Abstract
Selenium binding protein 1 (SELENBP1) messenger RNA (mRNA) has previously been shown to be upregulated in the brain and blood from subjects with schizophrenia. We aimed to validate these findings in a new cohort using real-time PCR in Brodmann’s Area (BA) 9, and to determine the disease specificity of increased SELENBP1 expression by measuring SELENBP1 mRNA in subjects with major depressive disorder and bipolar disorder. We then extended the study to include other cortical regions such as BA8 and BA44. SELENBP1 mRNA was higher in BA9 (P=0.001), BA8 (P=0.003) and BA44 (P=0.0007) from subjects with schizophrenia. Conversely, in affective disorders, there was no significant difference in SELENBP1 mRNA in BA9 (P=0.67), suggesting that the upregulation may be diagnosis specific. Measurement of SELENBP1 protein levels showed that changes in mRNA did not translate to changes in protein. In addition, chronic treatment of rats with antipsychotics did not significantly affect the expression of Selenbp1 in the cortex (P=0.24). Our data show that elevated SELENBP1 transcript expression is widespread throughout the prefrontal cortex in schizophrenia, and confirm that this change is a consistent feature of schizophrenia and not a simple drug effect.
Similar content being viewed by others
Introduction
Levels of selenium (Se) binding protein 1 (SELENBP1) expression have been shown to be higher in the dorsolateral prefrontal cortex from subjects with schizophrenia,1, 2 with the second study also showing higher levels of SELENBP1 messenger RNA (mRNA) in the dorsolateral prefrontal cortex from subjects with bipolar disorder (BP) who had psychosis,2 leading the authors to suggest that increased SELENBP1 expression may be associated with a psychotic state rather than a diagnostic criterion. Although the role of SELENBP1 in the central nervous system (CNS) remains largely unknown, there is evidence to suggest it may be involved in neurite growth and remodelling.3 Given that dendritic and synaptic proteins are altered in BP and schizophrenia,4, 5, 6, 7, 8 SELENBP1 may have a role in the aetiology of these disorders.
To challenge this hypothesis, we investigated whether SELENBP1 is differentially expressed in the cortex from subjects with schizophrenia, BP or major depressive disorder (MDD). We also addressed the extent of changes in cortical SELENBP1 expression by measuring mRNA levels in multiple cortical regions, determined whether a two-marker haplotype of SELENBP1, previously shown to be nominally associated with risk for schizophrenia (rs10788804, allele A; and rs2800953, allele A or G),9 influenced brain SELENBP1 expression, and whether changes in SELENBP1 mRNA translated to changes in protein levels. Finally, we determined whether CNS Selenbp1 expression was altered by chronic exposure to antipsychotic drugs to determine whether changes in the expression of that gene could be involved in their mechanism of action.
Materials and methods
Human post-mortem tissue collection
Consent for this study was obtained from the Ethics Committee of the Victorian Institute of Forensic Medicine and the Mental Health Research and Ethics Committee of Melbourne Health. All tissues were obtained from the Victorian branch of the Australian Brain Bank Network held at the Florey Institute of Neuroscience and Mental Health. Psychiatric diagnoses were made according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria10 by consensus between two senior psychiatrists and a psychologist after an extensive case history review using the Diagnostic Instrument for Brain Studies.11, 12 Informed consent for each tissue collection was obtained from the donor or senior next of kin. All subjects were coded to remove subject identities.
Total RNA and protein was obtained from tissue excised from Brodmann’s Area (BA) 9 (lateral surface of the frontal lobe, including the middle frontal gyrus superior to the inferior frontal sulcus), BA8 (primarily the superior frontal gyrus extending from the cingulate sulcus on the medial surface to the middle frontal gyrus laterally, bounded caudally by the agranular frontal region (BA6) and ventrally by the granular frontal region (BA9)) and BA44 (opercular region of the inferior frontal gyrus, bounded rostrally by the ascending limb of the lateral sulcus and caudally by the inferior pre-central sulcus), according to Brodmann’s criteria, in a cohort of 30 subjects with schizophrenia and 30 control subjects with no history of psychiatric illness, matched closely for age, sex, post-mortem interval and brain pH (schizophrenia cohort; Table 1). Total RNA was also extracted from BA9 from a further 10 subjects with MDD, 10 subjects with BP and 9 control subjects (affective disorder cohort; Table 1). These control subjects were different from those in the schizophrenia cohort in order to match closely for age, sex and brain pH (Table 1). Our previous studies have shown that cohorts of these sizes can detect significant differences of 10% or 20%, respectively, in the mean value of experimental variables.13, 14 For genotyping, DNA was extracted from cerebellum tissue taken from the same 30 schizophrenia and 30 control subjects as the schizophrenia cohort. Demographic data of all individuals are provided in Supplementary Table 1. Investigators were kept blinded to diagnosis allocations during sample preparation and subsequent experimentation.
