Abstract
Recent evidence has shown an increase in recurrence and a decrease in overall survival in patients treated with laparoscopic radical hysterectomy (LRH) and robotic assisted radical hysterectomy (RRH) open techniques (ORH). In addition, several high quality trials were recently published regarding the laparoscopic treatment of early stage cervical cancer. We sought out to reassess the recurrence rates, overall survival, complications and outcomes associated with laparoscopic radical hysterectomy (LRH) techniques against open techniques (ORH) when robotic assisted techniques were excluded. We searched PubMed, Medline, Cochrane CENTRAL, SCOPUS, ClinicalTrials.Gov and Web of Science for relevant clinical trials and observational studies. We included all studies that compared with early stage cervical cancer receiving LRH compared with ORH. We included randomized clinical trials, prospective cohort, and retrospective cohort trials. We included studies that included LRH and RRH as long as data was available to separate the two arms. We excluded studies that combined LRH and RRH without supplying data to differentiate. Of 1244 total studies, we used a manual three step screening process. Sixty studies ultimately met our criteria. We performed this review in accordance with PRISMA guidelines. We analyzed continuous data using mean difference (MD) and a 95% confidence interval (CI), while dichotomous data were analyzed using odds ratio (OR) and a 95% CI. Review Manager and Endnote software were utilized in the synthesis. We found that when excluding RRH, the was no significant difference regarding 5-year overall Survival (OR = 1.24 [0.94, 1.64], (P = 0.12), disease free survival (OR = 1.00 [0.80, 1.26], (P = 0.98), recurrence (OR = 1.01 [0.81, 1.25], (P = 0.95), or intraoperative complications (OR = 1.38 [0.94, 2.04], (P = 0.10). LRH was statistically better than ORH in terms of estimated blood loss (MD = − 325.55 [− 386.16, − 264.94] (P < 0.001), blood transfusion rate (OR = 0.28 [0.14, 0.55], (P = 0.002), postoperative complication rate (OR = 0.70 [0.55, 0.90], (P = 0.005), and length of hospital stay (MD = − 3.64[− 4.27, − 3.01], (P < 0.001). ORH was superior in terms of operating time (MD = 20.48 [8.62, 32.35], (P = 0.007) and number of resected lymph nodes (MD = − 2.80 [− 4.35, − 1.24], (P = 0.004). The previously seen increase recurrence and decrease in survival is not seen in LRH when robotic assisted techniques are included and all new high quality is considered. LRH is also associated with a significantly shorter hospital stay, less blood loss and lower complication rate.
Prospero Prospective Registration Number: CRD42022267138.
Similar content being viewed by others
Introduction
Cervical cancer is the third most common malignancy and the second most common cause of death from cancer among women in the USA1. The incidence and mortality of cervical cancer have a large geographic variation as it is more common and more fatal in developing countries2. Cervical cancer is classified into early-stage and advanced-stage cancer3. Although staged clinically, the surgical treatment of early-stage cervical treatment is critical for optimizing patient survival4,5. Radical hysterectomy with pelvic lymphadenectomy is a commonly performed procedure for the treatment of cervical cancer6. Patients who are found to be candidates for surgical intervention may see 5-year survival rate increased to as high as 87%7. Abdominal, laparoscopic, robotic-assisted laparoscopic and vaginal approaches to radical hysterectomy have all been described and performed by many authors8. Secondary to the risk of spreading cancers, several authors have stated that gynecologic oncology has been slower to adopt minimally invasive techniques than other specialties, sometimes reserving these techniques for only risk reducing procedures9. The first laparoscopic radical hysterectomy was performed in 199310 and since that time the minimally invasive surgery (both laparoscopic and robotic-assisted laparoscopic) have been increasingly used.11,12. Over the last two decades, many studies have compared the survival outcomes and operative morbidity of the minimally invasive and open surgery for the management of cervical cancer13. Open radical hysterectomy shows significant morbidity including bladder dysfunction, blood loss, and complications of blood transfusion14. Many retrospective studies15,16,17,18,19 have shown that laparoscopic surgery causes perioperative complications less than open surgery. Complicating this, a recent randomized clinical multicenter trial proved that minimally invasive surgery was accompanied by a high rate of recurrence and a worse disease-free survival rate than open surgery20. In addition, a recent retrospective study that included 2461 patients with cervical cancer showed that minimally invasive surgery is associated with a higher risk of death than open surgery21. The culmination of these results was a change in the National Comprehensive Cancer Network (NCCN) guidelines with regards to minimally invasive radical hysterectomy22. The current NCCN guidelines and European Society of Gynaecological Oncology (ESGO) guidelines recommended that the standard surgical approach for management of early-stage cervical cancer is abdominal radical hysterectomy23.
In addition to this recent evidence against minimally invasive radical hysterectomy techniques, several new high quality trials regarding LRH and RRH have been published. For this reason, we were motivated to investigate LRH versus ORH while excluding robotic assisted techniques. We conducted this meta-analysis with late breaking, high quality data to again compare the efficacy and safety of laparoscopic radical hysterectomy with that of open radical hysterectomy for the management of cervical cancer. We have meticulously excluded all robotic assisted cases, and attempted to obtain data to use as many high quality trials as possible, while excluding robotic assisted cases.
