes and the width of the lines both represent the number of studies. RAGT, robot-assisted gait training; BWSTT, body weight-supported treadmill training; BWSOGT, body weight-supported overground training.
Forest plot of ambulatory assessments. The SMDs and 95% CIs of comparison between the control intervention and other body weight-supported gait training therapies were as follows: RAGT = 0.30 (0.11, 0.50); BWSTT = 0.09 (− 0.40, 0.58); and BWSOGT = 0.09 (− 0.55, 0.73). RAGT, robot-assisted gait training; BWSTT, body weight-supported treadmill training; BWSOGT, body weight-supported overground training; SMD, standard mean difference; 95% CI, 95% credible interval.
Distribution of probabilities in the ranking of each body weight–supported gait training strategy. The ranking probabilities indicated that RAGT was the most effective, followed by BWSOGT, BWSTT, and the control intervention. RAGT, robot-assisted gait training; BWSTT, body weight-supported treadmill training; BWSOGT, body weight–supported overground training.
Network consistency
Network plots contain nodes, which represent the interventions in the network, and lines, which highlight the available direct comparisons between pairs of interventions25. The size of nodes and the width of lines both represent the number of studies. Our network plot (Fig. 3) depicts two triangle loops (RAGT-BWSTT-control intervention and BWSTT-BWSOGT-control intervention), and the loop-specific heterogeneity revealed no significant inconsistency between the results of direct and indirect comparisons (Table 3).
Furthermore, the differences between the traditional pairwise meta-analyses and network meta-analyses were determined and are presented as forest plots (Fig. 6); none of the differences were significant.
Adverse events
Of the 15 selected RCTs, six reported on adverse events29,30,33,35,39,41. No adverse events were observed in four studies30,33,35,41, and two reported that some participants had experienced pain29,39. The investigated interventions were relatively safe and well tolerated by participants.
Discussion
Our results revealed that the body weight-supported gait training protocol with the highest ranking was RAGT followed by BWSOGT, BWSTT, and the control intervention. However, only RAGT was significantly more effective than the control intervention. No significant inconsistency was noted in our network. Moreover, our quality assessment results revealed that most of the included studies had an acceptable risk of bias.
In our network meta-analysis, RAGT ranked first as a body weight-supported gait training protocol for patients with SCI. According to a systematic review conducted by Antonio et al., many rehabilitation robots are available and can be classified as grounded exoskeletons, end-effector devices, wearable exoskeletons, and soft exoskeletons42. Although all these devices are robot assisted, some provide only guidance and gait modulation without body weight support. To ensure comparability with other body weight-supported gait training devices, we focused on body weight-supported grounded exoskeletons.
RAGT aims to improve the walking ability of patients with SCI. Our results supported its use by these patients. Robotic training has become readily accessible in rehabilitation centres, and robot-assisted gait rehabilitation has received much attention owing to its benefits for people with neurological conditions30,43. Robotic training may be an attractive option for patients and their families because of its sophistication and use of computer interface that offers a virtual reality experience both biofeedback43. This technology is also appealing to therapists because they potentially require fewer staff members and cause less physical strain than conventional therapy44. Robotic orthoses provide guidance on lower limb movement during walking training, facilitating prolonged walking training with the afferent input of a normal gait pattern30. This extensive exposure within task-specific repetitive training promotes the reorganisation of the primary motor cortex, and functional outcomes can be improved in patients with neurological conditions45.
An effective gait requires multifactorial system control, including control of the neuromuscular, musculoskeletal, cardiopulmonary, sensory, and cognitive systems. Robot-assisted devices may help improve neural plasticity—the tendency of synapses and neural circuits to change in response to activity—by providing intensive locomotor gait training46,47. Furthermore, intensive training helps prevent the age-related process of deconditioning, the onset and progression of impairment, functional limitation, disability, and changes in physical function and health resulting from injury, disease, and other causes48.
Body weight-supported gait training has been widely advocated for people with SCI and has been demonstrated to improve ambulatory ability29,35,49,50. It enables patients to walk with improved gait pattern while their weight bearing stress is relieved35. BWSOGT combines body weight-supported training and over ground training, whereas BWSTT is a combination of body weight-supported gait training and treadmill training. In BWSTT, walking speed is almost constant owing to the use of a treadmill belt, thereby emphasising the rhythmicity of voluntary movements29. Although these two training strategies may improve ambulatory ability, few studies have illustrated their significance. Several novel devices are available, such as Rysen, the Float, and ZeroG. However, no RCTs have evaluated gait training for SCI by focusing on these devices. Therefore, future high-quality RCTs, especially those focusing on novel devices, are required to evaluate their potential for improving ambulatory function in patients with SCI.
