Abstract
Proteins of the Bcl-2 family are critical regulators of apoptosis. Proapoptotic members, like Bax, contain three of the four Bcl-2 homology regions (BH1-3), while BH3-only proteins, like Bim, possess only the short BH3 motif. Database searches revealed Bfk, an unusual novel member of the Bcl-2 family that contains a BH2 and BH3 region but not BH1 or BH4. Bfk is thus most closely related to Bcl-GL. It lacks a C-terminal membrane anchor and is cytosolic. Enforced expression of Bfk weakly promoted apoptosis and antagonized Bcl-2's prosurvival function. Like Bcl-GL, Bfk did not bind to any Bcl-2 family members, even though its BH3 motif can mediate association with prosurvival proteins. Low amounts of Bfk were found in stomach, ovary, bone marrow and spleen, but its level in the mammary gland rose markedly during pregnancy, suggesting that Bfk may play a role in mammary development.
Similar content being viewed by others
Introduction
The bcl-2 gene family encodes a divergent group of proteins that regulate programmed cell death (apoptosis) by an evolutionarily conserved mechanism found in species as distantly related as humans and nematodes.1 Members that promote survival, including Bcl-2 itself, several close mammalian relatives (Bcl-xL, Mcl-1, A1 and Bcl-w), CED-9 of nematodes and viral proteins such as E1B 19K and BHRF-1, share three or four regions of homology, the so-called Bcl-2 homology (BH) regions 1–4. In mammals, their action is opposed by at least a dozen proapoptotic family members.1 They fall into two distinct subgroups based on structure and function: the BH3-only proteins (Bik/Nbk/Blk, Bad, Hrk/DP5, Bid, Bim/Bod, Noxa, Puma/BBc3 and Bmf), which share with other family members only the short BH3 protein interaction motif,2 and the Bax family (Bax, Bak and Bok/Mtd), which possess three BH regions (BH1-3) and, perhaps surprisingly, have a structure closely resembling that of their prosurvival relatives.3 Experiments with transfected cells indicate that the BH3-only proteins are required for initiation of apoptosis, whereas the Bax/Bak-like proteins act further downstream in the signalling pathway.4,5 It is still not known how these proteins cooperate to promote apoptosis or how Bcl-2 acts to keep cells alive.1
As well as the three major subfamilies of prosurvival, BH3-only and Bax-like proteins, the Bcl-2 family also seems to contain at least two members that cannot be readily classified. Bcl-GL is weakly proapoptotic but differs from other proapoptotic Bcl-2 proteins in that it contains both a BH2 and BH3 but lacks a clearly identifiable BH1 region.6 However, alternate splicing of the bcl-g gene leads to production of a potent proapoptotic BH3-only protein called Bcl-GS.6 On the other hand, Bcl-rambo reportedly contains all the four BH regions characteristic of prosurvival Bcl-2 family members, but is proapoptotic because of a unique C-terminal extension.7 In striking contrast to other Bcl-2 family proteins, neither Bcl-GL nor Bcl-rambo binds to any other Bcl-2 family member.6,7
Through a search of the GenBank EST databases for new Bcl-2 family genes, we have uncovered an unusual novel family member, denoted Bfk. Like Bcl-GL, Bfk contains a BH3 and BH2 region and has weak proapoptotic activity when over expressed, but does not bind to either prosurvival or proapoptotic Bcl-2 family members. Bfk localizes to the cytoplasm, but unlike Bcl-GL, was not associated with organelles. The protein was detected in stomach, bone marrow, spleen and ovary, and its expression was highly regulated in the mammary gland and uterus during pregnancy.
Results
Cloning of bfk genes from several species
In a search for novel genes with a BH3 motif, we compared the sequences of all known mammalian BH3-only proteins against GenBank databases, translated into all six possible reading frames (tblastn). 8 The search revealed an incomplete open reading frame (ORF) from the chick (GenBank accession AI982317) with a putative BH3 region similar to that of the rat Bim/Bod, but the absence of the dynein light-chain binding region present in Bim9 indicated that this was not the chick Bim ortholog. Using the chick sequence in tblastn searches, we identified multiple-related murine and human ESTs and assembled a mouse EST contig of 836 nucleotides with an ORF of 167 amino acids (predicted 18.3 kDa) and a human EST contig of 781 nucleotides with an ORF of 163 amino acids. Clear orthologs are also present in bovine, rat and swine sources (GenBank accession numbers BF652609, AI639530 and F22868).
