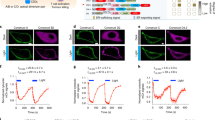
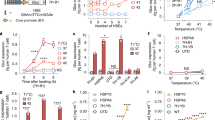
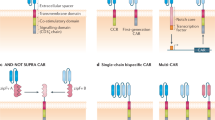
Abstract
Chimeric antigen receptor (CAR) T cell immunotherapy is emerging as a powerful tool for cancer treatment. However, the clinical application of CAR T cell therapy remains limited owing to on-target off-tumour toxicity and cytokine release syndrome, which can cause severe side effects. Remote and spatiotemporal control of CAR T cells could improve the safety and efficacy of CAR T cell therapy. Here, we discuss how CAR T cells can be externally controlled by combining stimuli-responsive nanotechnologies with immuno-engineering. We examine the use of different external stimuli, including light, ultrasound, magnetic fields, X-rays, electric fields and small molecules, to control the activity of CAR T cells against different malignancies and highlight the need for more efficient and biocompatible external stimuli as well as issues to be addressed to effectively treat solid tumours with CAR T cell therapy.
Key points
-
Chimeric antigen receptor (CAR) T cell therapies can be applied against various malignancies, with six CAR T cell therapies already approved by the FDA.
-
The clinical translation of CAR T cell immunotherapies, in particular against solid tumours, remains limited owing to low efficacy, potential cytotoxicity and considerable side effects, which may be addressed by precisely controlling the activation and antitumour effects of CAR T cells.
-
The combination of nanotechnologies and nano-optogenetic, chemogenetic and immuno-engineering approaches allows the remote control of CAR T cell therapies, using various external stimuli, including light, ultrasound, small molecules, magnetic energy, X-rays and electric fields.
-
Remote control strategies may prove particularly useful for the treatment of solid tumours, which are currently not susceptible to CAR T cell therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Milone, M. C. et al. Engineering-enhanced CAR T cells for improved cancer therapy. Nat. Cancer 2, 780–793 (2021).
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010). This article reports one of the first applications of CAR T cell therapy in human patients.
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Lim, W. A. & June, C. H. The principles of engineering immune cells to treat cancer. Cell 168, 724–740 (2017).
Chen, R., Jing, J., Siwko, S., Huang, Y. & Zhou, Y. Intelligent cell-based therapies for cancer and autoimmune disorders. Curr. Opin. Biotech. 66, 207–216 (2020).
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl Med. 5, 177ra138 (2013).
Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl Med. 6, 224ra225 (2014).
Neelapu, S. S. et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2018).
Mullard, A. FDA approves first CAR T therapy. Nat. Rev. Drug Discov. 16, 669 (2017).
Mullard, A. FDA approves first BCMA-targeted CAR-T cell therapy. Nat. Rev. Drug Discov. 20, 332 (2021).
Mullard, A. FDA approves second BCMA-targeted CAR-T cell therapy. Nat. Rev. Drug Discov. 21, 249 (2022).
Tokarew, N., Ogonek, J., Endres, S., von Bergwelt-Baildon, M. & Kobold, S. Teaching an old dog new tricks: next-generation CAR T cells. Br. J. Cancer 120, 26–37 (2019).
Saez-Ibañez, A. R. et al. Landscape of cancer cell therapies: trends and real-world data. Nat. Rev. Drug Discov. 21, 631–632 (2022).
Gust, J. et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 7, 1404–1419 (2017).
Morris, E. C., Neelapu, S. S., Giavridis, T. & Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 22, 85–96 (2022).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014).
Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016).
Losi, A., Gardner, K. H. & Möglich, A. Blue-light receptors for optogenetics. Chem. Rev. 118, 10659–10709 (2018).
Tan, P., He, L., Huang, Y. & Zhou, Y. Optophysiology: illuminating cell physiology with optogenetics. Physiol. Rev. 102, 1263–1325 (2022). This article presents a comprehensive summary of photogenetic tools used to control cells of the immune system and host cell physiology.
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005). This article reports the first use of microbial opsins to photo-control neuronal activity, providing the foundation of the optogenetics field.
Deisseroth, K. Optogenetics. Nat. Methods 8, 26–29 (2011).
Adamantidis, A. R., Zhang, F., Aravanis, A. M., Deisseroth, K. & de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424 (2007).
Tsai, H.-C. et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009).
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2–assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668 (2007).
Tan, P., He, L., Han, G. & Zhou, Y. Optogenetic Immunomodulation: shedding light on antitumor immunity. Trends Biotechnol. 35, 215–226 (2017).
Nguyen, N. T. et al. Nano-optogenetic engineering of CAR T cells for precision immunotherapy with enhanced safety. Nat. Nanotechnol. 16, 1424–1434 (2021). This article reports the application of UCNPs as nanotransducers to enable wireless control of light-switchable CAR T cells by NIR light.