Antipsychotic-treated rat tissue
Tissue was utilised from male Sprague–Dawley rats treated for a previous study.15 Ethics approval was obtained from the University of Melbourne Animal Ethics committee under the Animal Research Act 1985. Briefly, 6-week-old male rats (initially100–150 g), obtained from the breeding colony at Melbourne University, were randomly assigned to treatment groups and treated for a period of 12 months with either vehicle (H2O), 1.0 mg kg−1 per day haloperidol, 10 mg kg−1 per day chlorpromazine or 10 mg kg−1 per day thioridazine in the drinking water, a well-established method for long-term delivery of antipsychotics,16, 17, 18, 19, 20 in groups of five, with ad libitum access to food and water, and maintained at 19±4 °C on a 12-h light/dark cycle. The volume of water consumed was monitored daily to ensure adequate dosing and was renewed every 2 days to minimise any effects of drug degradation. We have previously shown that animal cohorts of this size allow significant differences in CNS expression of 11% or greater to be readily identified.15 These drugs were chosen as they were the most commonly recorded antipsychotics used by the subjects in our schizophrenia cohort, and doses were chosen based on previous studies to approximate clinically comparable dopamine D2 receptor occupancy.21, 22, 23 This route of drug administration is more similar to the most common way of delivering the drugs in the clinical situation; it has the added benefit of causing little stress to the animals. At the completion of the treatment period, all animals received drug-free drinking water for 72 h before being culled. The brains were rapidly removed, frozen in isopentane (Sigma-Aldrich, St Louis, MO, USA) on dry ice and stored at –70 °C. Brains were coded to render the investigator blind to grouping during subsequent testing. For measuring Selenbp1 mRNA, total RNA was extracted from a tissue section ~3mm thick excised from the right hemisphere immediately posterior to bregma.24
RNA extraction and first-strand complementary DNA synthesis
Total RNA was isolated from ~100 mg frozen tissue samples using 1.0 ml TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA was treated with DNase I (Life Technologies) at 37 °C for 25–30 min, then purified by phenol/chloroform extraction and stored at −80 ºC. RNA quantity and quality were analysed by spectrophotometer readings. DNA contamination was checked by PCR using primers specific for genomic DNA. RNA integrity numbers were determined using an Agilent 2100 bioanalyser (Agilent Technologies, Santa Clara, CA, USA).
First-strand complementary DNA was synthesised from 2 μg RNA using 100 units Moloney Murine Leukemia Virus reverse transcriptase with 2.5 μM random decamers and 2.5 μM oligo dT primers (Life Technologies), 0.5 mM of each dNTP and 20 units RNase inhibitor in 1 × reverse transcriptase buffer (50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2 and 5 mM dithiothreitol) in a final volume of 20 μl. The reaction was incubated at 44 °C for 1 h then inactivated at 92 °C, and the product was stored at −20 °C.
Real-time PCR
Complementary DNA was used as a template for real-time PCR performed with SYBR green detection using a Bio-Rad iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules CA, USA). Reactions were performed in triplicate in 50 μl volume containing complementary DNA diluted 1:125, 0.4 nM primers and 1 × IQ SYBR green supermix (Bio-Rad Laboratories), with cycling conditions of 95 °C for 3 min, 40 cycles of 30 s each at 95, 57 and 72 °C, followed by a melt curve. Quantitative PCR data were acquired using iQ5 optical system 2.0 software (Bio-Rad Laboratories).