Method
This meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)24 and the guidelines reported in the Cochrane Handbook for Systematic Reviews of Interventions25.
Eligibility criteria
The inclusion criteria included studies that met all of the following:
-
1.
Population: women with early stage cervical cancer
-
2.
Intervention: Laparoscopic radical hysterectomy (LRH)
-
3.
Comparator: Open radical hysterectomy (ORH)
-
4.
Included Study Designs: randomized clinical trials, prospective cohort, and retrospective cohort studies.
And included at least one of our primary or secondary outcomes.
Primary outcomes included:
(1.) Operative time, (2.) Estimated blood loss, (3.) Rate of intraoperative complications, (4.) Rate of postoperative complications, (5.) Rate of blood transfusions.
Secondary outcomes included:
(1.) Rate of recurrence, (2.) Postoperative or intraoperative mortality (defined as within 90 days of surgery), (3.) Five-year survival rate, (4.) Disease-free survival rate, (5.) Number of resected lymph nodes.
We made all efforts to obtain all data possible from all included studies in order to include as many high quality trials as possible, but ultimately excluded those trials that failed our inclusion critieria because they did not publish, or despite our best efforts we could not obtain the necessary data to differentiate which cases included robotic assistance and which did not. We excluded all secondary works, such as meta-analyses and reviews, all animal studies, conference abstracts, and studies with incomplete reported data.
Search and study selection
We searched PubMed, Medline, Scopus, Web of Science, ClinicalTrials.Gov and Cochrane CENTRAL databases from database inception until January 2nd 2022 for articles that matched our inclusion criteria.
We used the following search strategy in our search: (laparoscopic OR laparoscopy) AND (open OR abdominal) AND ((cervical cancer) OR (cancer cervix)) AND (hysterectomy). We screened the included articles in three steps. The first step implied importing the results from electronic databases to a Microsoft Excel26 sheet using EndNote Software27. The second step included a manual title and abstract screening of the articles imported to the Excel sheet. The third step was the full-text screening of the included citations from step 2. Additionally, we manually searched the references of the included papers for possible missed studies. Two researchers separately performed the literature search and eligibility match. Disagreements in the included studies was reached by consensus. A third member of the research team was assigned to settle disputes if consensus could not be reached on any study's eligibility, but was ultimately never needed.
Data collection
We collected three categories of data from each included study: the first category is the baseline and demographic characteristics of the included participants, such as the author, year, country, sample size, age, BMI, included stages, follow up period, time period, adenocarcinoma, squamous cell carcinoma, and positive lymph nodes. The second category included the outcomes of analysis, mainly: Operative time, estimated blood loss, Intraoperative complication, Postoperative complication, Blood transfusion rate, Recurrence, mortality, Five-year survival rate, Disease-free survival, and Resected lymph nodes. The third category included data for risk of bias assessment. The process of data collection was done using Microsoft Excel26.
Risk of bias assessment
We used the quality assessment tools from the National Heart, Lung, and Blood Institute (NHLB) to assess the risk of bias of observational studies28. We followed The Grading of Recommendations Assessment, Development and Evaluation (GRADE) Guidelines in assessing the quality of this study. We assessed the risk of bias of included trials using Cochrane’s risk of bias tool29. The tool assesses proper randomization of patients, allocation concealment, and adequate blinding through seven domains. Each domain was judged to be either “low”, “unclear”, or “high” risk of bias. The details of the GRADE assessment of each outcome can be found in Supplementary Table S1.
Analysis
We performed the meta-analysis of this study using Review Manager Software27. Our study included continuous and dichotomous outcomes. We analyzed continuous data using mean difference (MD) and 95% confidence interval (CI), while dichotomous data were analyzed using odds ratio (OR) and 95% CI. The fixed-effects model was used when data were homogeneous, while heterogeneous data were analyzed under a random-effects model. To measure the presence of inconsistency among the studies, we used the I2 and the p-value of the Chi-square tests30. Values of P < 0.1 or I2 > 50% were significant indicators of the presence of heterogeneity. We tried to solve the inconsistency of heterogeneous outcomes using Cochrane’s leave-one-out method30.
Ethics approval and consent to participate
This Manuscript has been reviewed by the institutional IRB board at Marchand Institute and was found to be exempt from IRB review. (January 2022). Data used was exempt from consent to participate or publish secondary to the nature of the study being a systematic review, retrospectively looking at previously published data.
Commitment to diversity
The Marchand Institute remains committed to diversity and tolerance in its research, and actively maintains a workplace free of racism and sexism. Greater than half of the authors for this study are female and many represent diverse backgrounds and under-represented ethnic groups.
Results
Summary of included studies
The literature search results are illustrated in the PRISMA flow diagram in Fig. 1. We included sixty studies that met our eligibility criteria from the different databases11,15,16,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87. We analyzed 42,994 patients with cervical cancer in different stages according to FIGO staging (2009 edition)3. A total of 15,995 patients were allocated to the laparoscopic group, while 26,999 patients were allocated to the laparotomy group. The mean age of patients in the laparoscopic group and laparotomy group was 46.5 and 46.7 years, respectively. A summary of the included studies, the demographic data of patients, the included stages, and follow-up duration are described in detail in Tables 1 and 2.
