Abstract
Graphite like C3N4 (g-C3N4) was synthesized facilely via the low temperature thermal condensation of melamine between 300–650°C. The results showed that the products maintained as melamine when the temperature is below 300°C. With the increase of temperature, the products were transformed into carbon nitride and amorphous g-C3N4 successively. The morphology of products was changed from spherical nanoparticles of melamine into layer carbon nitride and g-C3N4 with the increase of temperature. The photoluminescence spectra showed that the carbon nitride products have continuous tunable photoluminescence properties in the visible region with increasing temperature. With the help of steady state, transient state time-resolved photoluminescence spectra and Raman microstructural characterization, a novel tunable photoluminescence mechanism was founded systematically, which is mainly related to the two dimensional π-conjugated polymeric network and the lone pair of the carbon nitride.
Similar content being viewed by others
Introduction
Carbon nitride has become a research hot in the field of materials science since Liu and Cohen predicted that the carbon and nitride were the good candidates for the extreme hardness materials1. Due to the special electron structures, carbon nitride is a kind of novel organic and metal-free semiconductors, on the other hand, composing by carbon and nitride, as well as its special layer structure make it has better chemically and thermally stable in the ambient conditions2. Moreover, the most important is that we can handle the band gap of carbon nitride via doping or modification its morphology3. Owing to their special properties such as the super hardness, low density, reliable chemical inertness, water resistivity, wear resistance and biocompatibility2,3, carbon nitride was regarded as the promising materials applied in the emission devices, surface modification, medical science and photocatalysis, etc. Wang et al. have reported that using carbon nitride as photocatalysis to acquire the hydrogen from water2 and Iwano et al. have reported that the amorphous carbon nitride films can be applied to the white light emitting devices4. Among the carbon nitride materials, graphite like carbon nitride (g-C3N4) has attracted considerable attention due to its unique optical and electronic properties, which is promising to be applied as photoelectric nanodevices, chemical sensors, metal free photocatalysis, photovoltaic solar cells, etc.5,6,7,8.
Up to now, g-C3N4 with several different kinds of morphologies has been fabricated, such as nanoparticles, nanowires, nanobelts, hollow vessels, etc.5,6,9. And various techniques and methods, such as chemical vapor deposition (CVD), physical vapor deposition (PVD), solvothermal synthesis, thermal nitridation, etc., have been used to synthesize g-C3N4. However, most of the traditional techniques and methods need strict synthesized conditions, such as higher temperature, higher pressure or longer time pretreatment. Therefore, a brand new method with simple synthesized process and low cost is eagerly anticipated for the research of g-C3N4.
Nowadays, several works about the visible photoluminescence (PL) of carbon nitride have been reported4,10,11. However, the systematic investigation on the tunable PL of carbon nitride is scarce and it is much rarer for g-C3N4 compounds. Researchers have tried to propose the explanations about the mechanism of visible PL of carbon nitride4,10,12,13. But there is no unambiguous PL mechanism or tunable PL mechanism about carbon nitride, such as g-C3N4, has been proposed in detail so far, which restricts the preparation and design of carbon nitride with multicolor-emitting and efficient luminescence. Therefore, the mechanism investigation about the luminescence of carbon nitride is extremely important in this research field.
In this work, g-C3N4 nanopowders were conveniently synthesized through the low temperature thermal condensation of melamine. By changing the temperature of thermal condensation, continuously tunable emissions of g-C3N4 nanopowders, which are covering the blue to green light region, were obtained. And the tunable PL mechanism of g-C3N4 nanopowders was also proposed in detail, based on the steady state and transient state time-resolved PL spectra. The g-C3N4 nanopowders with tunable emissions were expected to extend their applications in photoelectric nanodevices.
