Abstract
During pregnancy immunolglobulin G (IgG) antibodies are transferred from mother to neonate across the placenta. Studies in high transmission areas have shown transfer of P. falciparum-specific IgG, but the extent and factors influencing maternal-foetal transfer in low transmission areas co-endemic for both P. falciparum and P. vivax are unknown. Pregnant women were screened weekly for Plasmodium infection. Mother-neonate paired serum samples at delivery were tested for IgG to antigens from P. falciparum, P. vivax and other infectious diseases. Antibodies to malarial and non-malarial antigens were highly correlated between maternal and neonatal samples (median [range] spearman ρ = 0.78 [0.57–0.93]), although Plasmodium spp. antibodies tended to be lower in neonates than mothers. Estimated gestational age at last P. falciparum infection, but not P. vivax infection, was positively associated with antibody levels in the neonate (P. falciparum merozoite, spearman ρ median [range] 0.42 [0.33–0.66], PfVAR2CSA 0.69; P. vivax ρ = 0.19 [0.09–0.3]). Maternal-foetal transfer of anti-malarial IgG to Plasmodium spp. antigens occurs in low transmission settings. P. vivax IgG acquisition is not associated with recent exposure unlike P. falciparum IgG, suggesting a difference in acquisition of antibodies. IgG transfer is greatest in the final weeks of pregnancy which has implications for the timing of future malaria vaccination strategies in pregnant women.
Similar content being viewed by others
Introduction
The blood stage of Plasmodium falciparum and P. vivax malaria infections are a major cause of mortality and morbidity, resulting in an estimated 584,000 deaths and 198 million clinical cases each year, predominantly young children under the age of five1. Despite these increased risks early in childhood, clinical malaria in the first six months of life is generally uncommon and infections tend to be asymptomatic with low density parasitaemia2. This protection in infancy is often attributed partially to the passive transfer of naturally acquired protective immunity to malaria from mother to child prior to the development of the infant’s own immune system2,3.
Naturally acquired immunity develops in individuals living in malaria endemic areas after repeated exposure to Plasmodium spp. infections. Immunity acts by reducing parasite densities and associated clinical symptoms rather than protecting against Plasmodium spp. infection per se4. Antibodies are an important component of malarial immunity5,6 and targets include antigens on the surface of sporozoites (pre-erythrocytic stage), merozoites (involved in erythrocyte invasion) and antigens on the surface of infected erythrocytes (IEs)1,6,7,8. Antibodies to the blood stage targets are associated with protection against clinical disease2,7,9,10 and individuals with substantial immunity are likely to possess a large repertoire of antibody responses2,3,11. At the time of their first pregnancy, women living in malaria endemic areas will have developed a degree of protective immunity. Despite this pre-existing immunity pregnant women typically develop higher P. falciparum and P. vivax densities, compared to non-pregnant adults4,12. This susceptibility has been attributed to immune modulation resulting in an impaired ability to limit parasite replication during pregnancy, and the lack of immunity to placental-binding variants of P. falciparum that accumulate in the placenta5,6,13. The sequestration of P. falciparum-IEs in the placenta is mediated in part by PfVAR2CSA, a specific variant of P. falciparum erythrocyte membrane protein (PfEMP1) expressed on the surface of P. falciparum-IEs14. Over successive pregnancies, women resident in malaria-endemic areas in Africa and Asia acquire PfVAR2CSA antibodies which have been associated with a decrease in the rates and density of placental infection13,15.
Although foetuses are capable of synthesising immunoglobulin from the twelfth week of gestation, the majority of foetal immunoglobulin is of maternal origin16,17. The maternal-foetal transfer of immunoglobulins begins in the sixteenth week of gestation16 and requires active transport via Fc receptors on placental syncytiotrophoblasts18. The immunoglobulin G (IgG) isotype is the only immunoglobulin to cross the placenta in significant amounts, facilitated by the neonatal Fc receptor18,19. Anti-P. falciparum IgG has been shown to correlate between maternal and cord samples, and detectable IgG titres and P. falciparum antigen-specific antibodies have been demonstrated in newborns living in high transmission areas of Africa and Papua New Guinea20,21,22,23,24. There is a paucity of maternal-foetal transfer studies of P. falciparum in low transmission settings and even fewer studies addressing the transfer of P. vivax antibodies. Importantly there are few studies comparing the maternal-foetal transfer of antibodies to Plasmodium spp. compared to other pathogens and vaccine-preventable diseases. In addition, very little is known about factors that influence infant antibody levels and, importantly, that influence the rate of maternal-foetal antibody transfer. Previous studies have shown that P. falciparum placental infection, HIV, gestational age at birth and hypergammaglobulinemia can reduce transplacental transfer of maternal antibodies25,26,27,28, but other factors may also play a role.