Several studies have illustrated the effectiveness of RAGT. Duan et al., through a pairwise meta-analysis, concluded that RAGT is more effective than conventional training in improving ambulation51. Furthermore, through a pairwise meta-analysis, Fang et al. reported that RAGT can improve spasticity and walking ability in people with SCI better than conventional training can52. In a systematic review of 13 RCTs, Mehrholz et al. compared different training strategies to improve gait in people with SCI and concluded that BWSTT and RAGT do not increase walking speed more than overground gait training and other forms of physiotherapy, but their effects on walking distance are unclear53. Although these studies have provided diverse outcomes on different training strategies for patients with SCI, they used only pairwise meta-analysis; these results lack indirect comparisons. Therefore, we performed both direct and indirect comparisons in the current network meta-analysis to investigate the efficacy of these interventions. Our network plot had two closed loops (Fig. 3). In a closed loop, each direct source of evidence is complemented by an indirect source of evidence for the same comparison25, thereby providing much more solid evidence than an open loop can. Thus, network meta-analyses have the advantage of estimating relative effects between any pair of interventions in the network, usually yielding more precise estimates than a single direct or indirect estimate.
The studies included in our analyses had high consistency and an acceptable risk of bias. This implies that the different sources of evidence (direct and indirect) agree with each other25. However, the transitivity of this study might be influenced by different characteristics among the participants and protocols of the selected RCTs. Regarding the AIS grade of the participants, one study included participants with AIS grade A7, whereas the others reported patients with grades C to D28,29,30,31,32,33,34,35,36,37,38,39,40 and one reported with grade A to C41. Furthermore, the treatment duration was different, ranging from 4 weeks36,41 to > 10 weeks29,32,38,39,40. These discrepancies may have affected the transitivity of this study.
This network meta-analysis has several strengths. First, this is the first network meta-analysis of RCTs that focused on the effect of different body weight-supported gait training approaches for patients with SCI. Second, no significant inconsistency was noted between the results of the direct trials and indirect comparisons, indicating favourable coherence. Third, multiple major databases were used to identify RCTs without language restrictions. Finally, the risk of bias of the selected RCTs was mostly acceptable.
This study also had several limitations. First, the number of included articles was relatively small, particularly those focused on BWSOGT and BWSTT, for conducting a network meta-analysis. This might make RAGT a dominant intervention in this study. Second, the transitivity of this study may have been influenced by the different treatment protocols and participant characteristics of the included studies. Thus, caution should be exercised when applying our results to other patient groups. Third, among the various robot-assisted devices available, we focused only on body weight-supported devices. To overcome these limitations, larger-scale studies focusing on BWSOGT or BWSTT are warranted to determine the effectiveness of these protocols. Moreover, future studies should attempt to include consistent protocols and participant characteristics.
Conclusion
We conducted the first network meta-analysis of RCTs focusing on body weight-supported gait training for patients with SCI. Among them, RAGT was the most effective, followed by BWSOGT, BWSTT, and the control intervention. We suggest that RAGT should be the training protocol of choice for improving walking ability in individuals with SCI. Because few studies have focused on BWSOGT and BWSTT, future studies should comprehensively evaluate their potential for improving the walking ability of patients with SCI. Moreover, further high-quality, large-scale RCTs are required to ensure the benefits and long-term effects of these interventions.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Nas, K., Yazmalar, L., Şah, V., Aydın, A. & Öneş, K. Rehabilitation of spinal cord injuries. World J. Orthop. 6, 8–16. https://doi.org/10.5312/wjo.v6.i1.8 (2015).
Anderson, K. D. Targeting recovery: Priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383. https://doi.org/10.1089/neu.2004.21.1371 (2004).
Eng, J. J. et al. Spinal cord injury rehabilitation evidence: Methods of the SCIRE systematic review. Top. Spinal Cord Inj. Rehabil. 13, 1–10. https://doi.org/10.1310/sci1301-1 (2007).
Harvey, L. A. Physiotherapy rehabilitation for people with spinal cord injuries. J. Physiother. 62, 4–11. https://doi.org/10.1016/j.jphys.2015.11.004 (2016).
