Abstract
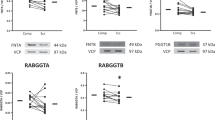
Combining in situ radioligand binding with autoradiography, we previously identified a reduction of [3H]phorbol 12,13-dibutyrate binding in the parahippocampal gyrus from schizophrenic subjects. To determine whether these changes were due to decreases in the level of protein kinase C, we measured [3H]phorbol 12,13-dibutyrate binding, levels of the protein kinase C isoforms α, β, δ, ε, γ, η and θ, as well as protein kinase C activity in crude particulate membranes from parahippocampal gyri of 15 schizophrenic and 15 control subjects. There was a significant decrease in the density (mean ± SEM: 6.56 ± 0.73 pmol mg−1 vs 9.68 ± 1.22 pmol mg−1; P < 0.05) and affinity (mean KD ± SEM: 4.64 ± 0.34 nM vs 2.95 ± 0.35 nM; P < 0.005) of [3H]phorbol 12,13-dibutyrate binding in homogenates from schizophrenic subjects. There were no significant changes in levels of the protein kinase C isoforms which are known to bind phorbol esters or in the activity of protein kinase C in membranes from schizophrenic subjects. These results suggest that there are changes in molecules capable of binding [3H]phorbol 12,13-dibutyrate, other than protein kinase C, in the parahippocampal gyrus from subjects with schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dean B, Opeskin K, Pavey G, Hill C, Keks N . Changes in protein kinase C and adenylate cyclase in the temporal lobe from subjects with schizophrenia J Neural Transm 1997; 104: 1371–1381
Opeskin K, Dean B, Pavey G, Hill C, Keks N, Copolov D . Neither protein kinase C nor adenylate cyclase are altered in the striatum from subjects with schizophrenia Schizophr Res 1996; 22: 159–164
Blumberg PM, Jaken S, Konig B, Sharkey NA, Leach KL, Jeng AY et al. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands Biochem Pharmacol 1984; 33: 933–940
Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y . Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters J Biol Chem 1982; 257: 7847–7851
Yamanishi J, Takai Y, Kaibuchi K, Sano K, Castagna M, Nishizuka Y . Synergistic functions of phorbol ester and calcium in serotonin release Biochem Biophys Res Commun 1999; 112: 778–786
Nishizuka Y . The molecular heterogeneity of protein kinase C and its implications for cellular regulation Nature 1988; 334: 661–665
Ways DK, Cook PP, Webster C, Parker PJ . Effect of phorbol esters on protein kinase C-zeta J Biol Chem 1992; 267: 4799–4805
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edn American Psychiatric Association: Washington DC 1994
Hill C, Keks N, Roberts S, Opeskin K, Dean B, Copolov D . Diagnostic Instrument for Brain Studies Mental Health Research Institute: Melbourne 1999
Foster P . Neuroleptic equivalence Pharmaceut J 1999; 243: 431–432
Kingsbury AE, Foster OJ, Nisbet AP, Cairns N, Bray L, Eve DJ et al. Tissue pH as an indicator of mRNA preservation in human post-mortem brain Brain Res Mol Brain Res 1995; 28: 311–318
Pandey GN, Dwivedi Y, Pandey SC, Conley RR, Roberts RC, Tamminga CA . Protein kinase C in the postmortem brain of teenage suicide victims Neurosci Lett 1997; 228: 111–114
Towbin H, Staehelin T, Gordon J . Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications Proc Natl Acad Sci U S A 1979; 76: 4350–4354
Dwivedi Y, Pandey GN . Effects of treatment with haloperidol, chlorpromazine, and clozapine on protein kinase C (PKC) andphosphoinositide-specific phospholipase C (PI-PLC) activity and on mRNA and protein expression of PKC and PLC isozymes in rat brain J Pharmacol Exp Ther 1999; 291: 688–704
Wan DC, Dean B, Pavey G, Copolov DL . Treatment with haloperidol or clozapine causes changes in dopamine receptors but not adenylate cyclase or protein kinase C in the rat forebrain Life Sci 1996; 59: 2001–2008
Areces LB, Kazanietz MG, Blumberg PM . Close similarity of baculovirus-expressed n-chimaerin and protein kinase C alpha as phorbol ester receptors J Biol Chem 1994; 269: 19553–19558
Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC et al. Munc13–1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release Neuron 1998; 21: 123–136
Ahmed S, Kozma R, Monfries C, Hall C, Lim HH, Smith P et al. Human brain n-chimaerin cDNA encodes a novel phorbol ester receptor Biochem J 1990; 272: 767–773
Lee J, Ahmed S, Kozma R, Teo M, Monfries C, Lim L . The N-terminal region of n-chimaerin allows lipid modulation of the C-terminal p21rac-GTPase activating domain Biochem Soc Trans 1992; 20: 310S
Diekmann D, Brill S, Garrett MD, Totty N, Hsuan J, Monfries C et al. Bcr encodes a GTPase-activating protein for p21rac Nature 1991; 351: 400–402
Augustin I, Rosenmund C, Sudhof TC, Brose N . Munc13–1 is essential for fusion competence of glutamatergic synaptic vesicles Nature 1999; 400: 457–461
Carlsson A, Hansson LO, Waters N, Carlsson ML . A glutamatergic deficiency model of schizophrenia Br J Psychiatry Suppl 1999; 37: 2–6
Song Y, Ailenberg M, Silverman M . Cloning of a novel gene in the human kidney homologous to rat munc13s: its potential role in diabetic nephropathy Kidney Int 1998; 53: 1689–1695
Acknowledgements
The authors would like to acknowledge the invaluable assistance of the Victorian Institute of Forensic Medicine in the procurement of tissue for this study, as well as Christine Hill and Nicholas Keks for the diagnosis of schizophrenia. They would also like to thank both Andrew McKinnon and Paul Dudgeon for their valuable advice regarding statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scarr, E., Pavey, G., Robinson, P. et al. Decreased phorbol ester binding in the parahippocampal gyrus from subjects with schizophrenia is not associated with changes in protein kinase C. Mol Psychiatry 7, 683–688 (2002). https://doi.org/10.1038/sj.mp.4001065
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001065
Keywords
This article is cited by
-
Die Wirkung von Antipsychotika auf glutamaterge Neurotransmission im Tiermodell
Der Nervenarzt (2004)