In human tissue, normalised relative quantities of SELENBP1 mRNA were determined relative to the geometric mean of three reference genes, peptidylprolyl isomerase A (PPIA), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and alpha synuclein (SNCA), that were chosen from nine genes tested in post-mortem CNS tissue as the most stable by geNorm analysis25 (M=1.398, 1.386 and 1.407, respectively). In rat tissue, normalised relative quantities of Selenbp1 mRNA were calculated using reference genes succinate dehydrogenase complex, subunit A, flavoprotein (Sdha), mitogen-activated protein kinase kinase 5 (Map2k5) and mitogen-activated protein kinase 6 (Mapk6). See Supplementary Table 2 for primer sequences. All amplicons were confirmed by sequencing before undertaking quantitative PCR. The relative quantities of the reference genes were not different between the analysis groups, and therefore were suitable reference genes for the present study.
Genotyping
Approximately 25 mg of frozen CNS tissue was homogenised using the Minilys homogeniser Precellys Minilys homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France) in 80 μl phosphate-buffered saline by agitating twice for 30 s at a velocity of 5000 r.p.m., with incubation on ice for 30 s between agitations. DNA was then extracted using the QIAamp DNA Mini kit (Qiagen, Hildon, Germany) according to the manufacturer’s instructions. The quality and concentration of the DNA was assessed by NanoDrop (Thermo Scientific, Waltham, MA, USA).
Samples were genotyped at single-nucleotide polymorphisms (SNPs) rs10788804 and rs2800953 with the Sequenom MassARRAY MALDI-TOF genotyping system (Sequenom, San Diego, CA, USA) using Sequenom iPLEX Gold chemistries, according to manufacturer’s instructions. Primer sequences are given in Supplementary Table 2. Data analysis was performed in a semi-automated manner using the Typer 4.0 Analyser Software (Sequenom). All genotype calls not assessed as ‘conservative’ by the analysis programme were manually checked and discarded if a clear call could not be made.
Western blotting
Homogenates were prepared at 5% (w/v) in 10 mM Tris (pH 7.4) containing 1% (w/v) SDS and 1 mM fresh sodium orthovanadate (Sigma-Aldrich), and the protein concentrations were measured using DC Protein Assay (Bio-Rad Laboratories). Twenty-five micrograms total protein of each sample were loaded in duplicate onto a polyacrylamide gel (4% stacking gel and 10% running gel) and separated by electrophoresis (150 V constant), then transferred (100 V constant, 1 h) onto a Hybond-ECL nitrocellulose membrane (GE Health Life Sciences, North Shore, NSW, Australia). Equal protein loading and transfer were confirmed by staining with 0.1% ponceau S in 3% trichloroacetic acid. Nitrocellulose membranes were then blocked in 5% non-fat milk powder/Tris-buffered saline with 0.1% Tween-20 (Sigma-Aldrich) for 1 h at room temperature and then incubated with mouse anti-SELENBP1 antibody (M061-3; MBL International, Woburn, MA, USA) at 4 °C overnight at 1/2000 dilution, followed by goat anti-mouse secondary antibody conjugated to horseradish peroxidase (Dako, Glostrup, Denmark) at 1/2000 for 1 h at room temperature. Antigenic bands were visualised using Pierce Supersignal West Pico chemiluminescent substrate (Thermo Scientific) and the image was captured with a Kodak 440 CF imaging system (Eastman Kodak, Rochester, NY, USA). The antibody specificity was confirmed by the presence of a single band in human CNS tissue homogenate at the known molecular weight of 56 kDa, and overexpression in cell lysate from SH-SY5Y cells transiently transfected with human SELENBP1 (Figure 1a).
SELENBP1 protein in schizophrenia. (a) Western blot of SELENPB1 expression in SH-SY5Y cells transfected with human SELENBP1 (lane 1, 5 μg and lane 2, 10 μg protein) and human central nervous system (CNS) tissue (lane 3, 20 μg and lane 4, 30 μg protein). (b) SELENBP1 protein expression in BA9, BA44 and BA8 from subjects with schizophrenia (Scz; ▴) and control (Cntrl; o). Error bars represent mean±s.e.m. ***P<0.001, two-way analysis of variance.