Results of risk of bias assessment
The result of the quality assessment yielded an overall moderate risk of bias according to Cochrane`s tool and the mean score for observational studies was 10.57 out of 14 according to NHLB. All clinical trials44,55,56,83 reported proper randomization therefore they were categorized as low risk of bias. All clinical trials were at low risk of bias regarding attrition bias and selective reporting. A detailed illustration of the quality assessment of the included studies is illustrated in Supplementary Table S2.
Analysis of outcomes
Operative time (minutes)
Operating time was reported by 42 studies. We found that ORH operating time was significantly lower than LRH operative time (MD = 20.48 [8.62, 32.35], (P = 0.007). Pooled data were heterogeneous (P < 0.001); I2 = 98% which could not be solved by the leave-one-out method or subgroup analysis (Fig. 2).
Estimated blood loss (ml)
We analyzed 6410 patients from 39 studies that reported the estimated blood loss. The overall mean difference showed that blood loss was significantly lower in LRH group than ORH group (MD = − 325.55 [− 386.16, − 264.94] (P < 0.001)), Pooled analysis was heterogeneous (P < 0.001); I2 = 97% (Fig. 3).
Intraoperative complication
Thirty two studies reported the rate of intraoperative complications. We found no significant difference between both groups (OR = 1.38 [0.94, 2.04], (P = 0.10)). Data was heterogeneous (P < 0.001); I2 = 69% as shown in Fig. 4A. We solved the heterogeneity by excluding Liang et al.87 (P = 0.15); I2 = 21%, the overall odds ratio after solving heterogeneity did not show any significant difference between both groups (OR = 1.14 [0.86, 1.51], (P = 0.37)) (Fig. 4B).
Postoperative complication
A total of 33,563 patients were analyzed from 43 studies that reported postoperative complications. The overall odds ratio showed that the LRH group had a postoperative complications rate significantly lower than that of ORH (OR = 0.70 [0.55, 0.90], (P = 0.005)). Pooled data was heterogeneous (P < 0.001); I2 = 78%. We could not solve heterogeneity by the leave-one-out method or subgroup analysis (Fig. 5).
Length of hospital stay (days)
Forty-one studies reported length of hospital stay. We found that patients in the LRH group stayed at hospital fewer days than patients in the ORH group (MD = − 3.64 [− 4.27, − 3.01], (P < 0.001)). We found heterogeneity which could not be solved by the leave-one-out method or subgroup analysis (Fig. 6).
Resected lymph nodes
Thirty six studies reported the number of resected lymph nodes as an outcome. Pooled analysis showed that the LRH group was associated with fewer resected lymph nodes than the ORH group (MD = − 2.80 [− 4.35, − 1.24], (P = 0.004)). We found heterogeneity among studies (P < 0.001); I2 = 93% (Fig. 7).
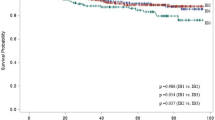
Five-year Overall Survival
A total of 8610 patients were analyzed from 22 studies. The combined analysis did not show any significant difference between both groups (OR = 1.24 [0.94, 1.64], (P = 0.12)). A little heterogeneity was found among studies (P = 0.03); I2 = 40% (Fig. 8A). We solved the heterogeneity by excluding Corrado et al.59 (P = 0.21); I2 = 19%. The overall analysis after solving heterogeneity also showed no significant difference between both groups (OR = 1.10 [0.87, 1.40], (P = 0.43)) (Fig. 8B).
Disease free survival
Twenty seven studies reported DFS. Pooled analysis did not show any difference between both groups (OR = 1.00 [0.80, 1.26], (P = 0.98)). Data was heterogeneous (P = 0.002); I2 = 50% (Fig. 9).
Postoperative or Intraoperative Mortality (Defined as within 90 days postop)
Twenty four studies reported mortality as an outcome. The overall odds ratio showed no significant difference between either group (OR = 0.86 [0.69, 1.06], (P = 0.15). Pooled analysis was homogeneous (P = 0.14); I2 = 24% (Fig. 10).
Recurrence
A total of 19,610 patients were analyzed from 40 studies. We found no significant difference between the two groups (OR = 1.01 [0.81, 1.25], (P = 0.95). Data was heterogeneous (P < 0.001); I2 = 56% (Fig. 11).
Blood transfusion rate
The combined analysis of 12,673 patients from 29 studies favored the LRH group significantly (OR = 0.28 [0.14, 0.55], (P = 0.002). Pooled analysis was heterogeneous (P < 0.001); I2 = 96% (Fig. 12).
A funnel plot and Egger test of all outcomes can be found in Supplementary File S1. A separate sensitivity analysis involving only the studies with a low risk of bias can also be found in Supplementary File S2. The sensitivity analysis showed different findings only in the outcome of postoperative complications, where the result changed from statistically significant to insignificant. There were no changes in any other outcomes within the sensitivity analysis.