Fehlings, M. G., Cadotte, D. W. & Fehlings, L. N. A series of systematic reviews on the treatment of acute spinal cord injury: A foundation for best medical practice. J. Neurotrauma 28, 1329–1333. https://doi.org/10.1089/neu.2011.1955 (2011).
Nasser, M. E. T., Reda, M. A. E. H., Awad, M. R., Amin, I. R. & Assem, S. A. Effect of massed practice and somatosensory stimulation on the upper extremity function in patients with incomplete cervical spinal cord injury. Alexandria J. Med. 50, 189–196. https://doi.org/10.1016/j.ajme.2014.02.001 (2014).
Yıldırım, M. A., Öneş, K. & Gökşenoğlu, G. Early term effects of robotic assisted gait training on ambulation and functional capacity in patients with spinal cord injury. Turk. J. Med. Sci. 49, 838–843. https://doi.org/10.3906/sag-1809-7 (2019).
Kurz, M. J., Stuberg, W., Dejong, S. & Arpin, D. J. Overground body-weight-supported gait training for children and youth with neuromuscular impairments. Phys. Occup. Ther. Pediatr. 33, 353–365. https://doi.org/10.3109/01942638.2013.771719 (2013).
Sousa, C. O., Barela, J. A., Prado-Medeiros, C. L., Salvini, T. F. & Barela, A. M. The use of body weight support on ground level: An alternative strategy for gait training of individuals with stroke. J. Neuroeng. Rehabil. 6, 43. https://doi.org/10.1186/1743-0003-6-43 (2009).
Sousa, C. O., Barela, J. A., Prado-Medeiros, C. L., Salvini, T. F. & Barela, A. M. Gait training with partial body weight support during overground walking for individuals with chronic stroke: A pilot study. J. Neuroeng. Rehabil. 8, 48. https://doi.org/10.1186/1743-0003-8-48 (2011).
Ada, L., Dean, C. M., Vargas, J. & Ennis, S. Mechanically assisted walking with body weight support results in more independent walking than assisted overground walking in non-ambulatory patients early after stroke: A systematic review. J. Physiother. 56, 153–161. https://doi.org/10.1016/s1836-9553(10)70020-5 (2010).
Wirz, M. et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: A multicenter trial. Arch. Phys. Med. Rehabil. 86, 672–680. https://doi.org/10.1016/j.apmr.2004.08.004 (2005).
Dobkin, B. H. An overview of treadmill locomotor training with partial body weight support: A neurophysiologically sound approach whose time has come for randomized clinical trials. Neurorehabil. Neural Repair 13, 157–165. https://doi.org/10.1177/154596839901300301 (1999).
Tefertiller, C., Pharo, B., Evans, N. & Winchester, P. Efficacy of rehabilitation robotics for walking training in neurological disorders: A review. J. Rehabil. Res. Dev. 48, 387–416. https://doi.org/10.1682/jrrd.2010.04.0055 (2011).
Dobkin, B. H. & Duncan, P. W. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate?. Neurorehabil. Neural Repair 26, 308–317. https://doi.org/10.1177/1545968312439687 (2012).
AuYong, N. & Lu, D. C. Neuromodulation of the lumbar spinal locomotor circuit. Neurosurg. Clin. N. Am. 25, 15–23. https://doi.org/10.1016/j.nec.2013.08.007 (2014).
Nam, K. Y. et al. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: A systematic review. J. Neuroeng. Rehabil. 14, 24. https://doi.org/10.1186/s12984-017-0232-3 (2017).
Vaney, C. et al. Robotic-assisted step training (lokomat) not superior to equal intensity of over-ground rehabilitation in patients with multiple sclerosis. Neurorehabil. Neural Repair 26, 212–221. https://doi.org/10.1177/1545968311425923 (2012).
Hutton, B. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 162, 777–784. https://doi.org/10.7326/m14-2385 (2015).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. https://doi.org/10.1136/bmj.n71 (2021).
Scivoletto, G. et al. Validity and reliability of the 10-m walk test and the 6-min walk test in spinal cord injury patients. Spinal Cord 49, 736–740. https://doi.org/10.1038/sc.2010.180 (2011).