To control for inter-blot variation, an internal control membrane sample, prepared from the cerebellum of a human subject that was not part of the cohorts used, was run in 12 wells on two gels to establish intra- and inter-blot variation for SELENBP1 levels.26 The internal control was run in duplicate on every gel and gels were imaged so that the optical density of this sample fell within the mean±1 s.d. obtained from the initial two gels. The density of SELENBP1 protein in each sample was then expressed as a ratio to the internal control.
Statistical analysis
Data from subjects and rats were grouped according to diagnosis or treatment with the identity of the groups kept blind during analyses. Demographic data of the cohorts were analysed by Student’s t-test or one-way analysis of variance across the diagnostic groups for continuous variables, which were all normally distributed, and χ2-test for non-continuous variables. Correlations between experimental data and continuous potential confounding factors were determined by Pearson correlation. Owing to the small cohort size, of the relationships that reached significance (P<0.05), only those with a r2 value of more than 0.49 were considered a strong correlation27 for further analysis by analysis of covariance, provided the factor was not different between the groups.28 Normality of the data distributions were assessed using D’Agostino and Pearson omnibus normality tests for the human data and Kolmogorov-Smirnov normality tests for the rat data. Variance in SELENPB1 mRNA with diagnosis or treatment was analysed using Student’s t-test or one-way analysis of variance. These parametric tests were chosen due to their low sensitivity to deviations from normality and low false-positive rates.29, 30, 31 To correct for multiple testing in human tissue, Bonferroni corrections were applied, resulting in P<0.013, considered statistically significant (analysis performed in schizophrenia cohort in three regions and in affective disorder cohort in one region; n=4). Two-way analysis of variance was used to analyse variation in SELENBP1 protein, with diagnosis and region as primary variables, and to assess SNP × mRNA interactions using diagnosis and genotype or allele as primary variables. All analyses were performed using GraphPad Prism version 5.01 (GraphPad Software, San Diego, CA, USA). Values are represented as mean±s.e.m.
Results
Demographic data
Schizophrenia
There were no significant differences in mean age (P=0.91), post-mortem interval (P=0.48), RNA integrity number (P=0.52 BA9, P=0.92 BA44 and P=0.74 BA8), brain hemisphere (χ21=2.07, P=0.15) or sex ratio (χ21=0.0, P=1) between subjects with schizophrenia and controls (Table 1). There was a significant difference in the number of subjects that died by suicide (χ21=9.23, P=0.002), thus SELENBP1 data were analysed comparing non-suicide with suicide completers. There was significant variation in brain pH between schizophrenia and control (P=0.04), rendering this an irresolvable confound; however, the experimental data showed no strong correlation with pH or any other potential confounds (r2<0.17; Supplementary Tables 3 and 4), indicating these factors would not affect the outcome.
Affective disorders
There were no significant differences in age (F2,28=0.11, P=0.90), post-mortem interval (F2,28=2.47, P=0.10), RNA integrity number (F2,27=1.40, P=0.26) or sex ratio (χ22=0.11, P=0.94) between groups, and duration of illness was not different between BP and MDD (P=0.62; Table 1). There was significant variance in the rate of suicide (χ22=14.2, P<0.001). pH was higher in MDD compared with control (F2,28=5.38, P=0.01; Table 1); however, it showed no strong correlation with SELENBP1 mRNA (r2=0.24; Supplementary Table 3). All other variables also showed no strong correlation with the experimental data (Supplementary Table 3).
SELENBP1 mRNA in schizophrenia and affective disorders
Schizophrenia
Levels of mRNA for individual reference genes varied between cortical region (F2,531=47.63, P<0.0001; not shown), and therefore each brain region was analysed individually.
The expression data did not meet normality criteria in the control group in BA9 or BA8 or in the schizophrenia group in BA44. In BA9, SELENBP1 mRNA was significantly higher in subjects with schizophrenia (4.20±0.47) compared with control (2.36±0.25; t58=3.47, P=0.001; Figure 2a). SELENBP1 mRNA was also higher in subjects with schizophrenia in BA44 (2.01±0.16 schizophrenia, 1.25±0.15 control; t58=3.57, P=0.0007; Figure 2b) and BA8 (1.77±0.17 schizophrenia, 1.04±0.15 control; t58=3.15, P=0.003; Figure 2c).