Discussion
This is the largest scale meta-analysis to date that has compared LRH to ORH through an evaluation of the available evidence, and the first, to our knowledge, to attempt to do so while specifically excluding robotic assisted techniques. In our study, we found that LRH was associated with a significantly lower incidence of estimated blood loss, postoperative complications. In addition, predictably, the LRH group had a short period of postoperative hospital stay. The number of the resected in the LRH group lymph node was significantly fewer than that of the ORH group. The combined analysis did not show any significant difference between both groups regarding 5-year overall survival, disease-free survival, and postoperative or intraoperative mortality. The duration of the surgical procedure was significantly longer in the LRH compared to ORH. This stands in contrast to previous studies that showed decreased survival as a result of minimally invasive radical hysterectomy techniques20.
Although isolated studies have disagreed with these results, the majority of previous systematic reviews and meta-analyses have not. In 2015, Wang et al.88 performed a meta-analysis of 12 cohort studies. They demonstrated similar results to our study. They conclude that LRH was better than ORH regarding the short-term outcomes such as faster functional recovery, estimated blood loss, and postoperative complications. The survival outcomes were also similar in both groups.
Smith et al.89 performed another meta-analysis to compare the minimally invasive hysterectomy with abdominal radical hysterectomy. They found that in more than 22,000 women with early-stage cervical cancer the progression-free survival was significantly worse for women who underwent the minimally invasive radical hysterectomy. This finding is strengthened with longer follow-up. The pooled relative risk for postoperative or intraoperative mortality was lower in the minimally invasive group.
In 2020, Kampers et al.90 performed the first meta-analysis stratifying the patients subjected to different operation techniques according to their risk factors. They compared the survival rates of open hysterectomy and laparoscopic hysterectomy in different risk groups. The study demonstrated that protective techniques in laparoscopy result in improved survival.
Kong et al.84 conducted a retrospective analysis of 88 patients with a cervical cancer diameter of 3 cm or greater. They found that LRH can be a feasible alternative surgical procedure for the management of FIGO stage IB and IIA cervical cancer. However, the institution at which the study was performed was introduced with the laparoscopic approach much later than the laparotomy approach. This in turn made the follow-up period in the LRH group relatively short to make any conclusive remarks on survival benefits.
Liang et al.87 reviewed the records of 18,447 patients undergoing radical hysterectomy for cervical cancer. They demonstrated that laparoscopic hysterectomy was associated with a higher risk of major complications than conventional laparotomy. Prior to our review, this study probably represented the largest scale evidence that assessed surgical complications after radical hysterectomy. Other studies to attempt this were single-center studies with small sample sizes15,60,74. In reconciling why the findings of Liang et al. did not also appear as statistically significant in our analysis, we hypothesize that this had to do with limitations on Liang’s study design. This study relied on inpatient medical records or readmission records to obtain information on complications without regular follow-up of the patients. Therefore, without any first-hand knowledge of the circumstances of this study we would hypothesize that perhaps this their conclusion may not have been as statistically significant if direct outreach to patients had been attempted, consistent with other cohort analyses.
In 2021, Campos et al.55 performed a single-center randomized controlled trial on 30 patients with cervical cancer and lymphovascular invasion. They demonstrated a non-significant trend of worse outcomes for LRH. The overall survival time and disease-free survival time were longer in the LRH group. However, the main limitation facing the study was the small sample size.
In 2018, the LACC study by Ramirez et al.20 found that the minimally invasive radical hysterectomy had lower rates of overall survival and disease-free survival than abdominal radical hysterectomy. It was largely based on this study that the National Comprehensive Cancer Network (NCCN) recommended careful counseling of the patient about short-term versus long-term outcomes and oncologic risks of the different surgical approaches.
Limitations
Although the main limitation facing us in this study is the heterogeneity in some outcomes, we managed to understand most of the attributing factors. We believe the use of studies with different designs and disparity in follow-up periods was responsible for the heterogeneity, which is understandable. Another possible limitation of our study includes selection bias, which is difficult to account for. With observational studies, there is always the possibility that surgeons have intentionally selected out cases for laparoscopic radical approaches that appear easier or have different characteristics than those chosen to be performed open. This could affect data. Our analysis represents the most recent and wide-scale evidence that compares LRH to ORH among women with cervical cancer. In light of this controversy it would be desirable to have more well designed prospective randomized trials in order to strengthen the evidence and dissolve any remaining controversy.
Conclusion
We conclude that LRH is associated with a significantly lower incidence of estimated blood loss, postoperative complications. In addition, the LRH had a short period of postoperative hospital stay. In contrast to previous studies that mixed robotic assisted cases we found no difference regarding 5-year overall survival and recurrence excluding robotic assisted cases.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 63, 11–30 (2019).
Kuhry, E., Schwenk, W., Gaupset, R., Romild, U. & Bonjer, J. Long-term outcome of laparoscopic surgery for colorectal cancer: A cochrane systematic review of randomised controlled trials. Cancer Treat. Rev. https://doi.org/10.1002/14651858.CD003432.pub2 (2008).
Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 105(2), 103–104 (2009).
Ginaldi, S., Wallace, S., Jing, B. S. & Bernardino, M. E. Carcinoma of the cervix: Lymphangiography and computer tomography. Am. J. Roentgenol. https://doi.org/10.2214/ajr.136.6.1087 (1981).
Togashi, K. et al. Carcinoma of the cervix: Staging with MR imaging. Radiology https://doi.org/10.1148/radiology.171.1.2928532 (1989).