Shin, J. C., Yoo, J. H., Jung, T. H. & Goo, H. R. Comparison of lower extremity motor score parameters for patients with motor incomplete spinal cord injury using gait parameters. Spinal Cord 49, 529–533. https://doi.org/10.1038/sc.2010.158 (2011).
Ditunno, J. F. et al. The Walking Index for Spinal Cord Injury (WISCI/WISCI II): Nature, metric properties, use and misuse. Spinal Cord 51, 346–355. https://doi.org/10.1038/sc.2013.9 (2013).
Higgins, J. P. T. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. https://doi.org/10.1136/bmj.d5928 (2011).
Higgins JPT TJ CJ CM, L. T., Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane http://www.training.cochrane.org/handbook (2021).
WEI-MING H, R.-W., LIOU., & SHEN-HUA, LIN. ShinyNMA. https://jerryljw.shinyapps.io/ShinyNMA_/ (Accessed 7 Aug 2021).
Rücker, G. & Schwarzer, G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 15, 58. https://doi.org/10.1186/s12874-015-0060-8 (2015).
Alcobendas-Maestro, M. et al. Lokomat robotic-assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: Randomized controlled trial. Neurorehabil. Neural Repair 26, 1058–1063. https://doi.org/10.1177/1545968312448232 (2012).
Alexeeva, N. et al. Comparison of training methods to improve walking in persons with chronic spinal cord injury: A randomized clinical trial. J. Spinal Cord Med. 34, 362–379. https://doi.org/10.1179/2045772311y.0000000018 (2011).
Cheung, E. Y. Y. et al. Effect of EMG-biofeedback robotic-assisted body weight supported treadmill training on walking ability and cardiopulmonary function on people with subacute spinal cord injuries—A randomized controlled trial. BMC Neurol. 19, 140. https://doi.org/10.1186/s12883-019-1361-z (2019).
Esclarín-Ruz, A. et al. A comparison of robotic walking therapy and conventional walking therapy in individuals with upper versus lower motor neuron lesions: A randomized controlled trial. Arch. Phys. Med. Rehabil. 95, 1023–1031. https://doi.org/10.1016/j.apmr.2013.12.017 (2014).
Field-Fote, E. C. & Roach, K. E. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: A randomized clinical trial. Phys. Ther. 91, 48–60. https://doi.org/10.2522/ptj.20090359 (2011).
Labruyère, R. & van Hedel, H. J. Strength training versus robot-assisted gait training after incomplete spinal cord injury: A randomized pilot study in patients depending on walking assistance. J. Neuroeng. Rehabil. 11, 4. https://doi.org/10.1186/1743-0003-11-4 (2014).
Mıdık, M., Paker, N., Buğdaycı, D. & Mıdık, A. C. Effects of robot-assisted gait training on lower extremity strength, functional independence, and walking function in men with incomplete traumatic spinal cord injury. Turk. J. Phys. Med. Rehabil. 66, 54–59. https://doi.org/10.5606/tftrd.2020.3316 (2020).
Senthilvelkumar, T., Magimairaj, H., Fletcher, J., Tharion, G. & George, J. Comparison of body weight-supported treadmill training versus body weight-supported overground training in people with incomplete tetraplegia: A pilot randomized trial. Clin. Rehabil. 29, 42–49. https://doi.org/10.1177/0269215514538068 (2015).
Shin, J. C., Kim, J. Y., Park, H. K. & Kim, N. Y. Effect of robotic-assisted gait training in patients with incomplete spinal cord injury. Ann. Rehabil. Med. 38, 719–725. https://doi.org/10.5535/arm.2014.38.6.719 (2014).
Wu, M., Kim, J. & Wei, F. Facilitating weight shifting during treadmill training improves walking function in humans with spinal cord injury: A randomized controlled pilot study. Am. J. Phys. Med. Rehabil. 97, 585–592. https://doi.org/10.1097/phm.0000000000000927 (2018).
Lin, H. D., Zhang, T. & Chen, Q. Effect of robot-assisted gait training on walking ability in patients with incomplete spinal cord injury. Acta Automatica Sinica 42, 1832–1838. https://doi.org/10.16383/j.aas.2016.c160173 (2016).
Edwards, D. J. et al. Walking improvement in chronic incomplete spinal cord injury with exoskeleton robotic training (WISE): A randomized controlled trial. Spinal Cord 60, 522–532. https://doi.org/10.1038/s41393-022-00751-8 (2022).