Amitrano, A. M. et al. Optical control of CD8+ T cell metabolism and effector functions. Front. Immunol. 12, 666231 (2021).
Kim, K.-D. et al. Targeted calcium influx boosts cytotoxic T lymphocyte function in the tumour microenvironment. Nat. Commun. 8, 15365 (2017).
Xu, Y. et al. Optogenetic control of chemokine receptor signal and T-cell migration. Proc. Natl Acad. Sci. USA 111, 6371–6376 (2014).
He, L. et al. Optogenetic control of non-apoptotic cell death. Adv. Sci. 8, 2100424 (2021).
Tan, P., He, L. & Zhou, Y. Engineering supramolecular organizing centers for optogenetic control of innate immune responses. Adv. Biol. 5, 2000147 (2021).
He, L. et al. Circularly permuted LOV2 as a modular photoswitch for optogenetic engineering. Nat. Chem. Biol. 17, 915–923 (2021).
He, L. et al. Near-infrared photoactivatable control of Ca2+ signaling and optogenetic immunomodulation. eLife 4, e10024 (2015).
Shah, S. et al. Hybrid upconversion nanomaterials for optogenetic neuronal control. Nanoscale 7, 16571–16577 (2015).
Hososhima, S. et al. Near-infrared (NIR) up-conversion optogenetics. Sci. Rep. 5, 16533 (2015).
Ibsen, S., Tong, A., Schutt, C., Esener, S. & Chalasani, S. H. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat. Commun. 6, 8264 (2015).
Nagel, G. et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl Acad. Sci. USA 100, 13940–13945 (2003).
Kim, C. K., Adhikari, A. & Deisseroth, K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci. 18, 222–235 (2017).
Levskaya, A., Weiner, O. D., Lim, W. A. & Voigt, C. A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461, 997–1001 (2009).
Zhou, X. X., Fan, L. Z., Li, P., Shen, K. & Lin, M. Z. Optical control of cell signaling by single-chain photoswitchable kinases. Science 355, 836–842 (2017).
Mansouri, M. & Fussenegger, M. Synthetic biology-based optogenetic approaches to control therapeutic designer cells. Curr. Opin. Syst. Biol. 28, 100396 (2021).
Fenno, L., Yizhar, O. & Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412 (2011).
Toettcher, J. E., Weiner, O. D. & Lim, W. A. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 155, 1422–1434 (2013).
Lan, T.-H., He, L., Huang, Y. & Zhou, Y. Optogenetics for transcriptional programming and genetic engineering. Trends Genet. 38, 1253–1270 (2022).
Li, X., Lovell, J. F., Yoon, J. & Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17, 657–674 (2020).
Kolar, K., Knobloch, C., Stork, H., Žnidarič, M. & Weber, W. OptoBase: a web platform for molecular optogenetics. ACS Synth. Biol. 7, 1825–1828 (2018).
Zhang, K. & Cui, B. Optogenetic control of intracellular signaling pathways. Trends Biotechnol. 33, 92–100 (2015).
Crefcoeur, R. P., Yin, R., Ulm, R. & Halazonetis, T. D. Ultraviolet-B-mediated induction of protein–protein interactions in mammalian cells. Nat. Commun. 4, 1779 (2013).
Strickland, D. et al. TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat. Methods 9, 379–384 (2012).
Guntas, G. et al. Engineering an improved light-induced dimer (iLID) for controlling the localization and activity of signaling proteins. Proc. Natl Acad. Sci. USA 112, 112–117 (2015).
Kawano, F., Suzuki, H., Furuya, A. & Sato, M. Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins. Nat. Commun. 6, 6256 (2015).
Liu, R. et al. Optogenetic control of RNA function and metabolism using engineered light-switchable RNA-binding proteins. Nat. Biotechnol. 40, 779–786 (2022).
Wang, H. et al. LOVTRAP: an optogenetic system for photoinduced protein dissociation. Nat. Methods 13, 755–758 (2016).
Kennedy, M. J. et al. Rapid blue-light-mediated induction of protein interactions in living cells. Nat. Methods 7, 973–975 (2010).
Zhou, Y. et al. A small and highly sensitive red/far-red optogenetic switch for applications in mammals. Nat. Biotechnol. 40, 262–272 (2022).
Niopek, D. et al. Engineering light-inducible nuclear localization signals for precise spatiotemporal control of protein dynamics in living cells. Nat. Commun. 5, 4404 (2014).
Inutsuka, A., Kimizuka, N., Takanohashi, N., Yakabu, H. & Onaka, T. Visualization of a blue light transmission area in living animals using light-induced nuclear translocation of fluorescent proteins. Biochem. Biophys. Res. Commun. 522, 138–143 (2020).
de Mena, L., Rizk, P. & Rincon-Limas, D. E. Bringing light to transcription: the optogenetics repertoire. Front. Genet. 9, 518 (2018).