Jones, W. B., Mercer, G. O., Lewis, J. L., Rubin, S. C. & Hoskins, W. J. Early invasive carcinoma of the cervix. Gynecol. Oncol. https://doi.org/10.1006/gyno.1993.1241 (1993).
Cibula, D. et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the management of patients with cervical cancer. Int. J. Gynecol. Cancer https://doi.org/10.1016/j.radonc.2018.03.003 (2018).
Bhatla, N., Aoki, D., Sharma, D. N. & Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynecol. Obstet. https://doi.org/10.1002/ijgo.12611 (2018).
Husain, A. et al. The role of laparoscopy in second-look evaluations for ovarian cancer. Gynecol. Oncol. https://doi.org/10.1006/gyno.2000.6036 (2001).
Nezhat, C. R., Burrell, M. O., Nezhat, F. R., Benigno, B. B. & Welander, C. E. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am. J. Obstet. Gynecol. https://doi.org/10.1016/0002-9378(92)91351-A (1992).
Abu-Rustum, N. R. et al. Total laparoscopic radical hysterectomy with pelvic lymphadenectomy using the argon-beam coagulator: Pilot data and comparison to laparotomy. Gynecol. Oncol. 91(2), 402–409 (2003).
Diver, E. et al. Minimally invasive radical hysterectomy for cervical cancer is associated with reduced morbidity and similar survival outcomes compared with laparotomy. J. Minim. Invasive Gynecol. https://doi.org/10.1016/j.ygyno.2016.04.485 (2017).
Colombo, N. et al. Endometrial cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. https://doi.org/10.1093/annonc/mdt353 (2013).
Ayhan, A., Tuncer, Z. S. & Yarali, H. Complications of radical hysterectomy in women with early stage cervical cancer: Clinical analysis of 270 cases. Eur. J. Surg/ Oncol. 17, 492–494 (1991).
Nam, J. H. et al. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: Long-term survival outcomes in a matched cohort study. Ann. Oncol. https://doi.org/10.1093/annonc/mdr360 (2012).
Toptas, T. & Simsek, T. Total laparoscopic versus open radical hysterectomy in stage IA2-IB1 cervical cancer: Disease recurrence and survival comparison. J. Laparoendosc. Adv. Surg. Tech. 24(6), 373–378 (2014).
Wang, W. et al. Long-term oncological outcomes after laparoscopic versus abdominal radical hysterectomy in stage I a2- II a2 cervical cancer: A matched cohort study. Zhonghua Fu Chan Ke Za Zhi https://doi.org/10.1097/IGC.0000000000000749 (2015).
Zhu, T. et al. Surgical and pathological outcomes of laparoscopic versus abdominal radical hysterectomy with pelvic lymphadenectomy and/or para-aortic lymph node sampling for bulky early-stage cervical cancer. Int. J. Gynecol. Cancer https://doi.org/10.1097/IGC.0000000000000716 (2017).
Li, X. et al. The survival rate and surgical morbidity of abdominal radical trachelectomy versus abdominal radical hysterectomy for stage IB1 cervical cancer. Ann. Surg. Oncol. https://doi.org/10.1245/s10434-016-5216-1 (2016).
Ramirez, P. T. et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N. Engl. J. Med. 379(20), 1895–1904 (2018).
Melamed, A. et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa1804923 (2018).
Koh, W.-J. et al. Cervical cancer, Version 3.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. https://doi.org/10.6004/jnccn.2019.0001 (2019).
Querleu, D. et al. Laparoscopic radical hysterectomy: A European Society of Gynaecological Oncology (ESGO) statement. Int. J. Gynecol. Cancer https://doi.org/10.1136/ijgc-2019-000775 (2020).
Moher, D. et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Rev. Esp. Nutr. Humana y Diet https://doi.org/10.1186/2046-4053-4-1 (2014).
Higgins, J. P. T. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series Vol. Version 5, 1 (Wiley, 2008).
Katz, A. Microsoft Excel 2010. Style (DeKalb, IL) (2010).
Lebowitz, F. Endnote (Aperture, 2021).
Pearson, G. D. et al. National Heart, Lung, and Blood Institute cardiovascular clinical trial perspective. Am. Heart J. 224, 25–34 (2020).
Munder, T. & Barth, J. Cochrane’s risk of bias tool in the context of psychotherapy outcome research. Psychother. Res. https://doi.org/10.1080/10503307.2017.1411628 (2018).
Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Handbook for Systematic Reviews of Interventions (2019).
Lee, C. L., Huang, K. G., Jain, S., Lee, P. S. & Soong, Y. K. Comparison of laparoscopic and conventional surgery in the treatment of early cervical cancer. J. Am. Assoc. Gynecol. Laparosc. 9(4), 481–487 (2002).
Li, Z. et al. Comparison of oncological outcomes and major complications between laparoscopic radical hysterectomy and abdominal radical hysterectomy for stage IB1 cervical cancer with a tumour size less than 2 cm. Eur. J. Surg. Oncol. 47(8), 2125–2133 (2021).