Sequence similarities with Bcl-2 family members led us to name the putative novel protein Bfk (Bcl-2 family kin). Both the human and mouse protein, which have 68.7% sequence identity overall, contain the BH3-like region (Figure 1a, b). It lies close to their N-termini, whereas the BH3 regions of known BH3-only proteins are more central or C-terminal.2 Inspection of the sequences C-terminal to the BH3 region revealed a BH2 region but not a defined BH1 region or hydrophobic tail (Figure 1b). The composition of Bfk is thus most similar to that of Bcl-GL,6 which also has a BH3 and BH2 region, but no BH1, BH4 or hydrophobic tail (Figure 1c). Significantly, the BH3 and BH2 regions of Bfk, Bcl-GL, Bcl-2 and Bax are all equidistant (Figure 1c). Furthermore, despite the absence of a true BH1 region in either Bfk or Bcl-GL, several highly conserved BH1 region residues are present in the regions of both proteins where a BH1 region would be expected to lie (Figure 1b). Whether the vestigial BH1 homology has functional significance is not known (see Discussion).
Sequence analysis of the bfk gene. (a) Alignment of human (H. sapiens), mouse (M. musculus) and known chick (G. gallus) Bfk protein sequences using Clustal alignment program (DNAStar). Identical residues are highlighted in black, functionally similar residues in gray. The BH3 and BH2 regions are underlined. (b) Sequence comparison of known BH3, BH1 and BH2 regions from mouse and viral (E1B 19K) Bcl-2 family proteins to homologous regions in mouse Bfk. (c) Schematic diagram comparing positions of BH regions of known Bcl-2 family proteins to Bfk (drawn to scale). (d) The genomic organization of the murine and human bfk loci. Open boxes represent coding exons, closed boxes noncoding exons. The BH3 region is encoded by coding exon 2 and the BH2 region is split between coding exons 3 and 4
The sequence of the human bfk gene places it at chromosome 1p13.1. Comparison of the genomic sequences spanning the human and mouse bfk genes (human: Celera accession number GA_x5J8B7NT9TV; GenBank accession number AL137856; mouse: Celera accession number GA_x5J8B7W87HU) showed that both genes are composed of four coding exons, with the BH3 region on exon 2 and the BH2 region split between exons 3 and 4 (Figure 1d). This organization, with the small BH3 region on a single exon and the BH2 region divided between two exons, is similar to that of other Bcl-2 family genes, including bcl-2 itself,10 bcl-xl,11 bax,12 bak13 and ced-9.14
Bfk expression pattern
In order to explore whether bfk constitutes an expressed gene, we made antibodies to the protein. Rabbits were immunized with full-length mouse Bfk protein and Bfk-specific antibodies were purified from their serum by affinity chromatography on a Bfk-sepharose column. The specificity of these antibodies for Bfk was confirmed by Western blot analysis of lysates from cells transfected with retroviral expression vectors encoding hemagglutinin (HA) epitope-tagged Bfk (MiT/HA-Bfk), untagged Bfk (MiT/Bfk) or, as a control, empty vector (MiT). The cells overexpressing tagged and untagged Bfk displayed prominent bands corresponding to the expected polypeptides of 20.3 and 18.3 kDa, respectively, that were absent from the control cells (Figure 2a). These antibodies revealed low levels of an ∼18 kDa Bfk protein in bone marrow, stomach, ovaries and (on longer exposure) spleen (Figure 2b). The thymus also contained a polypeptide of higher molecular weight. This might well represent an alternate isoform of Bfk, but we have not yet identified a corresponding transcript. Alternatively, Bfk could be subjected to some form of post-translational modification in thymocytes but not in other cell types.
Expression analysis of Bfk protein. (a) Specificity of rabbit anti-Bfk antibodies was confirmed by Western blot analyzing lysates from Phoenix packaging cells transfected with MiT retroviral plasmid encoding HA-tagged Bfk (MiT/HA-Bfk), untagged Bfk (MiT/Bfk) or empty vector (MiT). The blot was probed with preimmune serum, then reprobed with immune serum. Hsp70 was probed as a loading control. (b) Bfk expression in various tissues (40 μg of protein) was determined using the anti-Bfk antibody. As a control, lysate from Phoenix packaging cells transfected with MiT/Bfk is included (+ve). The faint higher molecular weight band seen in the positive control lane lysates from Phoenix packaging cells transfected with MiT/Bfk (indicated by asterisk) is an artifact and appears to be unrelated to the higher molecular weight band detected by the anti-Bfk antibodies in thymocyte lysates. (c) Expression of bfk mRNA in mammary glands from various stages of development. Total RNA (15 μg) from glands of 6-, 8- and 12-week-old virgin, 8, 12, 16 and 18 days pregnant (preg), 1 and 8 days lactating (lact) and 1, 2 and 5 days involution (inv) females was assessed using a bfk coding region probe. 18S rRNA is included as a loading control. (d) Bfk protein expression in mammary glands of adult virgin, 12 or 18 days pregnant (preg), 1 and 7 days lactating (lact) and 1 or 4 days involution (inv) females was assessed by Western blotting using the anti-Bfk antibody. As a control, lysate from Phoenix packaging cells transfected with MiT/Bfk is included. (e) Bfk protein expression in uterine tissue from virgin, 14 days pregnant and pseudopregnant (pseudo) females (40 μg of protein) determined using the anti-Bfk antibody
The bfk ESTs in the GenBank database include entries from small intestine, spleen, stomach and uterus, but 14 of the 26 ESTs derived from mammary tissue of lactating females and three others from mammary tumors. This predominance of mammary entries (65% overall) prompted us to investigate bfk expression in the mammary gland during pregnancy, lactation and involution (Figure 2c, d). In adult virgin females, bfk mRNA was below the level of detection, but it became readily detectable around 8 days postcoitum in pregnant females as a single transcript of approximately 2.0 kb. Both bfk mRNA and protein expression peaked around day 1 of lactation and then began to decline. By 2 days of involution, bfk mRNA was no longer detectable. The protein declined more slowly and remained detectable until day 4 of involution. The effects of pregnancy on bfk expression were not confined to mammary glands. Bfk protein was also abundant in the uterine horns from nulliparous females but barely detectable in postimplantation horns 13 days postcoitum, or in horns from pseudopregnant females (Figure 2e).