SELENBP1 messenger RNA (mRNA) in schizophrenia and affective disorders. SELENBP1 mRNA expression in (a) BA9, (b) BA44 and (c) BA8 from subjects with schizophrenia (Scz; ▴) and control (Cntrl; O), as well as in (d) BA9 from subjects with major depressive disorder (MDD; ♦), bipolar disorder (BP; ▪) and control (□). Error bars represent mean±s.e.m. **P<0.01 and ***P<0.001; t-test.
Comparing suicide completers with non-suicide subjects showed no significant differences in SELENBP1 mRNA (BA9 t58=1.97, P=0.053; BA44 t58=0.17, P=0.17; BA8 t58=0.13, P=0.90; Supplementary Figure 1, left); therefore, suicide was unlikely to affect the results. Removing the control subjects and comparing suicide with non-suicide within the diagnosis of schizophrenia also showed no significant difference (BA9 t28=0.63, P=0.53; BA44 t28=0.12, P=0.91; BA8 t28=1.13, P=0.27; Supplementary Figure 1, right).
Affective disorders
To determine the diagnostic specificity of mRNA changes observed in schizophrenia, SELENBP1 mRNA was measured in BA9 from subjects with MDD and BP. Data were not normally distributed in MDD and control. There was no significant variance in SELENBP1 mRNA with diagnosis (1.64±0.68 MDD, 1.70±0.31 BP, 2.74±1.16 control; F2,27=0.55, P=0.54; Figure 2d).
SELENBP1 mRNA was not different between suicide completers and non-suicide subjects, with or without controls included (t27=0.63, P=0.53 and t18=0.07, P=0.95, respectively; data not shown).
SELENBP1 protein in schizophrenia
The data distribution did not reach normality criteria in the schizophrenia group in BA9 and BA44 or in the control group in BA8. Two-way analysis of variance of SELENBP1 protein showed variance with region (F2,174=13.76, P<0.0001), but not diagnosis (F1,174=2.53, P=0.11; Figure 1b), and no interaction between the two variables (F2,174=0.30, P=0.74).
Comparing suicide completers with non-suicide subjects showed variance with region (F2,174=7.98, P=0.0006), but not suicide status (F1,174=0.01, P=0.91), and no interaction between the two variables (F2,174=1.98, P=0.14; Supplementary Figure 2, top). Upon removal of the control subjects from the analysis, there was still variance with region (F2,84=6.50, P=0.002) and not suicide status (F1,84=0.28, P=0.60), and no interaction between the two (F2,84=1.77, P=0.18; Supplementary Figure 2, bottom).
Genotype effect on SELENBP1 expression
Of the two SNPs tested, the primer set for rs2800953 did not allow for the elucidation of genotype in any of the samples, possibly due to inefficient primer binding. Analysis of the data for rs10788804 showed no deviation from Hardy–Weinberg equilibrium (χ2=0.04, P<0.05).32 There was no effect of genotype (BA9 F2,50=0.32, P=0.73; BA44 F2,50=2.42, P=0.10; BA8 F2,50=0.87, P=0.43; Table 2) or allele (0.06<P<0.78; Supplementary Table 5) on SELENBP1 mRNA levels.
Selenbp1 mRNA in antipsychotic-treated animals
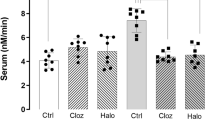
Data were normally distributed for each treatment group. Antipsychotic drugs had no effect on Selenbp1 mRNA levels in rats (0.61±0.01 vehicle, 0.56±0.03 chlorpromazine, 0.65±0.03 thioridazine and 0.58±0.03 haloperidol; F1,19=1.57, P=0.24; Figure 3).
Selenbp1 messenger RNA (mRNA) in antipsychotic-treated animals. Selenbp1 mRNA expression in central nervous system from rats treated with vehicle (Veh), 10 mg kg−1 per day chlorpromazine (Clp), 10 mg kg−1 per day thioridazine (Thd) or 1.0 mg kg−1 per day haloperidol (Hal). Error bars represent mean±s.e.m.