Huang, Z. et al. Engineering light-controllable CAR T cells for cancer immunotherapy. Sci. Adv. 6, eaay9209 (2020). This article reports blue light-controllable expression of CAR.
Xin, H., Namgung, B. & Lee, L. P. Nanoplasmonic optical antennas for life sciences and medicine. Nat. Rev. Mater. 3, 228–243 (2018).
Carrow, J. K. et al. Photothermal modulation of human stem cells using light-responsive 2D nanomaterials. Proc. Natl Acad. Sci. USA 117, 13329–13338 (2020).
Wang, Y. et al. Photothermal-responsive conjugated polymer nanoparticles for remote control of gene expression in living cells. Adv. Mater. 30, 1705418 (2018).
Lyu, Y. et al. Dendronized semiconducting polymer as photothermal nanocarrier for remote activation of gene expression. Angew. Chem. Int. Ed. 56, 9155–9159 (2017).
Anikeeva, P. & Deisseroth, K. Photothermal genetic engineering. ACS Nano 6, 7548–7552 (2012).
Huang, K., Li, Z., Lin, J., Han, G. & Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 47, 5109–5124 (2018).
Zhang, Y., Wu, M., Wu, M., Zhu, J. & Zhang, X. Multifunctional carbon-based nanomaterials: applications in biomolecular imaging and therapy. ACS Omega 3, 9126–9145 (2018).
Miller, I. C. et al. Enhanced intratumoural activity of CAR T cells engineered to produce immunomodulators under photothermal control. Nat. Biomed. Eng. 5, 1348–1359 (2021). This article reports NIR light-induced photothermal activation of heat-sensitive expression of CAR.
Amin, J., Ananthan, J. & Voellmy, R. Key features of heat shock regulatory elements. Mol. Cell Biol. 8, 3761–3769 (1988).
von Maltzahn, G. et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat. Mater. 10, 545–552 (2011).
O’Donoghue, G. P. et al. T cells selectively filter oscillatory signals on the minutes timescale. Proc. Natl Acad. Sci. USA 118, e2019285118 (2021). This article reports blue light-controllable assembly of split CAR.
Chen, G., Qiu, H., Prasad, P. N. & Chen, X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem. Rev. 114, 5161–5214 (2014).
Wen, S. et al. Advances in highly doped upconversion nanoparticles. Nat. Commun. 9, 2415 (2018).
Chen, B. & Wang, F. Emerging frontiers of upconversion nanoparticles. Trends Chem. 2, 427–439 (2020).
Sun, L.-D., Dong, H., Zhang, P.-Z. & Yan, C.-H. Upconversion of rare earth nanomaterials. Annu. Rev. Phys. Chem. 66, 619–642 (2015).
Rabut, C. et al. Ultrasound technologies for imaging and modulating neural activity. Neuron 108, 93–110 (2020).
Farhadi, A., Ho Gabrielle, H., Sawyer Daniel, P., Bourdeau Raymond, W. & Shapiro Mikhail, G. Ultrasound imaging of gene expression in mammalian cells. Science 365, 1469–1475 (2019).
Szablowski, J. O., Bar-Zion, A. & Shapiro, M. G. Achieving spatial and molecular specificity with ultrasound-targeted biomolecular nanotherapeutics. Acc. Chem. Res. 52, 2427–2434 (2019).
Flax, S. W., Pelc, N. J., Glover, G. H., Gutmann, F. D. & McLachlan, M. Spectral characterization and attenuation measurements in ultrasound. Ultrason. Imaging 5, 95–116 (1983).
Culjat, M. O., Goldenberg, D., Tewari, P. & Singh, R. S. A review of tissue substitutes for ultrasound imaging. Ultrasound Med. Biol. 36, 861–873 (2010).
Guo, J., Song, X., Chen, X., Xu, M. & Ming, D. Mathematical model of ultrasound attenuation with skull thickness for transcranial-focused ultrasound. Front. Neurosci. 15, 778616 (2022).
Kremkau, F. W., Barnes, R. W. & McGraw, C. P. Ultrasonic attenuation and propagation speed in normal human brain. J. Acoust. Soc. Am. 70, 29–38 (1981).
Nassiri, D. K., Nicholas, D. & Hill, C. R. Attenuation of ultrasound in skeletal muscle. Ultrasonics 17, 230–232 (1979).
Chivers, R. C. & Hill, C. R. Ultrasonic attenuation in human tissue. Ultrasound Med. Biol. 2, 25–29 (1975).
Maresca, D. et al. Biomolecular ultrasound and sonogenetics. Annu. Rev. Chem. Biomol. Eng. 9, 229–252 (2018).