In this study we determined antibodies to a panel of P. falciparum and P. vivax antigens representing different life-cycle stages in maternal, umbilical cord, and neonatal samples at delivery, in Karen women attending antenatal clinics at the Thai-Myanmar border. In this setting both P. falciparum and P. vivax transmission is low and placental infection is relatively rare as is the presence of HIV (<0.2%)29. We investigated maternal-foetal transfer of antibodies towards sporozoites, P. falciparum and P. vivax merozoite antigens, and antigens on the surface of Pf-IEs and gametocytes as well as to tetanus, measles and cytomegalovirus (CMV). We investigated how factors such as species-specific Plasmodium exposure (and timing of exposure), gravidity, chemoprophylaxis and gestational age influenced maternal-foetal transfer and neonatal antibody levels.
Materials and Methods
Study population
This study took place in the antenatal clinics (ANCs) of the Shoklo Malaria Research Unit (SMRU) in north-west Thailand from November 1998 to January 2000. More than 90% of pregnant women in the camps attended SMRU ANCs on a weekly basis30. All women are invited to come to an ANC as soon as they are aware of their pregnancy. All women who attend ANCs are screened weekly for Plasmodium spp. infection by light microscopy using a finger prick blood sample, and every second week for anaemia by haematocrit. All women are invited to deliver at SMRU although Karen women traditionally deliver at home. The epidemiology of malaria in this area, and the effects of P. falciparum and P. vivax malaria during pregnancy and on birth outcomes, have been described in detail previously30,31,32.
Study design and data collection
Mother-neonate pairs at delivery were selected from women included in a case-control study of Plasmodium spp. immunity, nested in a placebo-controlled trial of chloroquine prophylaxis33,34. Briefly, four tablets of chloroquine (153 mg base) or placebo were given at enrolment, and 2 tablets of the same type on a weekly basis until delivery. For more details on treatment refer to Villegas et al., 200734. Plasma samples at delivery were taken from peripheral samples of the mother and neonate (heel prick) as well as cord samples. Samples were available for 57/136 cases (women with Plasmodium spp. parasitaemia detected by light microscopy at any time during pregnancy) and 111/331 controls (women with no parasitaemia at any time during pregnancy). The controls represented a subset, selected on IgG response to schizont extract at enrolment for determination of antibody responses during pregnancy33. These were 74 of the most reactive individuals (‘high responder controls’) together with 37 randomly selected sero-negative individuals (see flow chart Supplemental Fig. 1). Apart from the selected subset, unavailable samples are the result of sample not being taken as the women delivered at home, or insufficient sample volume available for antibody determination. Estimated gestational age (EGA) was primarily assessed by the Dubowitz method35 and, when possible, neonates had a newborn neurological examination36. When a Dubowitz evaluation could not be done, EGA was calculated by a formula developed from a cohort of Karen pregnant women with known gestation age [(fundal height on admission ×0.887) + 4.968 weeks]31 which performs similarly to the Dubowitz method in this population37. A premature birth was defined as delivery before 37.0 estimated weeks of pregnancy.
Written informed consent was obtained from all participants. The study was performed in accordance with the guidelines approved by the Ethics Committee of the Faculty of Tropical Medicine of Mahidol University, Thailand; the Ethics Committee of the London School of Hygiene & Tropical Medicine, UK; and the Walter and Eliza Hall Institute of Medical Research, and the Alfred Hospital, Australia.
Antibody determination
Total IgG was determined to antigens representing a range of life-cycle stages from both P. falciparum and P. vivax that represented both biomarkers of exposure and protective immunity9,10. P. falciparum (3D7 allele unless otherwise stated) merozoite antigens apical membrane antigen 1, (PfAMA1), erythrocyte binding antigen 175 (PfEBA175) regions III-V, merozoite surface protein (MSP) 2 (PfMSP2), PfMSP3; infected erythrocyte antigen PfVAR2CSA (Duffy binding like (DBL) 5ε domain as it is recognized across geographically diverse isolates compared to other domains which are highly strain-specific38, 7G8 allelle), and P. vivax merozoite antigen (PvAMA1) was measured by high-throughput ELISA as previously described33.
Total IgG was also determined to P. falciparum merozoite antigens EBA 140 regions II and III-V (PfEBA140-RII and PfEBA140-RIII-V)39, reticulocyte binding protein homologue 2 (PfRh2)40 and P. vivax merozoite antigens PvMSP11941, and Duffy binding protein (PvDBP)42, P. falciparum-IE surface antigen PfDBLα (IT4var14 NTSB3-DBLa00.23)43 and the P. falciparum sporozoite protein (PfCSP)44 and gametocyte antigens (Pfs230)45, and antigens to tetanus toxoid, cytomegalovirus (CMV) and measles (PROSPECbio) using a Janus robotic platform but otherwise performed as described previously33. Antigens were coated on plates at 0.5 μg/mL and human sera were tested to each antigen at the following dilutions; PfDBLα, PfDBL5ε, Pfs230, PvAMA1 and measles at 1:250, PfMSP2, PfMSP3, PfEBA140-RII, PfEBA140-RIII-V, PfEBA175, PfRh2, PfCSP, tetanus toxoid and CMV at 1:500, PvMSP119 and PvDBP at 1:1000 and PfAMA1 at 1:2000 (Supplemental Table 1). Seropositivity was defined as an optical density (OD) greater than the mean plus three standard deviations of 17 negative controls (non-exposed Melbourne donors).