Bfk does not interact with other Bcl-2 protein family members
Heterotypic interaction between prosurvival and proapoptotic members of the Bcl-2 family plays a critical regulatory role in the control of apoptosis.1 We sought to identify potential binding partners of Bfk by testing their coimmunoprecipitation from lysates. Bfk carrying an N-terminal HA epitope tag was transiently overexpressed in metabolically labelled 293T cells together with various anti- or proapoptotic FLAG-tagged Bcl-2 family members. As expected,15 the BH3-only protein BimL strongly interacted with Bcl-2, but we found no binding of Bfk with Bcl-2, Bcl-xL, adenovirus E1B19 K protein, Bax, Bmf, BimEL, Bad or Puma/Bbc3 (Figure 3a and data not shown). Thus, it appears that Bfk, like Bcl-GL,6 does not interact with either prosurvival or proapoptotic family members. This failure does not reflect a defective BH3 interaction domain. When the BH3 region of BimL was replaced by that of Bfk, the chimeric protein bound to Bcl-2, Bcl-w and Bcl-xL in the yeast two-hybrid system (Figure 3b), whereas BimL mutants lacking a BH3 region were inactive (data not shown), as in previous studies.15
Bfk does not bind to known Bcl-2 family proteins but contains a functional BH3 region. (a) Interaction of Bfk with known Bcl-2 family proteins was assessed by immunoprecipitation of HA-tagged Bfk, FLAG-tagged Bcl-2, Bcl-xL, Bax or EE-tagged Puma, BimEL, Bad or Bmf from 35S-Met/35S-Cys labelled 293T cells cotransfected with the appropriate expression constructs. Coimmunoprecipitation of FLAG-tagged Bcl-2 with EE-tagged BimL is included as a positive control. (b) Yeast cells were cotransformed with expression constructs for various Bcl-2 family proteins fused to the GAL4 transactivation domain and either BimL or a mutant of BimL whose BH3 region has been replaced with that of Bfk (BimL(BH3bfk)) fused to the GAL4 DNA binding domain. Cells were grown on selective medium and assayed for protein interaction by β-galactosidase activity
Bfk is cytosolic
To investigate the subcellular localization of Bfk, we used the retroviral vector MiT to generate constructs that express either untagged Bfk (MiT/Bfk) or Bfk with an N-terminal HA epitope (Mit/HA-Bfk). Since the Bfk is expressed on a bi-cistronic message upstream of IRES-Thy1.1, infected cells expressing Bfk can be isolated by cell surface staining with antibodies to Thy1.1. NIH3T3 cells infected with the MiT/HA-Bfk retroviruses were examined by immunofluorescence staining with anti-HA antibodies and confocal microscopy. Consistent with the absence of a membrane-inserting hydrophobic tail, HA-Bfk (green) gave a cytosolic distribution, clearly distinct from the punctate mitochondrial signal produced by MitoTracker Red (Figure 4aA). Subcellular fractionation of digitonin-lysed NIH3T3 cells expressing HA-Bfk confirmed that the protein localized predominantly in the soluble (s) fraction. The efficacy of fractionation was confirmed by immunoblots with antibodies to cytochrome c, which appeared only in the pellet (p) fraction (containing mitochondria), and to Bax, which was largely recovered in the cytosolic fraction. Similarly, with FDC-P1 cells expressing untagged Bfk from the MiT retrovirus, digitonin fractionation yielded both the Bfk and endogenous Bax exclusively in the cytosolic fraction (data not shown).