Wang, S. et al. Ultrasonic neuromodulation and sonogenetics: a new era for neural modulation. Front. Physiol. 11, 787 (2020).
Kennedy, J. E., ter Haar, G. R. & Cranston, D. High intensity focused ultrasound: surgery of the future? Br. J. Radiol. 76, 590–599 (2003).
Illing, R. O. et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br. J. Cancer 93, 890–895 (2005).
Maloney, E. & Hwang, J. H. Emerging HIFU applications in cancer therapy. Int. J. Hyperth. 31, 302–309 (2015).
Bar-Zion, A. et al. Acoustically triggered mechanotherapy using genetically encoded gas vesicles. Nat. Nanotechnol. 16, 1403–1412 (2021).
Pan, Y. et al. Mechanogenetics for the remote and noninvasive control of cancer immunotherapy. Proc. Natl Acad. Sci. USA 115, 992–997 (2018). This article reports ultrasound stimulation of CAR expression through cell signalling by a mechanosensitive ion channel.
Macian, F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484 (2005).
Müller, M. R. & Rao, A. NFAT, immunity and cancer: a transcription factor comes of age. Nat. Rev. Immunol. 10, 645–656 (2010).
Guilhon, E. et al. Spatial and temporal control of transgene expression in vivo using a heat-sensitive promoter and MRI-guided focused ultrasound. J. Gene Med. 5, 333–342 (2003).
Deckers, R. et al. Image-guided, noninvasive, spatiotemporal control of gene expression. Proc. Natl Acad. Sci. USA 106, 1175–1180 (2009).
Kruse, D. E., Mackanos, M. A., O’Connell-Rodwell, C. E., Contag, C. H. & Ferrara, K. W. Short-duration-focused ultrasound stimulation of Hsp70 expression in vivo. Phys. Med. Biol. 53, 3641–3660 (2008).
Abedi, M. H., Lee, J., Piraner, D. I. & Shapiro, M. G. Thermal control of engineered T-cells. ACS Synth. Biol. 9, 1941–1950 (2020).
Wu, Y. et al. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat. Biomed. Eng. 5, 1336–1347 (2021). This article reports ultrasound-induced heat for the activation of heat shock response promoters to drive CAR expression.
Abedi, M. H. et al. Ultrasound-controllable engineered bacteria for cancer immunotherapy. Nat. Commun. 13, 1585 (2022).
Piraner, D. I., Abedi, M. H., Moser, B. A., Lee-Gosselin, A. & Shapiro, M. G. Tunable thermal bioswitches for in vivo control of microbial therapeutics. Nat. Chem. Biol. 13, 75–80 (2017).
Gurbatri, C. R. et al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci. Transl Med. 12, eaax0876 (2020).
Leventhal, D. S. et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat. Commun. 11, 2739 (2020).
Zhuang, Y. & Xie, R.-J. Mechanoluminescence rebrightening the prospects of stress sensing: a review. Adv. Mater. 33, 2005925 (2021).
Wu, W., Duan, Y. & Liu, B. Mechanoluminescence: quantitative pressure-brightness relationship enables new applications. Matter 2, 291–293 (2020).
Wang, C. et al. Heartbeat-sensing mechanoluminescent device based on a quantitative relationship between pressure and emissive intensity. Matter 2, 181–193 (2020).
Xiong, P., Peng, M. & Yang, Z. Near-infrared mechanoluminescence crystals: a review. iScience 24, 101944 (2021).
Wu, X. et al. Sono-optogenetics facilitated by a circulation-delivered rechargeable light source for minimally invasive optogenetics. Proc. Natl Acad. Sci. USA 116, 26332–26342 (2019).
Chen, R., Romero, G., Christiansen Michael, G., Mohr, A. & Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 347, 1477–1480 (2015). This article reports alternating magnetic field-induced magnetothermia for wireless deep-brain neurostimulation.
Formica, D. & Silvestri, S. Biological effects of exposure to magnetic resonance imaging: an overview. Biomed. Eng. Online 3, 11 (2004).
Schenck, J. F. Physical interactions of static magnetic fields with living tissues. Prog. Biophys. Mol. Biol. 87, 185–204 (2005).
Joines, W. T. Frequency-dependent absorption of electromagnetic energy in biological tissue. IEEE Trans. Biomed. Eng. 31, 17–20 (1984).
Sharma, P. K. & Guha, S. K. Transmission of time varying magnetic field through body tissue. J. Biol. Phys. 3, 95–102 (1975).
Perica, K. et al. Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano 8, 2252–2260 (2014).
Sanz-Ortega, L. et al. T cells loaded with magnetic nanoparticles are retained in peripheral lymph nodes by the application of a magnetic field. J. Nanobiotechnol. 17, 14 (2019).
Tay, A., Kunze, A., Murray, C. & Di Carlo, D. Induction of calcium influx in cortical neural networks by nanomagnetic forces. ACS Nano 10, 2331–2341 (2016).