Statistical Analysis
Spearman’s rank correlation coefficients (ρ) were calculated to measure the association between antibody levels derived from maternal, cord and neonatal blood, and for those mothers with a malaria infection during pregnancy, between antibody levels and estimated gestational age of last exposure to malaria infection. To investigate potential predictors of neonatal antibody levels we used multivariable robust linear regression (to reduce the influence of multivariate model outliers) on loge transformed antibody levels. Variables of interest included maternal antibody levels (loge transformed), gravidity (primigravid, multigravida), chloroquine prophylaxis (chloroquine, placebo) and EGA at delivery in weeks (continuous). In addition a new variable was created from case-control status to represent species-specific exposure. We defined an “exposed case” as a woman who had been exposed during pregnancy to the species representing the antigen, so that P. falciparum antibodies could be related to P. falciparum exposure and P. vivax antibodies to P. vivax exposure. Each species-specific case may also have had infection with the other parasite species. In addition controls were divided into high and low responders which were selection criteria for inclusion in the immunological study33. Variables were investigated in two separate multivariable models for each antigen. The first model excluded maternal IgG levels at delivery to investigate whether Plasmodium spp. exposure, gestational age at delivery, gravidity or chloroquine prophylaxis are associated (either directly or indirectly via maternal IgG levels at delivery) with neonatal IgG levels. The second model included maternal IgG levels to investigate whether variables of interest were associated with transfer of maternal antibodies to the foetus. To investigate whether gestational age at delivery, gravidity or chloroquine prophylaxis modified the transfer of maternal antibodies to the foetus, an interaction term with maternal IgG levels and estimated gestational age (<40 weeks, 40–41 weeks, >41 weeks), or gravidity, or chloroquine prophylaxis were investigated. These multivariate analyses were also conducted on the ratio of neonatal/maternal antibodies in seropositive women. Sensitivity analysis was performed by running the model including and excluding the 7 preterm deliveries in the <40 weeks category. Coefficients were less than 10% different between each model therefore preterm deliveries data were included in the <40 weeks group rather than a separate category containing few observations. Final models included maternal IgG levels together with an interaction term with estimated gestational age with adjustments for gravidity, chloroquine prophylaxis and Plasmodium spp. exposure. Analyses were conducted with Stata version 13.0 (Stata Corporation, College Station, Texas, US).
Results
Maternal characteristics and birth outcomes of the 57 cases and 111 controls included in the current study are shown in Table 1. Briefly, the majority of women were multigravida (77.2% cases, 85.6% controls) and the median estimated gestational age at delivery was 40 weeks. In cases, 63.2% of women experienced a P. falciparum infection, and 57.9% experienced a P. vivax infection during pregnancy, the median number of infections for both species was one (Table 1).
Antibody levels were determined towards P. falciparum and P. vivax antigens in maternal, cord and neonatal samples (maternal seroprevalences are summarised in Table 1). In addition IgG to non-malarial antigens tetanus toxoid, CMV and measles were also determined. Both Plasmodium spp. antibody levels, and antibodies to non-malarial antigens, were highly correlated between maternal samples and cord (median [range] spearman ρ = 0.89 [0.68–0.94]) and neonatal samples (0.78 [0.57–0.93], Fig. 1). The absolute difference in antibody levels in mother and neonate samples was calculated within each mother-neonate pair and showed that neonate IgG levels were typically lower for Plasmodium spp. antigens, but for each antigen there was variation in the amount that was transferred to the neonate (Fig. 2A). Antibodies specific for measles were also lower in neonates, whereas levels of tetanus and CMV antibodies were comparable in maternal and neonate samples (Fig. 2). Analysis of maternal-foetal antibody ratios in seropositive women yielded similar findings (Fig. 2B).
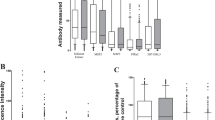
Scatterplots of maternal and neonatal antibody levels according to antigens representing (A) the surface of P. falciparum infected erythrocyte (PfEMP1 domains), (B) P. falciparum merozoite surface, (C) P. falciparum merozoite invasion ligands, (D) P. vivax merozoite, (E) P. falciparum sporozoite and gametocyte antigens, (F) other infectious diseases. Plasmodium spp. antibody levels, and antibodies to non-malarial antigens, were highly correlated between maternal and neonatal samples (median [range] spearman ρ = 0.78 [0.57–0.93]). Specific spearman ρ correlation results between maternal and neonate samples: PfVAR2CSA 0.77; PfDBLa 0.74; PfMSP2 0.78; PfMSP3 0.57; PfRh2 0.84; PfEBA175 0.91; PfEBA140-RII 0.93; PfEBA140-RIII-V 0.83; PfAMA1 0.84; PvDBP 0.78; PvAMA1 0.87; PvMSP1-19 0.68; Pfs230 0.75; PfCSP 0.75; Tetanus 0.67; CMV 0.84; Measles 0.75.