Results
The normalized XRD patterns of the carbon nitride compounds obtained under different temperatures were shown in Fig. 1. When the raw materials are heated at 300°C for 2 h, a series of sharp peaks are detected, which are corresponding to the crystalline melamine (C3H6N6) phase (JCPDS: 39-1950). The diffraction pattern in Fig. 1(a) indicates that the product maintains as melamine phase and no other impurity phase is detected. When the processing temperature rises to 350°C and 400°C, several new peaks appear and the peaks are broadened compared with those in Fig. 1(a), which indicates that the products have transformed from crystalline to amorphous. With increasing process temperature, three broad distinct diffraction peaks are observed when the processing temperature is higher than 500°C, which are located at 27.2°, 13.1°, 46.19°, respectively. It can be confirmed that all the diffraction peaks are in agreement with the recent reports on the XRD patterns of the amorphous g-C3N4 prepared via the thermal condensation of the melamine6,14,15,16. The XRD patterns indicate that the products maintained as crystalline melamine phase when the heating temperature is lower than 300°C. But, when the temperature rises to 350–400°C, it is transformed into crystalline or amorphous graphite like carbon nitride compounds and when the temperature is higher than 500°C, the products completely forms into amorphous g-C3N4.
Figure 2 shows the SEM images of the carbon nitride processed at 300°C, 400°C, 500°C, 600°C, respectively. The Fig. 2(a) shows that the morphology of melamine processed at 300°C is granular with a particle size of 50–80 nm. When the processed temperature is higher than 400°C, the morphology of products have transformed into layer. And with the increase of temperature, the size of the flake is obviously increased. Which can be clearly seen from the results shown in Fig. 2(b) (c) and (d), Fig. 2(b) shows that the size of the flake is located at a range of 0.5–0.8 μm when the temperature is 400°C. When the processed temperature rises to 500°C and 600°C, the size is also increased to 0.5–1.0 μm and 0.5–1.5 μm (as shown in Fig. 2(c) and (d)), respectively. Obviously, the evolution of the morphology from granular to layer is along with the chemical reaction process, the process of thermal condensation have happened since 400°C and the increasing temperature promotes this process, so that the degree of the condensation is increased which leads to the grow of the size of flake. In addition, from the SEM images, the size of most carbon nitride particles synthesized in this work is located at the submicron, which makes the products have a bright future in the application of bioengineering and photoelectric fields.
From the TEM image of the carbon nitride processed at 600°C, as shown in Fig. 3(a), it is obvious that the structure of the product is layer-like structure. The HR-TEM image of the carbon nitride is shown in Fig. 3(b). From the image, no obvious crystal fringe is observed for the product synthesized at 600°C, which indicates that the product is amorphous. What is more, the mist diffraction ring, shown in the selected area electron diffrantion (SEAD) in Fig. 3(c), further confirms that the product synthesized at 600°C is amorphous. And this result is in good agreement with those of HRTEM and XRD patterns.
Figure 4 shows the PL excitation spectra evolution with increasing process temperature from 300°C to 650°C for products and all the excitation spectra are monitored at the emission peak for each specimens. It can be seen that the evolution of the excitation spectra matchs well with the phase and microstructure evolution (as shown in Fig. 1). Interestingly, all the excitation spectra exhibit a broad excitation band covering 250 nm to 400 nm, which shows a slightly red shift with the increase of process temperature. Moreover, the effect of the processing temperature on the PL emission properties of carbon nitrideis is studied in this work. To investigate the PL emission properties of specimens under the same excitation, a shorter 280 nm excitation is selected as the excitation wavelength in the following works. Fig. 5 shows the normarized PL spactra of the multicolor-emitting carbon nitride products synthesized at 300, 350, 400, 450, 500, 550, 600 and 650°C for 2 h under the excitation wavelenth at 280 nm, respectively. The peak shape of the PL spectra are similar and the emission peak of PL spectra is located at 405, 425, 426, 458, 462, 475, 480 and 510 nm, respectively. Therefore, from this result, it can be clearly observed that with the processing temperature rising, the center of PL is red shift constantly. The center of PL spectra shifts from 400 nm to 510 nm with the increase of the processing temperatures, which stands for the blue-violet light region to the green light region. And the digital photos about the multicolor-emitting of carbon nitride products with (bottom) and without (upper) excitation are shown in the inset of Fig. 5, which also confirm that the PL emission is covering the blue-violet to green light region. On the other hand, the full width at half maximum (FWHM) is also became wider with the temperature increasing, which is related to the degree of the thermal condensation and the increase of disorder microstructure of the carbon nitride17. For the carbon nitride products synthesized at 650°C for 2 h, the broad emission band is even extended to the yellow light region.