Tay, A. & Di Carlo, D. Magnetic nanoparticle-based mechanical stimulation for restoration of mechano-sensitive ion channel equilibrium in neural networks. Nano Lett. 17, 886–892 (2017).
Rao, A., Luo, C. & Hogan, P. G. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15, 707–747 (1997).
Lee, J. et al. Magnetothermia-induced catalytic hollow nanoreactor for bioorthogonal organic synthesis in living cells. Nano Lett. 20, 6981–6988 (2020).
Munshi, R. et al. Magnetothermal genetic deep brain stimulation of motor behaviors in awake, freely moving mice. eLife 6, e27069 (2017).
Kunzler, J. E., Hsu, F. S. L. & Boyle, W. S. Magnetothermal oscillations. the oscillatory dependence of temperature on magnetic field. Phys. Rev. 128, 1084–1098 (1962).
Rosenfeld, D. et al. Transgene-free remote magnetothermal regulation of adrenal hormones. Sci. Adv. 6, eaaz3734 (2020).
Kosari, E. & Vafai, K. Thermal tissue damage analysis for magnetothermal neuromodulation and lesion size minimization. Brain Multiphys. 1, 100014 (2020).
Aichinger, H., Dierker, J., Joite-Barfuß, S. & Säbel, M. in Radiation Exposure and Image Quality in X-Ray Diagnostic Radiology: Physical Principles and Clinical Applications (eds Aichinger, H., Dierker, J., Joite-Barfuß, S. & Säbel, M.) 35–44 (Springer, 2004).
Fan, W. et al. Breaking the depth dependence by nanotechnology-enhanced X-ray-excited deep cancer theranostics. Adv. Mater. 31, 1806381 (2019).
Ahmad, M., Pratx, G., Bazalova, M. & Xing, L. X-ray luminescence and X-ray fluorescence computed tomography: new molecular imaging modalities. IEEE Access. 2, 1051–1061 (2014).
Blasse, G. Scintillator materials. Chem. Mater. 6, 1465–1475 (1994).
Chen, H., Rogalski, M. M. & Anker, J. N. Advances in functional X-ray imaging techniques and contrast agents. Phys. Chem. Chem. Phys. 14, 13469–13486 (2012).
Kamkaew, A., Chen, F., Zhan, Y., Majewski, R. L. & Cai, W. Scintillating nanoparticles as energy mediators for enhanced photodynamic therapy. ACS Nano 10, 3918–3935 (2016).
Huang, K. et al. Three-dimensional colloidal controlled growth of core–shell heterostructured persistent luminescence nanocrystals. Nano Lett. 21, 4903–4910 (2021).
Huang, K. et al. Enhancing light and X-ray charging in persistent luminescence nanocrystals for orthogonal afterglow anti-counterfeiting. Adv. Funct. Mater. 31, 2009920 (2021).
Chen, Q. et al. All-inorganic perovskite nanocrystal scintillators. Nature 561, 88–93 (2018).
Elmenoufy, A. H., Tang, Y. A., Hu, J., Xu, H. & Yang, X. A novel deep photodynamic therapy modality combined with CT imaging established via X-ray stimulated silica-modified lanthanide scintillating nanoparticles. Chem. Commun. 51, 12247–12250 (2015).
Tang, Y. A., Hu, J., Elmenoufy, A. H. & Yang, X. Highly efficient FRET system capable of deep photodynamic therapy established on X-ray excited mesoporous LaF3:Tb scintillating nanoparticles. ACS Appl. Mater. Interfaces 7, 12261–12269 (2015).
Villa, I. et al. Functionalized scintillating nanotubes for simultaneous radio- and photodynamic therapy of cancer. ACS Appl. Mater. Interfaces 13, 12997–13008 (2021).
Lu, K. et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng. 2, 600–610 (2018).
Chen, H. et al. Nanoscintillator-mediated X-ray inducible photodynamic therapy for in vivo cancer treatment. Nano Lett. 15, 2249–2256 (2015).
Volotskova, O. et al. Efficient radioisotope energy transfer by gold nanoclusters for molecular imaging. Small 11, 4002–4008 (2015).
Osakada, Y. et al. Hard X-ray-induced optical luminescence via biomolecule-directed metal clusters. Chem. Commun. 50, 3549–3551 (2014).
Zhang, X.-D. et al. Ultrasmall glutathione-protected gold nanoclusters as next generation radiotherapy sensitizers with high tumor uptake and high renal clearance. Sci. Rep. 5, 8669 (2015).
Krawczyk, K. et al. Electrogenetic cellular insulin release for real-time glycemic control in type 1 diabetic mice. Science 368, 993–1001 (2020).
Stanton, B. Z., Chory, E. J. & Crabtree, G. R. Chemically induced proximity in biology and medicine. Science 359, eaao5902 (2018).