Bfk is a cytosolic protein. (a) NIH 3T3 cells were infected with either empty MiT retrovirus or MiT retrovirus expressing HA-tagged Bfk (MiT/HA-Bfk). Subcellular distribution of Bfk was assessed by staining with an anti-HA antibody (green) and compared by confocal microscopy to the location of mitochondria, stained with MitoTracker (red), and nuclei, stained with DAPI (blue). (b) Cytosolic distribution of Bfk was confirmed by fractionating MiT/HA-Bfk infected NIH 3T3 cells using digitonin. Cytochrome c and Bax were assessed as controls for subcellular fractionation
Bfk weakly induces apoptosis
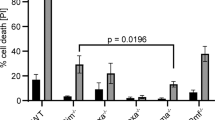
To investigate the impact of Bfk on cell death control, we transiently transfected 293T cells with constructs encoding N-terminally HA-epitope tagged Bfk and measured the proportion of dying cells by staining permeabilized cells with the DNA intercalating dye propidium iodide (PI) to reveal those with a DNA content of <2C. Bfk overexpression induced a low level of apoptosis (10.5%), marginally higher than that induced by vector controls (6%) and considerably less than that observed for Bax (38%) (Figure 5a). Consistent with this low apoptotic potency, Bfk could be expressed in 293T cells at levels considerably higher than Bax (Figure 5a). Mutating the highly conserved G64 and D65 residues in the Bfk BH3 to alanine abolished the weak proapoptotic activity of Bfk, suggesting that the BH3 region is essential for cell killing (Figure 5a). Consistent with the inability of Bcl-xL to interact with Bfk, it failed to block cell death caused by Bfk overexpression but readily antagonized that induced by Bax (Figure 5b). Bfk-induced apoptosis could, however, be inhibited by coexpression of the pan-caspase inhibitor p35, demonstrating that this death is caspase dependent (Figure 5b).
Bfk has weak proapoptotic activity. (a) HEK 293T cells transiently transfected with expression constructs for HA-tagged Bfk (1.0, 0.8 and 0.2 μg), HA-tagged Bfk with a BH3-region mutation (BfkGDAA, 1.0 μg) or HA-tagged Bax (0.5 μg). Empty vector DNA was added to standardize all transfection reactions to a total of 1 μg of DNA. Cellular DNA content was determined by staining with PI. Apoptotic cells were defined as having <2C DNA content. Protein expression from vectors in each transfection condition was confirmed by Western blot analysis using an anti-HA antibody. (b) HEK 293T cells were transiently cotransfected with expression constructs for HA-tagged Bfk (0.8 μg) or HA-tagged Bax (0.5 μg) together with FLAG-tagged antiapoptotic proteins Bcl-xL (0.2 μg) or the pan-caspase inhibitor p35 (0.7 μg). Empty vector DNA was added to bring all transfection reactions to 1.5 μg of total DNA. Apoptotic cells were measured and protein expression confirmed as in (a)
To further characterize the proapoptotic activity of Bfk, we infected the IL-3-dependent cell line FDC-P1 and a derivative overexpressing Bcl-2 (FD/Bcl-2) with the MiT-Bfk retrovirus or empty MiT retrovirus and FACS-sorted infected cells after staining with antibodies to Thy1.1 (Figure 6a, b). Supporting the notion that Bfk only weakly induces apoptosis, substantial levels of Bfk could be expressed stably in parental FDC-P1 cells (Figure 6a). To investigate how Bfk affects a physiological death stimulus, we starved FDC-P1 and FD/Bcl-2 cells expressing Bfk or a control vector of their requisite growth factor, IL-3. FDC-P1 cells expressing Bfk underwent apoptosis at a rate indistinguishable from control cells (Figure 6a). By comparison, in FD/Bcl-2 cells starved of IL-3, expression of Bfk did accelerate apoptosis. To address whether this effect was because of direct binding of Bcl-2 by Bfk, we tested for their coimmunoprecipitation following cytokine deprivation for 24 or 72 h. We found no evidence for binding of Bcl-2 to the 18.3 kDa Bfk protein (Figure 6b), suggesting that apoptotic stimuli do not promote their interaction.