Lee, E. J., Kang, H. & Kim, D. H. A comparative study of laparoscopic radical hysterectomy with radical abdominal hysterectomy for early-stage cervical cancer: A long-term follow-up study. Eur. J. Obstet. Gynecol. Reprod. Biol. 156(1), 83–86 (2011).
Lim, T. Y. K., Lin, K. K. M., Wong, W. L., Aggarwal, I. M. & Yam, P. K. L. Surgical and oncological outcome of total laparoscopic radical hysterectomy versus radical abdominal hysterectomy in early cervical cancer in Singapore. Gynecol. Minim. Invasive Ther. 8(2), 53–58 (2019).
Steed, H. et al. A comparison of laparascopic-assisted radical vaginal hysterectomy and radical abdominal hysterectomy in the treatment of cervical cancer. Gynecol. Oncol. 93(3), 588–593 (2004).
Shanmugam, S., Susikar, S., Hussain, S. A., Bharanidharan, T. & Michael, R. A retrospective comparison of the outcomes of laparoscopic and open radical hysterectomy for early and advanced cancer of the cervix, in the post-LACC era. Indian J. Gynecol. Oncol. https://doi.org/10.1007/s40944-020-00473-w (2020).
Rodriguez, J. et al. Oncological outcomes of laparoscopic radical hysterectomy versus radical abdominal hysterectomy in patients with early-stage cervical cancer: A multicenter analysis. Int. J. Gynecol. Cancer 31(4), 504–511 (2021).
Sert, M. B. & Abeler, V. Robot-assisted laparoscopic radical hysterectomy: Comparison with total laparoscopic hysterectomy and abdominal radical hysterectomy; One surgeon’s experience at the Norwegian Radium Hospital. Gynecol. Oncol. 121(3), 600–604 (2011).
Park, J. Y. et al. The role of laparoscopic radical hysterectomy in early-stage adenocarcinoma of the uterine cervix. Ann. Surg. Oncol. 23, 825–833 (2016).
Qin, M. et al. A comparison of laparoscopies and laparotomies for radical hysterectomy in stage IA1–IB1 cervical cancer patients: A single team with 18 years of experience. Front. Oncol. 10, 1–12 (2020).
Park, J. Y. et al. Laparoscopic versus open radical hysterectomy in patients with stage IB2 and IIA2 cervical cancer. J. Surg. Oncol. 108(1), 63–69 (2013).
Soliman, P. T. et al. Radical hysterectomy: A comparison of surgical approaches after adoption of robotic surgery in gynecologic oncology. Gynecol. Oncol. 123(2), 333–336 (2011).
Xiao, M., Gao, H., Bai, H. & Zhang, Z. Quality of life and sexuality in disease-free survivors of cervical cancer after radical hysterectomy alone: A comparison between total laparoscopy and laparotomy. Medicine (United States) 95(36), 4787 (2016).
Xu, Q. et al. Postoperative comparison of laparoscopic radical resection and open abdominal radical hysterectomy for cervical cancer patient. Arch. Gynecol. Obstet. 302(2), 473–479. https://doi.org/10.1007/s00404-020-05606-2 (2020).
Xiao, M. & Zhang, Z. Total laparoscopic versus laparotomic radical hysterectomy and lymphadenectomy in cervical cancer an observational study of 13-year experience. Medicine (United States) 94(30), 1–6 (2015).
Wang, W. et al. Laparoscopic vs. abdominal radical hysterectomy for locally advanced cervical cancer. Front. Oncol. 9, 1–10 (2019).
Wright, J. D. et al. Comparative effectiveness of minimally invasive and abdominal radical hysterectomy for cervical cancer. Gynecol. Oncol. 127(1), 11–17 (2012).
Suh, D. H., Cho, H. Y., Kim, K., No, J. H. & Kim, Y. B. Matched-case comparisons in a single institution to determine critical points for inexperienced surgeons’ successful performances of laparoscopic radical hysterectomy versus abdominal radical hysterectomy in stage IA2-IIA cervical cancer. PLoS ONE 10(6), 1–14 (2015).
Taylor, S. E., McBee, W. C., Richard, S. D. & Edwards, R. P. Radical hysterectomy for early stage cervical cancer: Laparoscopy versus laparotomy. J. Soc. Laparoendosc. Surg. 15(2), 213–217 (2011).
Sharma, R., Bailey, J., Anderson, R. & Murdoch, J. Laparoscopically assisted radical vaginal hysterectomy (Coelio-Schauta): A comparison with open Wertheim/Meigs hysterectomy. Int. J. Gynecol. Cancer 16(5), 1927–1932 (2006).
Zhao, W. & Yang, Q. Lymph-vascular space invasion in patients with stages ia2–iia2 cervical cancer treated with laparoscopic versus open radical hysterectomy. Cancer Manag. Res. 13, 1179–1186 (2021).
Zhang, S., Ma, L., Meng, Q. W., Zhou, D. & Moyiding, T. Comparison of laparoscopic-assisted radical vaginal hysterectomy and abdominal radical hysterectomy in patients with early stage cervical cancer. Medicine (United States) 96(36), e8005 (2017).
Yuan, Z. et al. Laparoscopic vs. Open abdominal radical hysterectomy for cervical cancer: A single-institution, propensity score matching study in China. Front. Oncol. 9, 1–7 (2019).