Box plot (median, interquartile range, range) of (A) the difference in total IgG between maternal and neonatal samples (mother-neonate), and (B) the ratio of IgG in neonate/mother samples in order of antigens representing i) P. falciparum infected erythrocyte, ii) P. falciparum merozoite surface, iii) P. falciparum merozoite apical (microneme and rhoptry), iv) P. vivax merozoite, v) P. falciparum transmission antigens, vi) infectious diseases. Line indicates no difference between maternal and neonatal IgG. There tends to be less neonatal IgG than maternal IgG to malaria antigens, which is not observed for CMV or Tetanus toxin antigens.
Species-specific infection and antibody transfer
To determine whether species-specific exposure during pregnancy resulted in species-specific antibody transfer we analysed the data by species of infection. Maternal Plasmodium species-specific exposure was associated with species-specific neonatal antibody levels, with higher neonatal P. falciparum antibody levels observed for those neonates whose mother experienced a P. falciparum episode in pregnancy (representative examples, Table 2). Similar associations were observed for P. vivax antibody levels in response to P. vivax infection, but were of lower magnitude than that observed for P. falciparum and only reached significance for PvAMA1 (Table 2). No associations with neonatal antibody levels were observed for gravidity, chloroquine prophylaxis and EGA at delivery, in line with our previous analyses of antibody responses in pregnancy33.
Due to the weekly parasitological assessment, we were able to assess how the timing of infections during pregnancy influenced the levels of neonatal antibodies and maternal transfer of antibodies in a subset of women who had been exposed to P. falciparum (n = 36) and P. vivax (n = 33). We examined the correlation of antibody levels in the neonate at delivery with estimated gestational age of the last exposure (P. falciparum - median [range] 23.7 weeks [7.6–40.9], P. vivax - 27.1 [12.2–40.3]). EGA at last P. falciparum infection was positively associated with antibody levels in the neonate, with higher levels seen in neonates whose mothers had been exposed later on in pregnancy (P. falciparum merozoite, spearman ρ median [range] 0.42 [0.33–0.66], PfVAR2CSA 0.69). There was no association between P. vivax antibody responses and EGA at the last P. vivax infection (ρ 0.19 [0.09–0.3]). There was no correlation between the time since maternal tetanus vaccination and neonatal tetanus antibodies at delivery (ρ −0.08).
Maternal-foetal transfer effect modifiers
To identify factors that modified the maternal-foetal transfer of antibody levels, log transformed maternal antibody levels, together with interaction terms between variables of interest, were added to the model predicting neonate antibody levels. Gravidity and chloroquine prophylaxis were not shown to modify the relationship between maternal and neonatal antibody levels (data not shown), but EGA was an effect modifier of the maternal-foetal transfer relationship. The increase in neonatal antibody levels per unit increase in maternal antibody levels were typically moderately greater in neonates born after 40 weeks gestation compared to neonates born before 40 weeks gestation, with varying levels of magnitude of this effect depending on antigen (Table 3). Analysis of maternal-foetal antibody ratios in seropositive women also demonstrated, increasing maternal-foetal transfer with increasing EGA (Supplemental Table 2).
Discussion
In a comprehensive study of the maternal-foetal transfer of P. falciparum and P. vivax antibody responses in an endemic low transmission area of South-East Asia, we demonstrated transfer of a broad range of both P. falciparum and P. vivax antibodies across the placenta during pregnancy. Anti-malarial IgG was slightly lower in neonates compared to mothers, but reached higher levels when gestation was longer. P. falciparum specific IgG was positively associated with recent P. falciparum infection, however P. vivax IgG was not as strongly linked with infection.
Antimalarial IgG levels were lower in neonates compared to mothers, a finding which has been reported in other malaria studies in high transmission areas21,24,46,47,48. Lower levels in neonates are potentially due to the saturation of neonatal Fc receptors which mediate the transfer of IgG across the syncytiotrophoblast3. Saturation of Fc receptors may be caused by maternal hypergammaglobulinemia (IgG > 15 g/L)3 which has been associated with reduced levels of anti-measles and anti-viral, but not tetanus, antibodies in the cord blood compared to the mother in several studies27,28,49,50. Similar findings were also observed in our study with lower level of anti-measles IgG observed in the neonate compared to the mother, but no differences in levels of anti-tetanus toxoid or anti-CMV. It is unclear what underpins differences in transfer according to antigen but similar to anti-measles antibody, the transfer of Plasmodium spp. antibody also appears impaired. Despite this impairment, our data would suggest that even in low transmission areas with prompt treatment of malaria, maternal IgG to malarial antigens is high and antimalarial antibodies are transferred to neonates at maximum capacity. Maternal-foetal transfer was dependent on EGA at delivery, with more maternal antibody transfer occurring at later stages of pregnancy. In healthy pregnancies (both term and preterm), neonatal total IgG is directly related to length of gestation3,18,51 with the maternal-foetal ratio increasing in the last four to six weeks suggesting an increase in active IgG transport during this time51,52. Our data provides evidence that increased transfer of anti-malarial antibodies also occurs in the last four weeks of pregnancy despite antibody levels being lower in the neonate at delivery.