The Commission Internationale de l'Eclairage (CIE) chromaticity diagram of the carbon nitride products syntherized at different temperature is depicted in Fig. 6. The CIE (x, y) coordinate of carbon nitrides synthesized at 300, 350, 400, 450, 500, 550, 600 and 650°C is located at (0.16, 0.08), (0.18, 0.075), (0.16, 0.16), (0.17, 0.20), (0.2, 0.25), (0.22, 0.31) and (0.28, 0.36), respectively, which further confirm that the PL emission is covering the blue-violet to green light region. Additionally, it is noteworthy that the emission of carbon nitride products obtained at 450–650°C is located at the edge of the central white-light region. Hence, it is expected to get the white light emitting after introducing red-light emission in the g-C3N4 products.
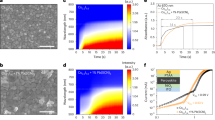
Figure 7 shows the decay curves of the carbon nitride products synthesized at different temperature for 2 h, which is measured at the emission peak with the 280 nm excitation. From the decay curves, with the increase of the temperature, the fluorescence lifetime of the products clearly decreases. Moreover, the decay curve of carbon nitride products in Fig. 7 show obvious deviation from the single-exponential decay, which indicates that the muitiple processes may be involved during the decay of the emission besides the radiative transition18. By the multi-exponential fitting, it can be estimated that the long fluorescence lifetime of carbon nitride products is between 106 ns and 259 ns. The nanosecond level lifetime of the multicolor-emitting carbon nitride products endows their potential application in display, lighting, etc.
The steady-state and transient PL emission spectra of the carbon nitride product synthesized at 400°C for 2 h are shown in the Fig. 8, which are excitated by Xe lamp and ns lamp, respectively. The steady-state PL spectrum exhibits a perfect single speak, while the transient PL sperctrum exhibits a board emission band included several peaks. The obvious difference between the steady-state and transient PL emission spectra implies that there is more than one PL emission center during the emission process of carbon nitride products.
Figure 9 shows the time-resolved emission spectra of carbon nitride products synthesized at 400°C for 2 h. It is obvious that there are at least two emission centers at 405 nm and 480 nm and the emission center located at 480 nm decays faster than that located at 405 nm. What is more, there is a little continuous blue shift for the 405 nm emission with the time decay, which is probably due to the remarkable change of the intensity between 405 nm and 480 nm emission bands18. Moreover, another time-resolved emission spectra of the carbon nitride products headed at 600°C for 2 h with the time scale from 0 to 100 ns are shown in Fig. 10. It has a single emission peak located at around 485 nm which is consistant to the PL emission peak of the product heated at 600°C obtained in Fig. 5. Compared with the spectra of the product obtained at 400°C, the emission peak located at 405 nm is disappeared or become fairly weak. The reason is listed as follows. From the XRD patterns results (Fig. 1), it is clear that the product obtained at 600°C is g-C3N4 phase has a higher degree of thermal condensation, compared with that obtained at 400°C. So that the structure of g-C3N4 is a plane network consisted of the tri-s-triazine structure3, which has a higher degree of disorder and bigger delocalization of electrons. The delocalization of electrons will lead to the overlap of the orbital, so that a bigger overlap of the π* antibonding state and δ* antibonding state will appear, which makes the transition probability between lone pair (LP) state formed through the LP electron of the nitride and the δ* conduction band becoming weaker. Therefore, this process causes the emission peak located at 405 nm of the carbon nitride disappeared. Furthermore, it can be deduced that the emission peak located at 480 nm is caused by the transition between LP to π* conduction band.
To investigate the effect of the processing time on the PL properties, the melamine processed at the same temperature for different time are taken. The results of PL spectra are shown in Fig. 11. When the raw materials are processed at the same temperature for different time, the peak shape of the PL spectra is almost similar, which indicates that the PL properties do not change obviously with the increase of processed time. It is noteworthy that, with the processing time increasing, the PL emission spectra of carbon nitride products exhibits a slight red shift as shown in Fig. 11(b). This result can be explained by considering that the longer processing time may cause an increasing of the degree of the thermal condensation, which will lead to the decrease of the optical band gap19. Moreover, the PL excitation spectra of the products processed at different temperatures with various treating times are shown in Fig. 11(a). which clearly indicated that the specimen processed at same temperature with different hours have almost the same excitation spectra. With these results, it can be deduced that the processing time have little effect on the PL properties.