Wu, C.-Y., Roybal Kole, T., Puchner Elias, M., Onuffer, J. & Lim Wendell, A. Remote control of therapeutic T cells through a small molecule–gated chimeric receptor. Science 350, aab4077 (2015).
Cho, J. H. et al. Engineering advanced logic and distributed computing in human CAR immune cells. Nat. Commun. 12, 792 (2021).
Cho, J. H., Collins, J. J. & Wong, W. W. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173, 1426–1438.e11 (2018).
Labanieh, L. et al. Enhanced safety and efficacy of protease-regulated CAR-T cell receptors. Cell 185, 1745–1763.e22 (2022).
Li, H. S. et al. High-performance multiplex drug-gated CAR circuits. Cancer Cell 40, 1294–1305.e4 (2022).
Chakravarti, D., Caraballo, L. D., Weinberg, B. H. & Wong, W. W. Inducible gene switches with memory in human T cells for cellular immunotherapy. ACS Synth. Biol. 8, 1744–1754 (2019).
Rodgers, D. T. et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc. Natl Acad. Sci. USA 113, E459–E468 (2016).
Peng, H. et al. ROR1-targeting switchable CAR-T cells for cancer therapy. Oncogene 41, 4104–4114 (2022).
Zajc, C. U. et al. A conformation-specific ON-switch for controlling CAR T cells with an orally available drug. Proc. Natl Acad. Sci. USA 117, 14926–14935 (2020).
Jacobs, C. L., Badiee, R. K. & Lin, M. Z. StaPLs: versatile genetically encoded modules for engineering drug-inducible proteins. Nat. Methods 15, 523–526 (2018).
Soumana, D. I., Ali, A. & Schiffer, C. A. Structural analysis of asunaprevir resistance in HCV NS3/4A protease. ACS Chem. Biol. 9, 2485–2490 (2014).
Romano, K. P. et al. The molecular basis of drug resistance against hepatitis C virus NS3/4A protease inhibitors. PLoS Pathog. 8, e1002832 (2012).
Cao, W. et al. A reversible chemogenetic switch for chimeric antigen receptor T cells. Angew. Chem. Int. Ed. 61, e202109550 (2022).
Nishimura, K., Fukagawa, T., Takisawa, H., Kakimoto, T. & Kanemaki, M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Methods 6, 917–922 (2009).
Nabet, B. et al. The dTAG system for immediate and target-specific protein degradation. Nat. Chem. Biol. 14, 431–441 (2018).
Koduri, V. et al. Peptidic degron for IMiD-induced degradation of heterologous proteins. Proc. Natl Acad. Sci. USA 116, 2539–2544 (2019).
Carbonneau, S. et al. An IMiD-inducible degron provides reversible regulation for chimeric antigen receptor expression and activity. Cell Chem. Biol. 28, 802–812.e6 (2021).
Jan, M. et al. Reversible ON- and OFF-switch chimeric antigen receptors controlled by lenalidomide. Sci. Transl Med. 13, eabb6295 (2021).
Straathof, K. C. et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 105, 4247–4254 (2005).
Di Stasi, A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 365, 1673–1683 (2011).
Zhou, X. et al. Long-term outcome after haploidentical stem cell transplant and infusion of T cells expressing the inducible caspase 9 safety transgene. Blood 123, 3895–3905 (2014).
Hoyos, V. et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 24, 1160–1170 (2010).
Diaconu, I. et al. Inducible Caspase-9 selectively modulates the toxicities of CD19-specific chimeric antigen receptor-modified T cells. Mol. Ther. 25, 580–592 (2017).
Budde, L. E. et al. Combining a CD20 chimeric antigen receptor and an inducible Caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS ONE 8, e82742 (2013).
Duong, M. T. et al. Two-dimensional regulation of CAR-T cell therapy with orthogonal switches. Mol. Ther. Oncolytics 12, 124–137 (2019).
Stavrou, M. et al. A rapamycin-activated Caspase 9-based suicide gene. Mol. Ther. 26, 1266–1276 (2018).
Minagawa, K. et al. In vitro pre-clinical validation of suicide gene modified anti-CD33 redirected chimeric antigen receptor T-cells for acute myeloid leukemia. PLoS ONE 11, e0166891 (2016).
Bonini, C. et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 276, 1719–1724 (1997).
Tiberghien, P. et al. Administration of herpes simplex–thymidine kinase-expressing donor T cells with a T-cell-depleted allogeneic marrow graft. Blood 97, 63–72 (2001).
Park, S. et al. Direct control of CAR T cells through small molecule-regulated antibodies. Nat. Commun. 12, 710 (2021).
Sockolosky, J. T. et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 359, 1037–1042 (2018).