Discussion
We found SELENPB1 mRNA to be higher in subjects with schizophrenia in all CNS regions examined, BA8, BA9 and BA44. By contrast, we showed no difference in levels of SELENBP1 mRNA in BA9 from subjects with MDD or BP compared with control, suggesting that there may be diagnosis selectivity in changes in cortical expression of that gene. Increased SELENBP1 mRNA levels did not translate to increases in protein levels. Rats treated with the same antipsychotics as those taken by subjects in this cohort showed no differences in CNS levels of Selenbp1 mRNA, indicating that changes in gene expression in human cortex are unlikely to be simply due to antipsychotic treatment. Finally, we found no association between SNP rs10788804 and SELENBP1 mRNA levels.
Our findings of higher levels of SELENBP1 mRNA in the dorsolateral prefrontal cortex of subjects with schizophrenia are in agreement with previous studies,1, 2 and we further show that these changes are likely to be widespread throughout the cortex of subjects with the disorder. We were unable to confirm higher SELENBP1 mRNA levels in BP with psychotic features2 owing to small sample size and insufficient case history. Although psychotic state was not well recorded in our subjects with BP, half of the subjects had been prescribed antipsychotics; separating BP subjects based on antipsychotic treatment showed no effect on SELENBP1 mRNA expression levels (t8=0.99, P=0.35; data not shown). In this regard, of interest is a small study of patients during their first hospitalisation with a schizophrenia spectrum disorder (schizophrenia, schizoaffective disorder or schizophreniform disorder) that reported no difference in levels of SELENBP1 mRNA in blood compared with controls.33 Significantly, that study involved patients in their first admission, whereas other studies utilised patients with an established diagnosis of schizophrenia, suggesting that differences in SELENBP1 expression may depend on disease state. Overall, our study adds to previous data showing upregulated SELENBP1 expression in schizophrenia; the consistency of this effect, together with mRNA being shown to be altered in blood as well as brain,1 indicates that it could be a candidate for inclusion in a panel of biomarkers that could be used to aid in diagnosis.
Rats treated with the same antipsychotics as those prescribed to subjects in the schizophrenia cohort showed no differences in CNS levels of Selenbp1 mRNA. Not measuring CNS levels of antipsychotic drugs is a limitation to this study that is common to many studies in rats that use this, and other, delivery methods. Importantly, there are a number of CNS-driven behavioural studies, particularly focussed on modulating dopaminergic activity, that show that the delivery of antipsychotic drugs in the drinking water does result in appropriate levels of antipsychotic drugs in rat CNS.19, 34, 35, 36, 37 In our current study, our results are in line with previous studies, and our data show no significant relationship between antipsychotic drug dose and SELENBP1 mRNA levels in schizophrenia,1, 2 thereby indicating that changes in SELENBP1 gene expression in human cortex is a feature of the disorder rather than an antipsychotic drug effect. Our finding of no association between genotype at rs10788804 and SELENBP1 mRNA levels suggests that changes in gene sequence at this site do not affect expression levels. However, previous studies showing association of polymorphisms9 and copy number variations38 in the SELENBP1 gene with schizophrenia support a role of this gene in the aetiology of the disorder.
Although there was a marked change in SELENBP1 mRNA expression in the CNS in schizophrenia, we saw no differences in total SELENBP1 protein with diagnosis. This could indicate that there would be no functional outcome from our findings on mRNA. However, it is now recognised that for some genes a neuron must rapidly synthesise protein in response to stimuli,39 particularly within specific compartments of the cell such as dendrites.40 Thus, the changed expression levels of SELENBP1 could affect the responsiveness of a neuron if it needs to rapidly increase levels of SELENBP1 protein. Findings from other studies indicate that there are altered SELENBP1 protein levels in subjects with schizophrenia. The difference in outcomes from our study and other studies could be related to the sensitivity of the techniques used to measure SELENBP1. For example, a proteomic study using 2-dimensional difference gel electrophoresis found that SELENBP1 protein levels were lower in post-mortem liver from subjects with schizophrenia, but higher in red blood cells from a separate group of patients,41 which is in line with mRNA findings in blood.1, 2 By using a similar technique, another study detected higher SELENBP1 protein in the prefrontal cortex from a small number of suicide subjects with no known neurodegenerative disease,42 whereas we saw no significant difference with suicide; however, that study used only suicide subjects that had died from hanging, and SELENBP1 is known to be induced under hypoxic conditions.43 Previously, increased glial and decreased neuronal immunohistochemical staining of SELENBP1 protein was observed in the dorsolateral prefrontal cortex from three subjects with schizophrenia compared with control.1 Although this study has significantly more power than that using immunohistochemistry, western blots do not allow us to examine localised expression; we may not be detecting changes occurring in a cell-type or -compartment-specific manner in this study. Thus, a more detailed examination of SELENBP1 protein in the CNS could reveal a physiological outcome of upregulated mRNA in schizophrenia.