To interpretate the PL emisson mechanism of carbon nitride and g-C3N4 products, microstructure evolution of carbon nitride products syntherized at different conditions are investigated by Raman spectra. The normalized Raman spectra of carbon nitride obtained at different temperature is shown in the Fig. 12. The evolution of the Raman spectra shown in Fig. 12(a) is generally due to the structural change of carbon nitride by the thermal condensation. From the spectra, it is confirmed that the products is still melamine crystal when the processing temperature is lower than 300°C. With the processing temperature rising into the region of 350°C to 450°C, a new crystal is formed and the compound phase slowly transforms into amorphous. When the the processing temperature is above 500°C, the products completely transform into amorphous g-C3N4. And the above results of Raman spectra is corresponding well with the XRD results (as shown in Fig. 1). What is more, owing to the strong fluorescent backgrounds, some of the structural peaks become inconspicuous. However, there are also two characteristic peaks related to the D and G bands located around 1405 cm−1 and 1570 cm−1 in Fig. 12(b). And the changing of the relative intensity of these two peaks is consistent with a trend that intensity radio of D (I(D)) band to G band (I(G)) becomes grater with the processing temperature rising. According to the equation20:

Where E(g) is the band gap of carbon nitride and C″ is a constant. It can be estimated that the E(g) is becoming narrower with the increase of temperature.
The Raman spectra of the carbon nitride obtained at different temperatures using the near-infared (NIR 785 nm) light for excitation.
(a) the normalized raman spectra of carbon nitride in the scanning region 50–2000 nm; (b) the normalized raman spectra of carbon nitride in the scanning region 1000–2000 nm.
Discussion
As shown in Fig. 5, the PL emission of the carbon nitride products exhibit continuous red shift with the processing temperature increasing. It is obvious that the PL property of the carbon nitride products synthesized by the thermal condensation of melamine is continuously tunable with the processed temperature rising. Hence, in the following part, the physical mechanism of this interesting tunable PL property of carbon nitride products is discussed in detail.
Compared with the results of Fig. 9 and Fig. 10, two emission peaks located at 405 nm and 480 nm are observed in Fig. 9. The emission center at 405 nm is caused by the transition between LP valence band and δ* conduction band which is related to the sp2 C-N bond21 and the emission band centered at 480 nm is attributed to the transition between the LP valance band and the π* conduction band. This result is consistent with that reported by Iwano et al., which indicate that the transition is caused by the π valence band and the π* conduction band, or the LP valence band and the δ* conduction band whose energy is more than 2 eV4. These two emission peaks compose the whole PL emission band. Hence, the evolution of these two emissions will alter the PL emission characteristics of the carbon nitride products.
The structure of the melamine and g-C3N4 is shown in the Fig. 133,22,23. It is clearly shown that the basic structure of the g-C3N4 is the tri-s-triazine ring structure, which is connected by the N atoms to form a π-conjugated polymeric network. Generally, the optical properties of all the disorder carbon-based materials are related to the π and π* antibonding states in the visible-near-ultraviolet energy range12. And the luminescence property of the carbon nitride materials is considered mainly effected by the size of the sp2 C-N clusters and the lone-pair electrons of the nitride12,21,24,25. In addition, the formation of the LP state is because the LP electrons of nitride is not hybridization with the carbon and it is located in the sp2 C-N π valence band12,21,26. Based on the above results and discussion, the tunable PL mechanism of the carbon nitride products are proposed as follows:
-
a
As the processing temperature increasing, the polymeric network of the g-C3N4 will extend by connecting more tri-s-triazine and the bond length of the sp2 C-N clusters becomes larger at higher temperature, both of them will increase the size of the sp2 C-N clusters. In a typical opinion, the larger size of the sp2 clusters has a small optical band gap4,21,27.