The only maternal factor associated with neonatal antibody levels was exposure to Plasmodium spp. during pregnancy. Increases in P. falciparum-specific neonatal antibody levels were strongly associated with P. falciparum maternal infection during pregnancy. However increases in P. vivax antibody levels, with respect to P. vivax maternal exposure, were of lower magnitude. We have previously demonstrated in these women that P. falciparum merozoite and pregnancy-specific responses are boosted with each successive P. falciparum infection in pregnancy but P. vivax responses are not boosted in response to P. vivax infection33. This apparent lack of boosting is most likely due to the lower parasite densities observed in P. vivax infections during pregnancy in this cohort which may not be detected with microscopy33 or the presence of sub-microscopic infections from liver stage relapse (occasionally detected by microscopy)53 which result in relatively constant antibody concentrations. The ability to mount an antibody response upon Plasmodium spp. exposure during pregnancy has a knock-on effect on neonatal antibody levels, but how differential species-specific antibody responses translates to differential species-specific risk in the first six months is yet to be determined.
The weekly sampling for the presence of parasites also enabled us to accurately classify when exposure to Plasmodium spp. occurred during pregnancy, and investigate the association between timing of infection and neonatal antibody levels as previous studies of infant antibodies have only examined parasitaemia at delivery26,46,47. We found that antibody levels were higher in neonates born to women who had been infected more recently (particularly in the last trimester). Conversely, there was no association between timing of maternal tetanus immunization and anti-tetanus antibodies in the neonate. This may be due to differences in antibody longevity between tetanus and Plasmodium spp. antibodies. We have previously shown that antibody half-life is shorter for Plasmodium spp. antibodies (1–2 years) compared to tetanus antibodies (10–12 years) in this cohort33,51,52,54. The implications of the aforementioned increase in maternal-foetal antibody transfer in later stages of pregnancy and short-lived responses are that foetuses of mothers that experience malaria exposure early on in pregnancy, particularly those before four months gestation when transfer begins16, may acquire lower levels of protective immunity compared to those with mothers infected in the latter stages of pregnancy. This finding suggests that if future malaria vaccine programs consider administering the vaccine to pregnant women then the third trimester may be the optimal time of vaccination to boost antibody levels in the infant.
Antibody transfer may be affected by drug regimens designed to protect women from malaria during pregnancy. Women included in the current study were participating in a randomised controlled trial of chloroquine prophylaxis34. Chloroquine has been proposed to be immunosuppressive55 but for our data no association between chloroquine and infant antibody levels or maternal-foetal transfer was observed which is in line with a previous study in Tanzania46. Chloroquine would most likely act indirectly on infant antibody levels by reducing exposure to Plasmodium spp. antigens. In the trial chloroquine prophylaxis successfully prevented P. vivax, but not P. falciparum infection during pregnancy34. Other studies of intermittent preventative treatment of malaria in pregnancy (IPTp) have shown that IPTp can reduce pregnancy-specific antibody levels in pregnant women living in malaria endemic areas of Africa56,57. A large reduction in antibody levels due to IPTp could result in lower antibody levels in the infant, however in our study it appears that transfer is at maximum capacity even in women who were not exposed to malaria during pregnancy. The impact of IPTp on malaria risk in infancy is unknown.
This study was designed to assess anti-malarial antibody transfer in women in a low transmission setting. These women are also quickly treated on the presence of Plasmodium in a blood smear. Rapid treatment upon diagnosis of Plasmodium spp. infection may mean that high parasitaemias and placental malaria are reached less often and consequently lower levels of antibody are observed in our study population than in both high transmission settings and populations in low transmission settings who do not receive intensive screening and treatment. In this setting there are low numbers of premature births (<37 weeks, n = 7), and low prevalence of placental malaria (histopathological placental parasite positivity 4.3% (3.2–5.6))29. It is possible that there are larger differences in maternal-foetal transfer in higher transmission areas where preterm birth and placental malaria are more frequent. It has also been shown that MSP1-42 and tetanus toxoid antigens can cross the placenta into the foetal circulation as immune complexes with IgG58,59. This raises the possibility that not all IgG is available for binding in an ELISA assay and we may have underestimated the level of maternal-foetal transfer. In this study we measured total IgG but the IgG subclass of transferred antibodies may also be important. Neonatal Fc receptors preferentially transport IgG1, followed by IgG3, and IgG23. The predominant subclasses against Plasmodium spp. antigens are IgG1 and IgG3 that mediate reductions in parasitemia by a number of mechanisms (e.g. opsonic phagocytosis, growth/invasion inhibition), but different Plasmodium spp. antigens have different IgG1/IgG3 bias60. Only one study has investigated functional immunity and shown that maternally transferred antibodies (MSP1-19) have growth-inhibitory activity61 but other mechanisms also need to be investigated. Our finding that anti-PfEMP1 antibodies are transferred may also suggest an ability for transferred IgG to protect the neonate from severe malaria potentially via parasite clearance or adherence inhibition62,63,64. Further studies of functional immunity and clinical protection are warranted to elucidate the role that maternally transferred antibodies have in protection from malaria in infancy.