Piira, A. et al. Quality of life and psychological outcomes of body-weight supported locomotor training in spinal cord injured persons with long-standing incomplete lesions. Spinal Cord 58, 560–569. https://doi.org/10.1038/s41393-019-0401-2 (2020).
Xiang, X. N. et al. Exoskeleton-assisted walking improves pulmonary function and walking parameters among individuals with spinal cord injury: A randomized controlled pilot study. J. Neuroeng. Rehabil. 18, 86. https://doi.org/10.1186/s12984-021-00880-w (2021).
Rodríguez-Fernández, A., Lobo-Prat, J. & Font-Llagunes, J. M. Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. J. Neuroeng. Rehabil. 18, 22. https://doi.org/10.1186/s12984-021-00815-5 (2021).
Pool, D., Valentine, J., Taylor, N. F., Bear, N. & Elliott, C. Locomotor and robotic assistive gait training for children with cerebral palsy. Dev. Med. Child Neurol. 63, 328–335. https://doi.org/10.1111/dmcn.14746 (2021).
Ammann-Reiffer, C., Bastiaenen, C. H., Meyer-Heim, A. D. & van Hedel, H. J. Effectiveness of robot-assisted gait training in children with cerebral palsy: A bicenter, pragmatic, randomized, cross-over trial (PeLoGAIT). BMC Pediatr. 17, 64. https://doi.org/10.1186/s12887-017-0815-y (2017).
Ungerleider, L. G., Doyon, J. & Karni, A. Imaging brain plasticity during motor skill learning. Neurobiol. Learn. Mem. 78, 553–564. https://doi.org/10.1006/nlme.2002.4091 (2002).
Wang, H. Y. & Yang, Y. H. Evaluating the responsiveness of 2 versions of the gross motor function measure for children with cerebral palsy. Arch. Phys. Med. Rehabil. 87, 51–56. https://doi.org/10.1016/j.apmr.2005.08.117 (2006).
Cauraugh, J. H. & Summers, J. J. Neural plasticity and bilateral movements: A rehabilitation approach for chronic stroke. Prog. Neurobiol. 75, 309–320. https://doi.org/10.1016/j.pneurobio.2005.04.001 (2005).
Guide to Physical Therapist Practice. Part 1: A description of patient/client management. Part 2: Preferred practice patterns. American Physical Therapy Association. Phys. Ther. 77, 1160–1656 (1997).
Wirz, M., Bastiaenen, C., de Bie, R. & Dietz, V. Effectiveness of automated locomotor training in patients with acute incomplete spinal cord injury: A randomized controlled multicenter trial. BMC Neurol. 11, 60. https://doi.org/10.1186/1471-2377-11-60 (2011).
Hicks, A. L. et al. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: Effects on functional walking ability and measures of subjective well-being. Spinal Cord 43, 291–298. https://doi.org/10.1038/sj.sc.3101710 (2005).
Duan, R. et al. Clinical benefit of rehabilitation training in spinal cord injury: A systematic review and meta-analysis. Spine 46, e398–e410. https://doi.org/10.1097/brs.0000000000003789 (2021).
Fang, C. Y., Tsai, J. L., Li, G. S., Lien, A. S. & Chang, Y. J. Effects of robot-assisted gait training in individuals with spinal cord injury: A meta-analysis. Biomed. Res. Int. 2020, 2102785. https://doi.org/10.1155/2020/2102785 (2020).
Mehrholz, J., Harvey, L. A., Thomas, S. & Elsner, B. Is body-weight-supported treadmill training or robotic-assisted gait training superior to overground gait training and other forms of physiotherapy in people with spinal cord injury? A systematic review. Spinal Cord 55, 722–729. https://doi.org/10.1038/sc.2017.31 (2017).
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
F-A.Y. conceptualized and designed the study and drafted the manuscript. H-C.C. critically revised the manuscript for intellectual content. S-C.C. and J-F.C. conducted a comprehensive search for articles that met the eligibility criteria. F-A.Y. and Y-C.S. extracted the relevant data and assessed the quality of the selected trials. T-H.L. and R.E. provided statistical expertise, analyzed and interpreted the data, and submitted the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, FA., Chen, SC., Chiu, JF. et al. Body weight-supported gait training for patients with spinal cord injury: a network meta-analysis of randomised controlled trials. Sci Rep 12, 19262 (2022). https://doi.org/10.1038/s41598-022-23873-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23873-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.