Aspuria, P.-J. et al. An orthogonal IL-2 and IL-2Rβ system drives persistence and activation of CAR T cells and clearance of bulky lymphoma. Sci. Transl Med. 13, eabg7565 (2021).
Zhang, Q. et al. A human orthogonal IL-2 and IL-2Rβ system enhances CAR T cell expansion and antitumor activity in a murine model of leukemia. Sci. Transl Med. 13, eabg6986 (2021).
Labanieh, L., Majzner, R. G. & Mackall, C. L. Programming CAR-T cells to kill cancer. Nat. Biomed. Eng. 2, 377–391 (2018).
Rafiq, S., Hackett, C. S. & Brentjens, R. J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 17, 147–167 (2020).
Akhoundi, M., Mohammadi, M., Sahraei, S. S., Sheykhhasan, M. & Fayazi, N. CAR T cell therapy as a promising approach in cancer immunotherapy: challenges and opportunities. Cell. Oncol. 44, 495–523 (2021).
Boroojerdi, M. H., Rahbarizadeh, F., Kozani, P. S., Kamali, E. & Kozani, P. S. Strategies for having a more effective and less toxic CAR T-cell therapy for acute lymphoblastic leukemia. Med. Oncol. 37, 100 (2020).
Wang, T., Liu, S., Huang, Y. & Zhou, Y. Red-shifted optogenetics comes to the spotlight. Clin. Transl Med. 12, e807 (2022).
Moon, E. K. et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 17, 4719–4730 (2011).
Sotoudeh, M., Shirvani, S. I., Merat, S., Ahmadbeigi, N. & Naderi, M. MSLN (Mesothelin), ANTXR1 (TEM8), and MUC3A are the potent antigenic targets for CAR T cell therapy of gastric adenocarcinoma. J. Cell Biochem. 120, 5010–5017 (2019).
Kozani, P. S., Kozani, P. S. & Rahbarizadeh, F. Novel antigens of CAR T cell therapy: new roads; old destination. Transl. Oncol. 14, 101079 (2021).
Zhang, Q. et al. The antitumor capacity of mesothelin-CAR-T cells in targeting solid tumors in mice. Mol. Ther. Oncolytics 20, 556–568 (2021).
Hou, L. et al. Nano-delivery of fraxinellone remodels tumor microenvironment and facilitates therapeutic vaccination in desmoplastic melanoma. Theranostics 8, 3781–3796 (2018).
Saha, S. et al. Gold nanoparticle reprograms pancreatic tumor microenvironment and inhibits tumor growth. ACS Nano 10, 10636–10651 (2016).
Miao, L. et al. Targeting tumor-associated fibroblasts for therapeutic delivery in desmoplastic tumors. Cancer Res. 77, 719–731 (2017).
Miao, L. et al. Nanoparticle modulation of the tumor microenvironment enhances therapeutic efficacy of cisplatin. J. Control. Release 217, 27–41 (2015).
Peng, J. et al. Adenoviral vector for enhanced prostate cancer specific transferrin conjugated drug targeted therapy. Nano Lett. 22, 4168–4175 (2022).
Zhao, Y. et al. Enhancing prostate-cancer-specific MRI by genetic amplified nanoparticle tumor homing. Adv. Mater. 31, 1900928 (2019).
Li, Y., Jiang, M., Deng, Z., Zeng, S. & Hao, J. Low dose soft X-ray remotely triggered lanthanide nanovaccine for deep tissue CO gas release and activation of systemic anti-tumor immunoresponse. Adv. Sci. 8, 2004391 (2021).
Park, J. et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 11, 895–905 (2012).
Chen, Q. et al. Intelligent albumin–MnO2 nanoparticles as pH-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv. Mater. 28, 7129–7136 (2016).
Yang, G. et al. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 8, 902 (2017).
Pellegatta, S. et al. Constitutive and TNFα-inducible expression of chondroitin sulfate proteoglycan 4 in glioblastoma and neurospheres: implications for CAR-T cell therapy. Sci. Transl Med. 10, eaao2731 (2018).
Curran, K. J. et al. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol. Ther. 23, 769–778 (2015).
Collinson-Pautz, M. R. et al. Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies. Leukemia 33, 2195–2207 (2019).
Chmielewski, M., Hombach, A. A. & Abken, H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol. Rev. 257, 83–90 (2014).
Eshhar, Z., Waks, T., Gross, G. & Schindler, D. G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl Acad. Sci. USA 90, 720–724 (1993). This article reports one of the first designs of CAR T cells.
Krause, A. et al. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J. Exp. Med. 188, 619–626 (1998).
Maher, J., Brentjens, R. J., Gunset, G., Rivière, I. & Sadelain, M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat. Biotechnol. 20, 70–75 (2002).
Imai, C. et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 18, 676–684 (2004).
Brentjens, R. J. et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 9, 279–286 (2003).