The clinical relevance of the antimalarial antibodies detected in infants is debated with conflicting evidence on the protective effects of passively transferred P. falciparum antibodies in the first year of life2,21,26,47,65,66,67. There are many possible reasons for these conflicting findings, such as differences in study design, host and environmental factors and malaria transmission. Rates of placental malaria may also be important because the presence of placental malaria is associated with foetal priming to blood stage antigens68,69,70. Importantly it is currently unclear how long maternal antibodies persist in the infant. Most transplacentally transferred IgG has a half-life of 21 days and is more-or-less undetectable by six months of age61. In addition, species-specific differences in potential protection due to antibody transfer are unknown. Exposure to P. falciparum led to increased levels of P. falciparum antibodies in the infant, but the effect of P. vivax exposure on P. vivax antibody levels was not as marked. How this translates to the differential risk of P. falciparum and P. vivax during the first six months of life is unknown and further studies are warranted.
Conclusions
In a low transmission area in Thailand, we demonstrated the effective maternal-foetal transfer of both P. falciparum and P. vivax antibodies in treated malaria episodes. Infant antibody levels were influenced by maternal exposure to infection, and timing of those exposures. Functional assays are needed to determine the validity of the passive immunity hypothesis that maternal transfer of antibodies leads to protection from disease in infants in the first months of life. If this is indeed the case this work indicates that gestational age is important for antibody transfer, and timing of vaccination during pregnancy would also be important. If protective immunity can be gained from the mother, studies to determine the length of time that infants remain protected will inform the optimal timing of vaccination for infants.
Additional Information
How to cite this article: Charnaud, S. C. et al. Maternal-foetal transfer of Plasmodium falciparum and Plasmodium vivax antibodies in a low transmission setting. Sci. Rep. 6, 20859; doi: 10.1038/srep20859 (2016).
References
World Health Organization. World Malaria Report 2014. 1–242 (World Health Organization, 2015, 2014). at < http://www.who.int/malaria/publications/world_malaria_report_2014/en/> Date of Access: 18/12/2015.
Riley, E. M., Wagner, G. E., Akanmori, B. D. & Koram, K. A. Do maternally acquired antibodies protect infants from malaria infection? Parasite Immunol. 23, 51–59 (2001).
Palmeira, P., Quinello, C., Silveira-Lessa, A. L., Zago, C. A. & Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 985646 (2012).
Marsh, K. & Kinyanjui, S. Immune effector mechanisms in malaria. Parasite Immunol. 28, 51–60 (2006).
Cohen, S., Butcher, G. A. & Crandall, R. B. Action of malarial antibody in vitro . Nature 223, 368–371 (1969).
Brown, G. V., Anders, R. F., Mitchell, A. J. & Heywood, P. F. Target antigens of purified human immunoglobulins which inhibit growth of Plasmodium falciparum in vitro . Nature 297, 591–593 (1982).
Chan, J.-A., Fowkes, F. J. I. & Beeson, J. G. Surface antigens of Plasmodium falciparum-infected erythrocytes as immune targets and malaria vaccine candidates. Cell. Mol. Life Sci. 71, 3633–3657 (2014).
Mueller, I. et al. Natural acquisition of immunity to Plasmodium vivax: epidemiological observations and potential targets. Adv. Parasitol. 81, 77–131 (2013).
Fowkes, F. J. I., Richards, J. S., Simpson, J. A. & Beeson, J. G. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 7, e1000218 (2010).
Cutts, J. C. et al. Immunological markers of Plasmodium vivax exposure and immunity: a systematic review and meta-analysis. BMC Med 12, 150 (2014).
Osier, F. H. A. et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect. Immun. 76, 2240–2248 (2008).
McLean, A. R. D., Ataide, R., Simpson, J. A., Beeson, J. G. & Fowkes, F. J. I. Malaria and immunity during pregnancy and postpartum: a tale of two species. Parasitology 142, 999–1015 (2015).
Beeson, J. G. & Duffy, P. E. The immunology and pathogenesis of malaria during pregnancy. Curr. Top. Microbiol. Immunol. 297, 187–227 (2005).
Salanti, A. et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 200, 1197–1203 (2004).
Fried, M., Nosten, F., Brockman, A., Brabin, B. J. & Duffy, P. E. Maternal antibodies block malaria. Nature 395, 851–852 (1998).
Toivanen, P., Mäntyjärvi, R. & Hirvonen, T. Maternal antibodies in human foetal sera at different stages of gestation. Immunology 15, 395–403 (1968).
Linnet-Jepson, P., Galatius-Jensen, F. & Hauge, M. On the inheritance of the Gm serum group. Acta Genet Stat Med 8, 164–196 (1958).
Simister, N. E., Story, C. M., Chen, H. L. & Hunt, J. S. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur. J. Immunol. 26, 1527–1531 (1996).
Brambell, F. W. R. The transmission of passive immunity from mother to young. (North-Holland Pub. Co., 1970).
Rasheed, F. N. et al. Relationships between maternal malaria and malarial immune responses in mothers and neonates. Parasite Immunol. 17, 1–10 (1995).
Riley, E. M. et al. Lack of association between maternal antibody and protection of African infants from malaria infection. Infect. Immun. 68, 5856–5863 (2000).
Williams, A. I. & McFarlane, H. Distribution of malarial antibody in maternal and cord sera. Arch. Dis. Child. 44, 511–514 (1969).