Effect of Bfk on cytokine withdrawal-induced apoptosis. (a) FDC-P1 cells were infected with either MiT retrovirus or MiT retrovirus expressing HA-tagged Bfk, sorted for Thy1.1 positive cells and stained with anti-Thy1.1 and analyzed by FACS to confirm Thy1.1 expression. Bfk protein expression was confirmed in MiT/Bfk infected FDC-P1 cells by Western blotting using the anti-Bfk antibody. Hsp70 was probed as a loading control. Sorted MiT/Bfk and MiT infected FDC-P1 cells were starved of IL-3 for 2 days and cell viability assessed by staining with PI at 0, 24 and 48 h poststarvation. (b) FD/Bcl-2 cells were infected, sorted, stained with anti-Thy1.1 and starved of IL-3 as described in (a). Bcl-2 protein was immunoprecipitated from FD/Bcl-2/Bfk cells 0, 24 and 72 h after IL-3 deprivation and immunoprecipitates were tested for the presence of the 18.3 kDa Bfk protein by Western blotting using the anti-Bfk antibody. Coimmunoprecipitation of BimL and BimS with Bcl-2 from FD/Bcl-2/BimL cells was included as a positive control. Western blots of total cell lysates prior to immunoprecipitation show both Bcl-2 and Bfk were expressed at all time points. Probing with an antibody to Hsp70 was used as a loading control
Discussion
Our databases search revealed that many vertebrates possess an unusual novel member of the Bcl-2 protein family, which we have named Bfk. Unlike most family members, Bfk lacks a C-terminal hydrophobic membrane anchor and has both a BH3 and a BH2 region but not a BH4 or defined BH1 region (Figure 1). Its structure therefore most closely resembles that of Bcl-GL.6 Also like Bcl-GL, overexpressed Bfk has weak proapoptotic activity, and this requires its BH3 region and caspase activation. It is therefore tempting to suggest that Bfk and Bcl-GL represent a new subgroup of the Bcl-2 family. However, whereas Bcl-GL6 was reported to associate with organelles, Bfk appears to be a cytosolic protein (Figure 4a, b). Since Bcl-rambo shares considerable sequence identity with Bcl-GL (at least within the region homologous to Bcl-2) and since it also fails to bind to other Bcl-2 family members, it might also be included in this proposed new subgroup. It should be noted however that Bcl-rambo is reported to also possess BH1 and BH4 regions.7
Perhaps surprisingly, Bfk, again like Bcl-GL,6 does not appear to bind to either prosurvival or proapoptotic family members (Figure 3a). It is therefore unclear how Bfk kills cells and even possible that it only kills cells when overexpressed. As postulated for Bcl-GL,6 the BH3 domain of Bfk may be buried by its native conformation and hence unable to interact with other Bcl-2 family members. In support of that notion, the Bfk BH3 region could mediate association with Bcl-2-like proteins when installed in another polypeptide (Figure 3b). Accordingly, the weak proapoptotic activity of full-length Bfk could indicate that it represents the latent form of a potent BH3-only protein. Like caspase-8 cleavage of Bid to tBid,16,17 proteolytic processing may transform Bfk into a highly toxic BH3-only protein. Although we have not found any evidence of caspase-mediated cleavage of Bfk, it might become activated by other intracellular proteases. Alternate splicing of bfk could also produce a potent cell death inducer, as occurs with bcl-g,6 bim15 and bcl-x.18 As yet we have no evidence for any truncated Bfk protein, but such products might appear only in certain cell types and/or after certain stimuli.
Bfk and Bcl-GL may well have functions independent of truncation. The position of the BH3 and BH2 regions in Bfk and Bcl-GL is conserved relative to Bax and Bcl-2 (Figure 1c), and several highly conserved BH1 region residues remain in Bfk and Bcl-GL, despite their lack of a formal BH1 region. Given the structural similarity of Bcl-2 and Bax,3 the conserved positioning of the BH3 and BH2 in Bfk and Bcl-GL makes it likely that their structures resemble those of Bcl-2 and Bax. If so, their role in promoting apoptosis may be more like that of Bax and Bak than that of BH3-only proteins. Determining the structure of Bfk would therefore provide insight into its role in regulating apoptosis.
The expression of Bfk during pregnancy is highly regulated, perhaps in direct response to pregnancy hormones. Characterization of the bfk promoter region may shed light on the mechanisms regulating bfk during pregnancy. The inverse effect of pregnancy on Bfk protein levels in mammary glands and uterus most likely reflects the different cell types in these tissues and their differing responses to those hormones. The increased expression of Bfk in the gland during pregnancy suggests that Bfk may help to launch apoptosis in this organ.19 Establishing the physiologic function of Bfk will probably only be revealed by generating knockout mice, but our expression analysis indicates that interesting abnormalities may arise in mammary glands and uterine horns of bfk−/−females.