Carpenito, C. et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl Acad. Sci. USA 106, 3360–3365 (2009).
Wang, J. et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum. Gene Ther. 18, 712–725 (2007).
Zhong, X.-S., Matsushita, M., Plotkin, J., Riviere, I. & Sadelain, M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol. Ther. 18, 413–420 (2010).
Pulè, M. A. et al. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol. Ther. 12, 933–941 (2005).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Till, B. G. et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 119, 3940–3950 (2012).
Chinnasamy, D. et al. Local delivery of lnterleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin. Cancer Res. 18, 1672–1683 (2012).
Pegram, H. J. et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119, 4133–4141 (2012).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Liu, X. et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 27, 154–157 (2017).
Depil, S., Duchateau, P., Grupp, S. A., Mufti, G. & Poirot, L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 19, 185–199 (2020).
Klein, J. & Sato, A. The HLA system. First of two parts. N. Engl. J. Med. 343, 702–709 (2000).
Klein, J. & Sato, A. The HLA system. Second of two parts. N. Engl. J. Med. 343, 782–786 (2000).
Shah, R. M. et al. T-cell receptor αβ+ and CD19+ cell–depleted haploidentical and mismatched hematopoietic stem cell transplantation in primary immune deficiency. J. Allergy Clin. Immunol. 141, 1417–1426.e1 (2018).
Poirot, L. et al. Multiplex genome-edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Cancer Res. 75, 3853–3864 (2015).
Torikai, H. et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 119, 5697–5705 (2012).
Kamiya, T., Wong, D., Png, Y. T. & Campana, D. A novel method to generate T-cell receptor-deficient chimeric antigen receptor T cells. Blood Adv. 2, 517–528 (2018).
Georgiadis, C. et al. Long terminal repeat CRISPR-CAR-coupled “Universal” T cells mediate potent anti-leukemic effects. Mol. Ther. 26, 1215–1227 (2018).
Fiorenza, S., Ritchie, D. S., Ramsey, S. D., Turtle, C. J. & Roth, J. A. Value and affordability of CAR T-cell therapy in the United States. Bone Marrow Transplant. 55, 1706–1715 (2020).
Lyman, G. H., Nguyen, A., Snyder, S., Gitlin, M. & Chung, K. C. Economic evaluation of chimeric antigen receptor T-cell therapy by site of care among patients with relapsed or refractory large B-cell lymphoma. JAMA Netw. Open 3, e202072 (2020).
Zhang, C., Hu, Y., Xiao, W. & Tian, Z. Chimeric antigen receptor- and natural killer cell receptor-engineered innate killer cells in cancer immunotherapy. Cell. Mol. Immunol. 18, 2083–2100 (2021).
Rezvani, K. Adoptive cell therapy using engineered natural killer cells. Bone Marrow Transplant. 54, 785–788 (2019).
Laskowski, T. J., Biederstädt, A. & Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 22, 557–575 (2022).
Shimasaki, N., Jain, A. & Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 19, 200–218 (2020).
Liu, S. et al. Harnessing natural killer cells to develop next-generation cellular immunotherapy. Chronic Dis. Transl Med. 8, 245–255 (2022).
Klichinsky, M. et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38, 947–953 (2020).
Maimon, B. E. et al. Optogenetic peripheral nerve immunogenicity. Sci. Rep. 8, 14076 (2018).
Huang, K., Liu, X., Han, G. & Zhou, Y. Nano-optogenetic immunotherapy. Clin. Transl Med. 12, e1020 (2022).
Acknowledgements
This work was supported by the National Institutes of Health (R01CA232017 to G.H. and Y.Z., R01CA240258 to Y.H., R01GM144986 to Y.Z., and R01EB029122 and R35GM140929 to Y. W.), the Welch Foundation (BE-1913-20220331 to Y.Z.) and the Cancer Prevention and Research Institutes of Texas (RP210070 to Y.Z.). The authors apologize to those whose primary work has not been cited here for lack of space.
Author information
Authors and Affiliations
Contributions
G.H., Y.Z. and Y.W. conceived the idea and supervised the work. K.H., Y.H., Y.Z. and G.H. drafted the initial manuscript with inputs and further editing from all other authors.
Corresponding authors
Ethics declarations
Competing interests
Y.W. is a scientific co-founder of Cell E&G and Acoustic Cell Therapy. These financial interests do not affect the design, conduct or reporting of this research. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Shu Wang, Michel Sadelain, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, K., Liu, L., Huang, Y. et al. Remote control of cellular immunotherapy. Nat Rev Bioeng 1, 440–455 (2023). https://doi.org/10.1038/s44222-023-00042-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44222-023-00042-8
This article is cited by
-
Programmable synthetic receptors: the next-generation of cell and gene therapies
Signal Transduction and Targeted Therapy (2024)