Logie, D. E., McGregor, I. A., Rowe, D. S. & Billewicz, W. Z. Plasma immunoglobulin concentrations in mothers and newborn children with special reference to placental malaria: Studies in the Gambia, Nigeria, and Switzerland. Bull. World Health Organ. 49, 547–554 (1973).
Desowitz, R. S., Elm, J. & Alpers, M. P. Plasmodium falciparum-specific immunoglobulin G (IgG), IgM, and IgE antibodies in paired maternal-cord sera from east Sepik Province, Papua New Guinea. Infect. Immun. 61, 988–993 (1993).
Brabin, B. J. et al. The sick placenta-the role of malaria. Placenta 25, 359–378 (2004).
Moro, L. et al. Malaria and HIV infection in Mozambican pregnant women are associated with reduced transfer of antimalarial antibodies to their newborns. J. Infect. Dis. 211, 1004–1014 (2015).
Okoko, B. J. et al. The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural West African population. J. Infect. Dis. 184, 627–632 (2001).
de Moraes-Pinto, M. I. et al. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch. Dis. Child. Fetal Neonatal Ed. 79, F202–5 (1998).
McGready, R. et al. The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. Am. J. Trop. Med. Hyg. 70, 398–407 (2004).
Nosten, F., Kuile, ter, F., Maelankirri, L., Decludt, B. & White, N. J. Malaria during pregnancy in an area of unstable endemicity. Trans. R. Soc. Trop. Med. Hyg. 85, 424–429 (1991).
Nosten, F. et al. Effects of Plasmodium vivax malaria in pregnancy. Lancet 354, 546–549 (1999).
Luxemburger, C. et al. Effects of malaria during pregnancy on infant mortality in an area of low malaria transmission. Am. J. Epidemiol. 154, 459–465 (2001).
Fowkes, F. J. I. et al. New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J. Infect. Dis. 206, 1612–1621 (2012).
Villegas, L. et al. Chloroquine prophylaxis against vivax malaria in pregnancy: a randomized, double-blind, placebo-controlled trial. Trop. Med. Int. Health 12, 209–218 (2007).
Dubowitz, L. M. S. & Dubowitz, V. Gestational age of the newborn: a clinical manual. (Addison Wesley Publishing Company, 1977).
Haataja, L. et al. A new approach for neurological evaluation of infants in resource-poor settings. Ann Trop Paediatr 22, 355–368 (2002).
Moore, K. A. et al. Estimating Gestational Age in Late Presenters to Antenatal Care in a Resource-Limited Setting on the Thai-Myanmar Border. PLoS ONE 10, e0131025 (2015).
Travassos, M. A. et al. Differential recognition of terminal extracellular Plasmodium falciparum VAR2CSA domains by sera from multigravid, malaria-exposed Malian women. Am. J. Trop. Med. Hyg. 92, 1190–1194 (2015).
Richards, J. S. et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J. Immunol. 191, 795–809 (2013).
Reiling, L. et al. Evidence that the erythrocyte invasion ligand PfRh2 is a target of protective immunity against Plasmodium falciparum malaria. J. Immunol. 185, 6157–6167 (2010).
Chen, J.-H. et al. Measurement of naturally acquired humoral immune responses against the C-terminal region of the Plasmodium vivax MSP1 protein using protein arrays. Parasitol. Res. 109, 1259–1266 (2011).
Li, J. & Han, E.-T. Dissection of the Plasmodium vivax reticulocyte binding-like proteins (PvRBPs). BBRC 426, 1–6 (2012).
Avril, M. et al. A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells. PNAS 109, E1782–90 (2012).
Tsuboi, T. et al. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect. Immun. 76, 1702–1708 (2008).
Tachibana, M. et al. N-terminal prodomain of Pfs230 synthesized using a cell-free system is sufficient to induce complement-dependent malaria transmission-blocking activity. Clin. Vaccine Immunol. 18, 1343–1350 (2011).
Mutabingwa, T. K., Malle, L. N., de Geus A & Oosting, J. Malaria chemosuppression in pregnancy. I. The effect of chemosuppressive drugs on maternal parasitaemia. Trop Geogr Med 45, 6–14 (1993).
Deloron, P. et al. Isotypic analysis of maternally transmitted Plasmodium falciparum-specific antibodies in Cameroon, and relationship with risk of P. falciparum infection. Clin. Exp. Immunol. 110, 212–218 (1997).
Campbell, C. C., Martinez, J. M. & Collins, W. E. Seroepidemiological studies of malaria in pregnant women and newborns from coastal El Salvador. Am. J. Trop. Med. Hyg. 29, 151–157 (1980).
de Moraes-Pinto, M. I. et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J. Infect. Dis. 173, 1077–1084 (1996).
Okoko, B. J. et al. Influence of placental malaria infection and maternal hypergammaglobulinaemia on materno-foetal transfer of measles and tetanus antibodies in a rural west African population. J Health Popul Nutr 19, 59–65 (2001).
Pitcher-Wilmott, R. W., Hindocha, P. & Wood, C. B. The placental transfer of IgG subclasses in human pregnancy. Clin. Exp. Immunol. 41, 303–308 (1980).