Materials and Methods
Bfk cloning and constructs
Murine bfk was amplified by PCR from total RNA isolated from day 8 lactating mammary glands of BALB/c mice. All oligonucleotides were supplied by Geneworks (Adelaide, SA) and introduced restriction sites are underlined. Amplification was performed with AmpliTaq (Applied Biosystems) using the 5′ oligonucleotide 5′-CCTGCTGAAGATCTTGGAGAATGTCTAAAATG, which introduces a BglII site, and 3′ oligonucleotide 5′-GCTCAGTCTAGATTAGAATAATCCTCTTC, which introduces an XbaI site, with the following cycle conditions: 96°C 10(min followed by 25 cycles of 96(C 30 s, 55°C 30 s, 72°C 1 min. The bfk cDNA was cloned as a BglII/XbaI fragment into the vector pEF HA PGK Hygro to generate the N-terminal HA-epitope tagged Bfk vector pEF/HA-mBfk and sequenced to confirm identity. The Bfk retroviral expression vectors pMiT/Bfk and pMiT/HA-Bfk were generated by cloning untagged Bfk or HA-tagged Bfk, respectively, from from pEF/HA-Bfk into the BglII/SalI sites of the vector pMSCV-IRES-Thy1.1 (pMiT) (a kind gift from Drs. D Hildeman and P Marrack). The bacterial Bfk expression vector pET-11a/Bfk was generated by cloning untagged Bfk from pEF/HA-Bfk into the NdeI/BglII sites of pET-11a (Novagen). The Bfk BH3 mutant expression vector pEF/HA-BfkGDAA was generated by PCR mutagenesis using the overlapping oligonucleotides 5′-TTCGGATCCTGGCTGCTCAGTTCAATGGAGAGCTG (sense) and 5′-CTGAGCAGCCAGGATCCGAAGGCGGCCAACAATG (anti-sense) to mutate both Gly64 and Asp65 to alanine. The yeast expression vector pGBT-9/BimL(BH3bfk) was generated by cloning the BH3 region of Bfk (created by annealing overlapping oligonucleotides with overhanging BglII and MluI compatible ends encoding the Bfk BH3-region (aa 49–73) 5′-GATCATTACTCTTTTGATGTGGCTATCATTGTTGGCCGCCTTCGAATACTGGGTGATCAGTTCAATGGAGAGCTGGAAGCT (sense) and 5′-CGCGAGCTTCCAGCTCTCCATTGAACTGATCACCCAGTATTCGAAGGCGGCCAACAATGATAGCCACATCAAAAGAGTAAT – (anti-sense) between the BglII/MluI sites engineered to flank the BimL BH3 region (aa 84–107) in pGBT-9/BimL-Sub-Cam (pGBT-9 supplied by Clontech).
Production of anti-Bfk antibodies
Full-length mouse Bfk protein was expressed in BL21 CodonPlus (DE3) E. coli (Stratagene) from the vector pET-11a/Bfk and purified by ion-exchange chromatography using Sepharose Q fast flow followed by gel filtration chromatography using a Superdex G-75 column. Pure, monomeric protein was obtained and used for immunization of New Zealand white rabbits. Bfk-specific antibodies were purified on an affinity column prepared by coupling Bfk protein to cyanogen bromide-activated sepharose (Amersham).
Northern blot analysis
Total RNA was isolated from mammary glands derived from BALB/c mice at different stages of development using RNAzol (Tel-Test) and 15 μg was fractionated on a 1% agarose–formaldehyde gel and transferred to Hybond N+ membrane (Amersham). It was hybridized with a 554 bp 32P-labelled fragment corresponding to the bfk coding region in ExpressHyb TM solution (Clontech) at 60°C. Washing was performed at 55°C in 2 × SSC/0.1% SDS (2 × 20 min washes), with final washes in 0.2 × SSC/0.1% SDS at 55°C (2 × 15 min). Subsequent hybridization with an oligonucleotide probe representing 18S rRNA (5′-TAATGATCCTTCCGCAGGTTCACCTACGGA-3′) provided a loading control. Hybridization with this oligonucleotide was performed in ExpressHyb solution at 60°C for 1 hour, before washing at RT in 2 × SSC (2 × 10min).
Cell culture, transfection, metabolic labelling and cell survival assays
The IL-3-dependent murine promyelocytic cell line FDC-P1 and its derivatives were cultured as described20 in high-glucose DME supplemented with 10% fetal calf serum (FCS), 50 μM 2-mercaptoethanol and 10−6 M asparagine (FMA). NIH 3T3 and HEK 293T cells were cultured in DME medium supplemented with 10% FCS. All cells were maintained at 37°C in a humidified 10% CO2 incubator. FDC-P1 cells were starved of IL-3 by washing in medium without IL-3 and centrifuging cells through a cushion of FCS three times. Survival of FDC-P1 cells was quantified by PI uptake by incubating cells with 2 μg/ml PI and then analyzing in a FACScan (Becton Dickinson). Transient transfection of HEK 293T cells was performed using Fugene reagent (Roche). HEK 293T cells transiently transfected with 2.5 μg of plasmid DNA in 10 cm dishes were metabolically labelled (Beckon Dickinson) for 24 h with 100 μCi/ml 35S-Met and 35S-Cys (NEN) added 24 h post- transfection. For survival assays, HEK 293T cells were transiently transfected in 5 cm dishes in triplicate for each experiment and each experiment was conducted on at least two independent occasions. Viability was assessed by DNA content analysis: adherent and detached cells were harvested 24 h post-transfection, and fixed in 70% ethanol. Cells were then washed and incubated in PI solution (69 μM PI, 38 mM sodium citrate pH 7.4 and 5 μg/ml RNaseA (Boehringer)) at 37°C for 20 min then room temperature for 15 min. DNA content was measured by analysis in a FACScan (Becton Dickinson), dead cells were scored as those with an apparent subdiploid (<2C) DNA content.