Although the exact function of SELENBP1 has yet to be characterised, it is known to bind Se,44, 45 a trace element involved in neuroprotection.46, 47, 48, 49, 50, 51 It is of note that Se deficiency has been associated with higher rates of schizophrenia52, 53 and some studies have shown lower plasma Se concentrations in patients with schizophrenia,54, 55 whereas others showed that Se is unchanged in the blood and serum of patients.56, 57 Thus, it would be beneficial to determine the Se concentrations in CNS regions in subjects with schizophrenia to ascertain whether there is a link between Se levels and SELENBP1 expression. The inverse relationship demonstrated between SELENBP1 expression and cell growth,58, 59 and the observed association of SELENBP1 with neuronal cell outgrowth,3 suggests that changes in central SELENBP1 could be linked to aberrant cell growth in the brains of people with schizophrenia;60, 61, 62 however, more detailed studies are needed to determine its exact function in the CNS and its implications in disease aetiology.
With this study, it has now been shown in three separate cohorts that SELENBP1 mRNA is upregulated in the frontal cortex of subjects with schizophrenia, an effect not attributed to antipsychotic medication. This supports the notion that SELENBP1 may have a role in the pathophysiology of schizophrenia. These findings warrant further characterisation of SELENBP1 and its role in CNS function.
References
Glatt SJ, Everall IP, Kremen WS, Corbeil J, Sasik R, Khanlou N et al. Comparative gene expression analysis of blood and brain provides concurrent validation of SELENBP1 up-regulation in schizophrenia. Proc Natl Acad Sci USA 2005; 102: 15533–15538.
Kanazawa T, Chana G, Glatt SJ, Mizuno H, Masliah E, Yoneda H et al. The utility of SELENBP1 gene expression as a biomarker for major psychotic disorders: replication in schizophrenia and extension to bipolar disorder with psychosis. Am J Med Genet B Neuropsychiatr Genet 2008; 147B: 686–689.
Miyaguchi K . Localization of selenium-binding protein at the tips of rapidly extending protrusions. Histochem Cell Biol 2004; 121: 371–376.
Konopaske GT, Lange N, Coyle JT, Benes FM . Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry 2014; 71: 1323–1331.
Bouras C, Kovari E, Hof PR, Riederer BM, Giannakopoulos P . Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol 2001; 102: 373–379.
Eastwood SL, Harrison PJ . Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull 2001; 55: 569–578.
Rosoklija G, Keilp JG, Toomayan G, Mancevski B, Haroutunian V, Liu D et al. Altered subicular MAP2 immunoreactivity in schizophrenia. Prilozi 2005; 26: 13–34.
Arnold SE, Lee VM, Gur RE, Trojanowski JQ . Abnormal expression of two microtubule-associated proteins (MAP2 and MAP5) in specific subfields of the hippocampal formation in schizophrenia. Proc Natl Acad Sci USA 1991; 88: 10850–10854.
Kanazawa T, Glatt SJ, Faraone SV, Hwu HG, Yoneda H, Tsuang MT . Family-based association study of SELENBP1 in schizophrenia. Schizophr Res 2009; 113: 268–272.
American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th edn American Psychiatric Association: Washington, DC, USA, 1994.
Hill C, Keks N, Roberts S, Opeskin K, Dean B, MacKinnon A et al. Problem of diagnosis in postmortem brain studies of schizophrenia. Am J Psychiatry 1996; 153: 533–537.
Roberts SB, Hill CA, Dean B, Keks NA, Opeskin K, Copolov DL . Confirmation of the diagnosis of schizophrenia after death using DSM-IV: a Victorian experience. Aust N Z J Psychiatry 1998; 32: 73–76.