-
b
The g-C3N4 is a type kind of extended solid materials condensation by the melamine single molecules and the thermal condensation increasing at a higher temperature, which will lead to the g-C3N4 composed by more tri-s-triazine ring. Therefore, the π-conjugated system is enhanced. These effects involve the condensation process and the increasing π states, which will result in the narrowing of band gap11,28,29.
-
c
The denser packing of the sp2 aromatic clusters is formed for the intensity of the thermal condensation. Hence, a stronger overlap of the orbital is appearing and consequently the band gap of g-C3N4 decreases.
All the above factors are in good agreement with the Raman results, which confirms that the red shift of the PL emission at high temperature is directly caused by the tunable narrowing of band gap.
Herein, for the good insight into the tunable PL mechanism of the carbon nitride, a simple schematic diagram corresponding to the band gap states of the carbon nitride are tentatively founded in Fig. 14. As the excitation spectra exhibit a broad excitation band and it is almost the same for the specimen obtained above 500°C which is nearly forming into g-C3N4, we assume that the electronic is excited from the δ valence band to the δ* conduction band as the process 1 in Fig. 14. This is because that the degree of thermal condensation has no obvious effect on the δ band region30. The emission center located at 405 nm and 480 nm for the carbon nitride obtained at 400°C is corresponded to the process 2 and 3, respectively. The band a represents the overlap of the δ* and the π* bands which provides a channel for the excited electrons to relax to the π* conduction band and the band a will be shifted to the direction close to π* conduction band. So, at the higher processing temperature, the delocalization of the electrons will be increased24. Therefore, a stronger overlap is appearing and then the band a is closer to the π* state, just as the a′ band in Fig. 14. Hence, the probability for the excited electrons located at δ* conduction band relaxing to the π* conduction band is increasing and consequently the π*→LP emission is slowly dominated the PL emission of the carbon nitride as the processing temperature rising. And this is consistent with the results discussed in Fig. 10. Furthermore, the higher processing temperature enhances the π-conjugated system as higher degree of thermal condensation. Therefore, π-conjugated number is increasing and the packing of the g-C3N4 becomes denser. Owing to all of the above factors will narrow the band gap (just indicated by the π and LP states will continuously move up to the location of π′ and LP′, respectively), the gap between the LP valence state and the δ* or π* conduction band is decreasing. Therefore, the PL emission of carbon nitride products is red shift with the increase of processing temperature.
In conclusion, carbon nitride and g-C3N4 nanopowders were synthesized by low temperature thermal condensation of melamine. With the increase of processing temperature, the phase have been transformed from the melamine (C3H6H6) phase to amorphous g-C3N4 and the morphology was also changed from granular to layer structure with different sizes of flakes. Moreover, the PL properties of the carbon nitride products synthesized at different temperatures indicate that there are two PL centers located at 405 nm and 480 nm under the 280 nm excitation. And with the temperature increasing, the carbon nitride products exhibit tunable PL properties, such as the emission peak is red shift from 400 nm to 510 nm and it can be expand to the range of the green light. The tunable PL mechanism of carbon nitride products is also proposed based on the steady and transient state PL spectroscopy. The PL properties of carbon nitride products are assigned to the change of the optical band gap which is related to the size of the sp2 clusters. Furthermore, it also has a relationship with the thermal condensation of the carbon nitride products and the LP electrons which can form the LP states. The tunable PL properties of the carbon nitride materials synthesized via convenient low temperature thermal condensation of melamine are expected to expand their applications in displays, lighting, bio-labels, etc.
Methods
Materials synthesis
Analytical grade melamine powders, purchased from Sinopharm Chemical Reagent (SCR) Co., Ltd, Shanghai, China, were first ground for 30 min in agate mortar and then 2 g ground melamine powders were put into a 20 ml alumina crucible, which was placed in the central region of a quartz tube furnace for heat treatment. High purity nitrogen (99.999%) was introduced into the quartz tube with a rate of 75–150 sscm. The melamine was heated at a designed temperature between 300°C and 650°C for 2–4 h. After cooling to room temperature, the product was taken out and ground into fine powers in the agate mortar.