Immunoprecipitation, subcellular fractionation and Western blotting
Immunoprecipitations were performed as described21 using 2 μg/ml of either anti-HA.11. (BabCo), anti-FLAG M2 (Sigma) or anti-human Bcl-2 (Bcl-2-100) antibodies. Cell fractionation was performed in 0.025% digitonin as described previously.21 For Western blot analysis, total cell lysates from tissues or cultured cells were prepared in RIPA buffer with protease inhibitors.21 Equal quantities of protein lysate were resolved by SDS-PAGE and subjected to Western blotting as described21 using either anti-Bax (5B7, Sigma), anti-HA.11 (BabCo), anti-FLAG M2 (Sigma), anti-Hsp70 (N15 or N6, a kind gift from Dr. W. Welch), rabbit anti-Bim (Stressgen) or anti-human Bcl-2 (Bcl-2-100) antibodies at 1 μg/ml or purified rabbit anti-Bfk antibodies at 2 μg/ml.
Yeast two-hybrid assays
Yeast cells (MAV 103) (Clontech) were transfected with pGBT-9/BimL(BH3bfk) or pGBT-9/BimL either with empty pGAD (Clontech), pGAD/Bcl-2ΔC22, pGAD/Bcl-w, pGAD/BimL pGAD/Bcl-xL or pGAD/Bax by heat shock in lithium acetate. Yeast was grown at 30°C on agar plates with uracil, but without tryptophan or leucine. Five independent colonies from each transformation were replica plated and then assayed for β-galactosidase activity by permeabilizsing in chloroform and overlaying with agarose containing 20 mg/ml 5-bromo, 4-chloro, 3-indolyl β-D-galactopyranoside (X-gal).
Virus production and cell infection
MSCV-IRES-Thy1.1 retrovirus was prepared using a modification of the protocol by Pear et al.,22 by transfecting Phoenix ecotrophic packaging cells (a kind gift from Dr. Garry Nolan, Stanford) with retroviral vectors using Fugene (Roche) and cultured in a humidified incubator at 37°C, 10% CO2 for 24 h in DME with 10% FCS. Medium was changed to FMA and cells were cultured for 24 h in a humidified incubator at 32°C, 10% CO2. Viral particles were harvested by filtering culture supernatant through 0.45 μm filters and used immediately for infection. NIH 3T3 cells were infected 24 h after plating at 1 × 105 cells per 6 cm dish (Becton Dickinson) by replacing culture medium with viral supernatant containing 4 μg/ml hexadimethrine bromide (PolyBrene, Sigma) and culturing in a humidified incubator for 24 h at 37°C, 10% CO2. FDC-P1 and FDC-P1/Bcl-2 cells were infected with retroviruses at 0.67 × 106 cells/ml using RetroNectin (Takara) for 48 h in 24-well plates (Falcon #1147, Becton Dickinson) in a humidified incubator for 24 h at 37°C, 10% CO2.
Immunofluorescence staining, cell sorting and confocal microscopy
For detection of Thy 1.1 expression, cells were washed once in balanced salt solution containing 10% FCS and incubated for 30min with a FITC-conjugated anti-Thy1.1 antibody (19XE2). Cells were analyzed in a FACScan (Becton Dickinson) or sorted for Thy1.1 positive cells using either a FACStar Plus (Becton Dickinson) or modified FACS II (Becton Dickinson) cell sorter. Sub cellular localization of HA-Bfk by confocal microscopy was performed as described23 except that the cells were also stained with DAPI at 2μg/ml (Molecular Probes).