Chen, C. H. et al. Comparing robotic surgery with conventional laparoscopy and laparotomy for cervical cancer management. Int. J. Gynecol. Cancer 24(6), 1105–1111 (2014).
Campos, L. S., Limberger, L. F., Stein, A. T. & Caldas, J. M. Survival after laparoscopic versus abdominal radical hysterectomy in early cervical cancer: A randomized controlled trial. Asian Pac. J. Cancer Prev. 22(1), 93–97 (2021).
Campos, L. S., Limberger, L. F., Stein, A. T. & Kalil, A. N. Postoperative pain and perioperative outcomes after laparoscopic radical hysterectomy and abdominal radical hysterectomy in patients with early cervical cancer: A randomised controlled trial. Trials https://doi.org/10.1186/1745-6215-14-293 (2013).
Pedone Anchora, L. et al. How to select early-stage cervical cancer patients still suitable for laparoscopic radical hysterectomy: A propensity-matched study. Ann. Surg. Oncol. 27(6), 1947–1955 (2020).
Bogani, G. et al. Patterns of recurrence after laparoscopic versus open abdominal radical hysterectomy in patients with cervical cancer: A propensity-matched analysis. Int. J. Gynecol Cancer 30(7), 987–992 (2020).
Corrado, G. et al. Comparison of different surgical approaches for stage IB1 cervical cancer patients: A multi-institution study and a review of the literature. Int. J. Gynecol. Cancer 28(5), 1020–1028 (2018).
Bogani, G. et al. Laparoscopic versus open abdominal management of cervical cancer: Long-term results from a propensity-matched analysis. J. Minim. Invasive Gynecol. https://doi.org/10.1016/j.jmig.2014.03.018 (2014).
Anagnostopoulos, A., Mitra, S., Decruze, B., Macdonald, R. & Kirwan, J. Safety and cost considerations during the introduction period of laparoscopic radical hysterectomy. Obstet. Gynecol. Int. https://doi.org/10.1155/2017/2103763 (2017).
Gil-Moreno, A. et al. Radical hysterectomy: Efficacy and safety in the dawn of minimally invasive techniques. J. Minim. Invasive Gynecol. 26(3), 492–500 (2019).
Gortchev, G., Tomov, S., Tantchev, L., Velkova, A. & Radionova, Z. Robot-assisted radical hysterectomy-perioperative and survival outcomes in patients with cervical cancer compared to laparoscopic and open radical surgery. Gynecol. Surg. 9(1), 81–88 (2012).
Li, G. et al. A comparison of laparoscopic radical hysterectomy and pelvic lymphadenectomy and laparotomy in the treatment of Ib–IIa cervical cancer. Gynecol. Oncol. 105(1), 176–180 (2007).
Guo, J. et al. Laparoscopic procedure compared with open radical hysterectomy with pelvic lymphadenectomy in early cervical cancer: A retrospective study. Onco Targets Ther. 11, 5903–5908 (2018).
He, J. et al. Comparison of laparoscopic and abdominal radical hysterectomy for early stage cervical cancer: Oncologic outcomes based on tumor diameter. Int. J. Gynecol. Cancer 30(9), 1308–1316 (2020).
Kanao, H. et al. Feasibility and outcome of total laparoscopic radical hysterectomy with no-look no-touch technique for FIGO IB1 cervical cancer. J. Gynecol. Oncol. 30(3), 1–12 (2019).
Chen, X. et al. Comparison of laparoscopic and open radical hysterectomy in cervical cancer patients with tumor size ≤2 cm. Int. J. Gynecol. Cancer 30(5), 564–571 (2020).
Kim, S. I. et al. Impact of laparoscopic radical hysterectomy on survival outcome in patients with FIGO stage IB cervical cancer: A matching study of two institutional hospitals in Korea. Gynecol. Oncol. 155(1), 75–82 (2019).
Kim, J. H. et al. Comparative effectiveness of abdominal versus laparoscopic radical hysterectomy for cervical cancer in the postdissemination era. Cancer Res. Treat. 51(2), 788–796 (2019).
Chen, C. et al. Laparoscopic versus abdominal radical hysterectomy for stage IB1 cervical cancer patients with tumor size ≤ 2 cm: A case-matched control study. Int. J. Clin. Oncol. 25(5), 937–947 (2020).
Estape, R. et al. A case matched analysis of robotic radical hysterectomy with lymphadenectomy compared with laparoscopy and laparotomy. Gynecol. Oncol. 113(3), 357–361 (2009).
Chen, X., Yu, J., Zhao, H., Hu, Y. & Zhu, H. Laparoscopic radical hysterectomy results in higher recurrence rate versus open abdominal surgery for stage IB1 cervical cancer patients with tumor size less than 2 centimeter: A retrospective propensity score-matched study. Front. Oncol. 11, 1–9 (2021).
Magrina, J. F., Kho, R. M., Weaver, A. L., Montero, R. P. & Magtibay, P. M. Robotic radical hysterectomy: Comparison with laparoscopy and laparotomy. Gynecol. Oncol. https://doi.org/10.1016/j.ygyno.2008.01.011 (2008).