Characterization methods
The structure of the products was studied by X-ray diffraction (XRD) patterns which were obtained by the D8 advance X-ray diffractometer (Bruker, Switzerland) with a Cu Kα radiation (λ = 1.54056 Å) and the Raman spectroscopy (Renishaw in Via) using a 785 nm excitation. The morphology of the products was determined by the field emission-scanning electron microscopy (FE-SEM, Nova NanoSEM430, FEI, Netherlands) and high-resolution transmission electron microscopy (HR-TEM, 2100F, JEOL, Japan). The PL properties was investigated using the high resolution spectrofluorometer Edinburgh Instruments FLS 920 equipped with a single photon counting photomultiplier (Hamamatsu R928P) in Peltier air-cooled house for ultraviolet to visible range. All the measurements were carried out at room temperature.
References
Liu, A. Y. & Cohen, M. L. Prediction of new low compressibility solids. Science 245, 841–842 (1989).
Wang, X. C. et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 8, 76–80 (2009).
Wang, Y., Wang, X. C. & Antonietti, M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 51, 68–89 (2012).
Iwano, Y. et al. Study of amorphous carbon nitride films aiming at white light emitting devices. Jpn. J. Appl. Phys. 47, 7842–7844 (2008).
Li, Y. G. et al. Nitrogen-rich carbon nitride hollow vessels: synthesis, characterization and their properties. J. Phys. Chem. B. 114, 9429–9434 (2010).
Yang, J. H. et al. Synthesis and characterization of nitrogen-rich carbon nitride nanobelts by pyrolysis of melamine. Appl. Phys. A. 105, 161–166 (2011).
Kawaguchi, M., Yagi, S. & Enomoto, H. Chemical preparation and characterization of nitrogen-rich carbon nitride powders. Carbon 42, 345–350 (2004).
Hong, J. D., Xia, X. Y., Wang, Y. S. & Xu, R. Mesoporous carbon nitride with in situ sulfur doping for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. 22, 15006–15012 (2012).
Bai, X. J., Cao, C. B., Xu, X. Y. & Yu, Q. Synthesis and characterization of crystalline carbon nitride nanowires. Solid State Commun. 150, 2148–2153 (2010).
Papadimitriou, D., Roupakas, G., Dimitriadis, C. A. & Logothetidis, S. Raman scattering and photoluminescence of nitrogenated amorphous carbon films. J. Appl. Phys. 92, 870–875 (2002).
Thomas, A. et al. Graphitic carbon nitride materials: variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 18, 4893–4908 (2008).
Fanchini, G., Tagliaferro, A., Conway, N. M. J. & Godet, C. Role of lone-pair interactions and local disorder in determining the interdependency of optical constants of a-CN:H thin films. Phys. Rev. B. 66, 195415 (2002).
Chen, L. C. et al. Preparation of graphite-like carbon nitride nanoflake film with strong fluorescent and electrochemiluminescent activity. Nanoscale 5, 225–230 (2013).
Yan, H. J., Chen, Y. & Xu, S. M. Synthesis of graphitic carbon nitride by directly heating sulfuric acid treated melamine for enhanced photocatalytic H2 production from water under visible light. Int. J. Hydrogen Energy 37, 125–133 (2012).
Fu, J., Chang, B. B., Tian, Y. L., Xi, F. N. & Dong, X. P. Novel C3N4-CdS composite photocatalysts with organic-inorganic heterojunctions: in situ synthesis, exceptional activity, high stability and photocatalytic mechanism. J. Mater. Chem. A. 1, 3083–3090 (2013).
Yan, S. C., Li, Z. S. & Zou, Z. G. Photodegradation of Rhodamine B and Methyl Orange over Boron-Doped g-C3N4 under Visible Light Irradiation. Langmuir 26, 3894–3901 (2010).
Zhao, Y. C. et al. Large-scale synthesis of nitrogen-rich carbon nitride microfibers by using graphitic carbon nitride as precursor. Adv. Mater. 20, 1777–1781 (2008).
Liu, X. F. et al. Spectroscopic investigation on BCNO-based phosphor: photoluminescence and long persistent phosphorescence. J. Phys. D: Appl. Phys. 42, 215409 (2009).