Abbreviations
- BH:
-
Bcl-2 homology
- Bfk:
-
Bcl-2 family kin
- EST:
-
expressed sequence tag
- ORF:
-
open reading frame
- HA:
-
hemagglutinin
- MiT:
-
MSCV-IRES-Thy1.1
- MSCV:
-
mouse stem cell virus
- IRES:
-
internal ribosome entry site
- PI:
-
propidium iodide
- PCR:
-
polymerase chain reaction
- SDS:
-
sodium dodecyl sulfate
- DME:
-
Dulbecco's modified Eagle
- FCS:
-
fetal calf serum
- PAGE:
-
polyacrylamide gel electrophoresis
- FITC:
-
fluorescein isothiocyanate
- DAPI:
-
4′6-diamidino-2-phenylindole dihydrochloride
References
Cory S and Adams JM (2002) The bcl-2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2: 647–656
Huang DCS and Strasser A (2000) BH3-only proteins – essential initiators of apoptotic cell death. Cell 103: 839–842
Suzuki M, Youle RJ and Tjandra N (2000) Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103: 645–654
Zong WX, Lindsten T, Ross AJ, MacGregor GR and Thompson CB (2001) BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 15: 1481–1486
Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T and Korsmeyer SJ (2001) BCL-2, BCL-xL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8: 705–711
Guo B, Godzik A and Reed JC (2001) Bcl-G, a novel pro-apoptotic member of the Bcl-2 family. J. Biol. Chem. 276: 2780–2785
Kataoka T, Holler N, Micheau O, Martinon F, Tinel A, Hofmann K and Tschopp J (2001) Bcl-rambo, a novel Bcl-2 homologue that induces apoptosis via its unique C-terminal extension. J. Biol. Chem. 276: 19548–19554
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W and Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402
Puthalakath H, Huang DCS, O'Reilly LA, King SM and Strasser A (1999) The pro-apoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3: 287–296
Negrini M, Silini E, Kozak C, Tsujimoto Y and Croce CM (1987) Molecular analysis of mbcl-2: structure and expression of the murine gene homologous to the human gene involved in follicular lymphoma. Cell 49: 455–463
Grillot DA, Gonzalez-Garcia M, Ekhterae D, Duan L, Inohara N, Ohta S, Seldin MF and Nunez G (1997) Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J. Immunol. 158: 4750–4757
Oltvai ZN, Milliman CL and Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74: 609–619
Herberg JA, Phillips S, Beck S, Jones T, Sheer D, Wu JJ, Prochazka V, Barr PJ, Kiefer MC and Trowsdale J (1998) Genomic structure and domain organisation of the human Bak gene. Gene 211: 87–94
Hengartner MO and Horvitz HR (1994) C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 76: 665–676
O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S and Huang DCS (1998) Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17: 384–395
Li H, Zhu H, Xu C-J and Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501
Luo X, Budlhardjo I, Zou H, Slaughter C and Wang X (1998) Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94: 481–490
Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G and Thompson CB (1993) bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74: 597–608
Kumar R, Vadlamudi RK and Adam L (2000) Apoptosis in mammary gland and cancer. Endocr. Relat. Cancer 7: 257–269
Huang DCS, Cory S and Strasser A (1997) Bcl-2, Bcl-XL and adenovirus protein E1B19kD are functionally equivalent in their ability to inhibit cell death. Oncogene 14: 405–414
Hausmann G, O'Reilly LA, van Driel R, Beaumont JG, Strasser A, Adams JM and Huang DCS (2000) Pro-apoptotic apoptosis protease-activating factor 1 (Apaf-1) has a cytoplasmic localization distinct from Bcl-2 or Bcl-xL . J. Cell Biol. 149: 623–634
Pear WS, Nolan GP, Scott ML and Baltimore D (1993) Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90: 8392–8396
O'Reilly LA, Print C, Hausmann G, Moriishi K, Cory S, Huang DCS and Strasser A (2001) Tissue expression and subcellular localization of the pro-survival molecule Bcl-w. Cell Death Differ. 8: 486–494
Acknowledgements
We thank Drs. D Hildeman and P Marrack for the MiT retroviral vector, C Chang and B Duscio for technical assistance, Dr. H Puthalakath for help with yeast two-hybrid analysis, Dr. L O'Reilly and J Jimenez for help with protein and antibody production and Dr. F Battye, C Tarlinton, J Chan, D Kaminaris, V Lapatis and Dr. L O'Reilly for help with flow cytometry and confocal microscopy. We are grateful to Drs. A Harris, H Puthalakath and P Bouillet for insightful discussions and critical comments on the manuscript. This work was supported by fellowships and grants from the NHMRC (Canberra), the Dr. Josef Steiner Cancer Research Foundation (Bern), the Sylvia and Charles Viertel Charitable Foundation, the Leukemia and Lymphoma Society of America and the NIH (CA80188).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Melino
Rights and permissions
About this article
Cite this article
Coultas, L., Pellegrini, M., Visvader, J. et al. Bfk: a novel weakly proapoptotic member of the Bcl-2 protein family with a BH3 and a BH2 region. Cell Death Differ 10, 185–192 (2003). https://doi.org/10.1038/sj.cdd.4401204
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401204
Keywords
This article is cited by
-
BCL-G: 20 years of research on a non-typical protein from the BCL-2 family
Cell Death & Differentiation (2023)
-
GWAS for autoimmune Addison’s disease identifies multiple risk loci and highlights AIRE in disease susceptibility
Nature Communications (2021)
-
Human BCL-G regulates secretion of inflammatory chemokines but is dispensable for induction of apoptosis by IFN-γ and TNF-α in intestinal epithelial cells
Cell Death & Disease (2020)
-
HDAC1 and HDAC2 independently regulate common and specific intrinsic responses in murine enteroids
Scientific Reports (2019)
-
Apolipoprotein L2 contains a BH3-like domain but it does not behave as a BH3-only protein
Cell Death & Disease (2014)