Liu, Y. et al. The impact of the surgical routes and learning curve of radical hysterectomy on the survival outcomes in stage IB cervical cancer: A retrospective cohort study. Int. J. Surg. 68, 72–77 (2019).
Ditto, A. et al. Implementation of laparoscopic approach for type B radical hysterectomy: A comparison with open surgical operations. Eur. J. Surg. Oncol. 41(1), 34–39 (2015).
Frumovitz, M. et al. Comparison of total laparoscopic and abdominal radical hysterectomy for patients with early-stage cervical cancer. Obstet. Gynecol. 110(1), 96–102 (2007).
Ghezzi, F. et al. Surgicopathologic outcome of laparoscopic versus open radical hysterectomy. Gynecol. Oncol. 106(3), 502–506 (2007).
Malzoni, M., Tinelli, R., Cosentino, F., Fusco, A. & Malzoni, C. Total laparoscopic radical hysterectomy versus abdominal radical hysterectomy with lymphadenectomy in patients with early cervical cancer: Our experience. Ann. Surg. Oncol. 16(5), 1316–1323 (2009).
Paik, E. S. et al. Comparison of laparoscopic and abdominal radical hysterectomy in early stage cervical cancer patients without adjuvant treatment: Ancillary analysis of a Korean Gynecologic Oncology Group Study (KGOG 1028). Gynecol. Oncol. 154(3), 547–553 (2019).
Mendivil, A. A. et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: A five year experience. Surg. Oncol. 25(1), 66–71 (2016).
Kim, S. et al. Learning curve could affect oncologic outcome of minimally invasive radical hysterectomy for cervical cancer. Asian J. Surg. 44(1), 174–180 (2021).
Naik, R., Jackson, K. S., Lopes, A., Cross, P. & Henry, J. A. Laparoscopic assisted radical vaginal hysterectomy versus radical abdominal hysterectomy—A randomised phase II trial: Perioperative outcomes and surgicopathological measurements. BJOG Int. J. Obstet. Gynaecol. 117(6), 746–751 (2010).
Kong, T. W., Chang, S. J., Lee, J., Paek, J. & Ryu, H. S. Comparison of laparoscopic versus abdominal radical hysterectomy for FIGO stage IB and IIA cervical cancer with tumor diameter of 3 cm or greater. Int. J. Gynecol. Cancer 24(2), 280–288 (2014).
Laterza, R. M. et al. Recurrence of early stage cervical cancer after laparoscopic versus open radical surgery. Int. J. Gynecol. Cancer 26(3), 547–552 (2016).
Lambaudie, E. et al. Role of robot-assisted laparoscopy in adjuvant surgery for locally advanced cervical cancer. Eur. J. Surg. Oncol. 36(4), 409–413 (2010).
Liang, C. et al. Effect of laparoscopic versus abdominal radical hysterectomy on major surgical complications in women with stage IA–IIB cervical cancer in China, 2004–2015. Gynecol. Oncol. 156(1), 115–123. https://doi.org/10.1016/j.ygyno.2019.10.032 (2020).
Wang, Y., Deng, L., Xu, H., Zhang, Y. & Liang, Z. Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer https://doi.org/10.1186/s12885-015-1818-4 (2015).
Smith, A. J. B., Jones, T. N., Miao, D. & Fader, A. N. Minimally invasive radical hysterectomy for cervical cancer: A systematic review and meta-analysis. J. Minim. Invasive Gynecol. 28(3), 544-555.e7. https://doi.org/10.1016/j.jmig.2020.12.023 (2021).
Kampers, J. et al. Protective operative techniques in radical hysterectomy in early cervical carcinoma and their influence on disease-free and overall survival: A systematic review and meta-analysis of risk groups. Arch. Gynecol. Obstet. 304(3), 577–587. https://doi.org/10.1007/s00404-021-06082-y (2021).
Acknowledgements
The Marchand Institute for Minimally Invasive Surgery would like to acknowledge the efforts of all of the students, researchers, residents and fellows at the institute who put their time and effort into these projects without compensation, only for the betterment of women’s health. We firmly assure them that the future of medicine belongs to them.
Author information
Authors and Affiliations
Contributions
All authors attest to significant contributions to this work. In particular the following duties were performed by the listed authors. This list does not preclude these authors from making other significant contributions to other portions of this work, and this Institute values all of the effort from our valuable team. (Middle initial corresponds to the second letter of the first name to avoid ambiguity.) Conceptualization was performed mostly by G.M., A.H.M., and A.H.A. Initial draft was composed mostly by A.K., H.U., A.M.A., C.C., S.G., C.M. and A.T.M. Data collection was performed mostly by C.C., S.G., and C.M. Data analysis was performed mostly by J.P., A.M.A., C.C., C.M., and A.T.M. Final draft and discussion was written mostly by G.M., A.H.M., and S.G. Data used was exempt from consent to participate or publish secondary to the nature of the study being a systematic review, retrospectively looking at previously published data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marchand, G., Masoud, A.T., Abdelsattar, A. et al. Meta-analysis of laparoscopic radical hysterectomy, excluding robotic assisted versus open radical hysterectomy for early stage cervical cancer. Sci Rep 13, 273 (2023). https://doi.org/10.1038/s41598-023-27430-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-27430-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.