Tyborski, T. et al. Tunable optical transition in polymeric carbon nitrides synthesized via bulk thermal condensation. J. Phys.: Condens. Matter 24, 162201 (2012).
Ferrari, A. C. & Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B. 61, 14095–14107 (2000).
Wang, B. B., Cheng, Q. J., Wang, L. H., Zheng, K. & Ostrikov, K. The effect of temperature on the mechanism of photoluminescence from plasma-nucleated, nitrogenated carbon nanotips. Carbon 50, 3561–3571 (2012).
Du, A. J. et al. Hybrid graphene and graphitic carbon nitride nanocomposite: gap opening, electron-hole puddle, interfacial charge transfer and enhanced visible light response. J. Am. Chem. Soc. 134, 4393–4397 (2012).
Jürgens et al. Melem (2,5,8-triamino-tri-s-triazine), an important intermediate during condensation of melamine rings to graphitic carbon nitride: synthesis, structure determination by X-ray powder diffractometry, solid-state NMR and theoretical studies. J. Am. Chem. Soc. 125, 10288–10300 (2003).
Alibart, F., Lejeune, M., Durand Drouhin, O., Zellama, K. & Benlahsen, M. Influence of disorder on localization and density of states in amorphous carbon nitride thin films systems rich in π-bonded carbon atoms. J. Appl. Phys. 108, 053504 (2010).
Sun, C. Q. et al. Bond contraction and lone pair interaction at nitride surfaces. J. Appl. Phys. 90, 2615–2617 (2001).
Robertson, J. & Davis, C. A. Nitrogen doping of tetrahedral amorphous carbon. Diam. Relat. Mater. 4, 441–444 (1995).
Robertson, J. Recombination and photoluminescence mechanism in hydrogenated amorphous carbon. Phys. Rev. B. 53, 16302–16305 (1996).
Ferrari, A. C., Rodil, S. E. & Robertson, J. Interpretation of infrared and Raman spectra of amorphous carbon nitrides. Phys. Rev. B. 67, 155306 (2003).
Huda, M. N. & Turnerb, J. A. Morphology-dependent optical absorption and conduction properties of photoelectrochemical photocatalysts for H2 production: a case study. J. Appl. Phys. 107, 123703 (2010).
Zheng, B. et al. First-principle study of nitrogen incorporation in amorphous carbon. Carbon 44, 962–968 (2006).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51102096, 51072054, 51072060 and 51132004), National Basic Research Program of China (2011CB808100) and UIRT of Guangdong Province.
Author information
Authors and Affiliations
Contributions
G.P.D. proposed and guided the overall project. Y.H.Z., Q.W.P., G.Q.C. and M.R.L. performed all the experiments and analyzed the results. All the authors discussed the results. Y.H.Z. and G.P.D. wrote the manuscript, with discussion from Q.Y.Z. and J.R.Q.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Zhang, Y., Pan, Q., Chai, G. et al. Synthesis and luminescence mechanism of multicolor-emitting g-C3N4 nanopowders by low temperature thermal condensation of melamine. Sci Rep 3, 1943 (2013). https://doi.org/10.1038/srep01943
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01943
This article is cited by
-
Photocatalytic Hydrogen Evolution Under Visible Light Using MoS2/g-C3N4 Nano-Photocatalysts
Catalysis Letters (2024)
-
The Effect of UV–Ozone Treatment on Structural, Optical, and Dielectric Properties of Thermally Evaporated Graphitic Carbon Nitride Thin Film
Journal of Electronic Materials (2023)
-
Carbon Nanofiber-Bridged Carbon Nitride-Fe2O3 Photocatalyst: Hydrogen Generation and Degradation of Aqueous Organics
Catalysis Letters (2023)
-
Construction and characterization of intramolecular tri-s-triazine heterostructures
Journal of Materials Science (2023)
-
FRET Mechanism of SnO2 Integrated Luminescent g-C3N4 Nanocomposite and Its Robust Chemical, Optical and Thermal States for Emissive Layer Application
Journal of Inorganic and Organometallic Polymers and Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.