Saji, F., Samejima, Y., Kamiura, S. & Koyama, M. Dynamics of immunoglobulins at the feto-maternal interface. Rev. Reprod. 4, 81–89 (1999).
Adekunle, A. I. et al. Modeling the Dynamics of Plasmodium vivax Infection and Hypnozoite Reactivation In Vivo . PLoS Negl Trop Dis 9, e0003595 (2015).
Fowkes, F. J. I., McGready, R., Johnstone-Robertson, S., Nosten, F. & Beeson, J. G. Antibody boosting and longevity following tetanus immunization during pregnancy. Clin Infect Dis 56, 749–750 (2013).
Bygbjerg, I. C. & Flachs, H. Effect of chloroquine on human lymphocyte proliferation. Trans. R. Soc. Trop. Med. Hyg. 80, 231–235 (1986).
Aitken, E. H. et al. Antibody to P. falciparum in pregnancy varies with intermittent preventive treatment regime and bed net use. PLoS ONE 7, e29874 (2012).
Staalsoe, T. et al. Intermittent preventive sulfadoxine-pyrimethamine treatment of primigravidae reduces levels of plasma immunoglobulin G, which protects against pregnancy-associated Plasmodium falciparum malaria. Infect. Immun. 72, 5027–5030 (2004).
May, K. et al. Antibody-dependent transplacental transfer of malaria blood-stage antigen using a human ex vivo placental perfusion model. PLoS ONE 4, e7986 (2009).
Malek, A., Sager, R. & Schneider, H. Transport of proteins across the human placenta. Am. J. Reprod. Immunol. 40, 347–351 (1998).
Stanisic, D. I. et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect. Immun. 77, 1165–1174 (2009).
Wilson, P. T., Malhotra, I., Mungai, P., King, C. L. & Dent, A. E. Transplacentally transferred functional antibodies against Plasmodium falciparum decrease with age. Acta Trop. 128, 149–153 (2013).
Bull, P. C. et al. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4, 358–360 (1998).
Chan, J.-A. et al. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J. Clin. Invest. 122, 3227–3238 (2012).
Yipp, B. G. et al. Recombinant PfEMP1 peptide inhibits and reverses cytoadherence of clinical Plasmodium falciparum isolates in vivo . Blood 101, 331–337 (2003).
Branch, O. H. et al. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kiloDalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am. J. Trop. Med. Hyg. 58, 211–219 (1998).
Achidi, E. A. et al. Lack of association between levels of transplacentally acquired Plasmodium falciparum-specific antibodies and age of onset of clinical malaria in infants in a malaria endemic area of Nigeria. Acta Trop. 61, 315–326 (1996).
Høgh, B., Marbiah, N. T., Burghaus, P. A. & Andersen, P. K. Relationship between maternally derived anti-Plasmodium falciparum antibodies and risk of infection and disease in infants living in an area of Liberia, west Africa, in which malaria is highly endemic. Infect. Immun. 63, 4034–4038 (1995).
Malhotra, I. et al. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med. 6, e1000116 (2009).
Fievet, N. et al. Malaria cellular immune responses in neonates from Cameroon. Parasite Immunol. 18, 483–490 (1996).
Metenou, S., Suguitan, A. L., Long, C., Leke, R. G. F. & Taylor, D. W. Fetal immune responses to Plasmodium falciparum antigens in a malaria-endemic region of Cameroon. J. Immunol. 178, 2770–2777 (2007).
Acknowledgements
We thank Julia McGuire for initial analyses. We thank Joe Smith for DBL5 and DBL-α antigens, Robin Anders for MSP2 antigen and Annie Mo for EBA175 antigen. This work was supported by the National Health and Medical Research Council of Australia (project grant 1049213, fellowships to FJIF and JGB, Infrastructure for Research Institutes Support Scheme Grant), Australian Research Council (Future Fellowship to FJIF and JGB), and Victorian State Government Operational Infrastructure Support grant. This research was supported in part by the Intramural Research Program of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.C.C. prepared tables and figures and analysed data using an analysis plan designed by F.J.I.F. and J.A.S. F.J.I.F., A.H.-C., R.P., A.G., J.S.R., J.G.B., P.G., C.L., T.T. and D.L.N. contributed to experimental work. R.M., K.C., M.P. and F.N. performed clinical work. F.J.I.F. wrote the first draft of the manuscript. All authors interpreted data and contributed to writing of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Charnaud, S., McGready, R., Herten-Crabb, A. et al. Maternal-foetal transfer of Plasmodium falciparum and Plasmodium vivax antibodies in a low transmission setting. Sci Rep 6, 20859 (2016). https://doi.org/10.1038/srep20859
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20859
This article is cited by
-
Community-based molecular and serological surveillance of subclinical malaria in Myanmar
BMC Medicine (2021)
-
Interaction between maternally derived antibodies and heterogeneity in exposure combined to determine time-to-first Plasmodium falciparum infection in Kenyan infants
Malaria Journal (2019)
-
Housing type and risk of malaria among under-five children in Nigeria: evidence from the malaria indicator survey
Malaria Journal (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.