Gibbons AS, Brooks L, Scarr E, Dean B . AMPA receptor expression is increased post-mortem samples of the anterior cingulate from subjects with major depressive disorder. J Affect Dis 2012; 136: 1232–1237.
Salah-Uddin H, Scarr E, Pavey G, Harris K, Hagan JJ, Dean B et al. Altered M(1) muscarinic acetylcholine receptor (CHRM1)-Galpha(q/11) coupling in a schizophrenia endophenotype. Neuropsychopharmacology 2009; 34: 2156–2166.
Crook JM, Dean B, Pavey G, Copolov D . The binding of [3H]AF-DX 384 is reduced in the caudate-putamen of subjects with schizophrenia. Life Sci 1999; 64: 1761–1771.
Andersson C, Hamer RM, Lawler CP, Mailman RB, Lieberman JA . Striatal volume changes in the rat following long-term administration of typical and atypical antipsychotic drugs. Neuropsychopharmacology 2002; 27: 143–151.
Lee H, Tarazi FI, Chakos M, Wu H, Redmond M, Alvir JM et al. Effects of chronic treatment with typical and atypical antipsychotic drugs on the rat striatum. Life Sci 1999; 64: 1595–1602.
Kaneda H, Shirakawa O, Dale J, Goodman L, Bachus SE, Tamminga CA . Co-administration of progabide inhibits haloperidol-induced oral dyskinesias in rats. Eur J Pharmacol 1992; 212: 43–49.
Muller AP, Tort AH, Gnoatto J, Moreira JD, Vinade ER, Perry ML et al. Metabolic and behavioral effects of chronic olanzapine treatment and cafeteria diet in rats. Behav Pharmacol 2010; 21: 668–675.
Kelley JJ, Gao XM, Tamminga CA, Roberts RC . The effect of chronic haloperidol treatment on dendritic spines in the rat striatum. Exp Neurol 1997; 146: 471–478.
Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G . Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry 1992; 49: 538–544.
Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN . Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther 2003; 305: 625–631.
Barth VN, Chernet E, Martin LJ, Need AB, Rash KS, Morin M et al. Comparison of rat dopamine D2 receptor occupancy for a series of antipsychotic drugs measured using radiolabeled or nonlabeled raclopride tracer. Life Sci 2006; 78: 3007–3012.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates. 6th edn, Elsevier Science Publishing Co Inc.: San Diego, CA, USA, 2006.
Vandesompele J. geNorm. http://medgen.ugent.be/~jvdesomp/genorm/ (accessed on 5th November 2007).
Dean B, Keriakous D, Scarr E, Thomas EA . Gene expression profiling in Brodmann's area 46 from subjects with schizophrenia. Aust N Z J Psychiatry 2007; 41: 308–320.
Cook RD, Weisberg S . Applied Regression Including Computing and Graphics New York. Wiley-Interscience: : NY, USA, 1999, p. 632.
Miller GA, Chapman JP . Misunderstanding analysis of covariance. J Abnorm Psychol 2001; 110: 40–48.
Glass GV, Peckham PD, Sanders JR . Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Rev Educ Res 1972; 42: 237–288.
Harwell MR, Rubinstein EN, Hayes WS, Olds CC . Summarizing Monte Carlo results in methodological research: the one- and two-factor fixed effects ANOVA cases. J Educ Stat 1992; 17: 315–339.
Lix LM, Keselman JC, Keselman HJ . Consequences of assumption violations revisited: a quantitative review of alternatives to the one-way analysis of variance F test. Rev Educ Res 1996; 66: 579–619.
Rodriguez S, Gaunt TR, Day IN . Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol 2009; 169: 505–514.
Yao Y, Schroder J, Karlsson H . Verification of proposed peripheral biomarkers in mononuclear cells of individuals with schizophrenia. J Psychiatr Res 2008; 42: 639–643.
Terry AV Jr., Mahadik SP . Time-dependent cognitive deficits associated with first and second generation antipsychotics: cholinergic dysregulation as a potential mechanism. J Pharmacol Exp Ther 2007; 320: 961–968.
Gao XM, Cooper T, Suckow RF, Tamminga CA . Multidose risperidone treatment evaluated in a rodent model of tardive dyskinesia. Neuropsychopharmacology 2006; 31: 1864–1868.
Terry AV Jr., Hill WD, Parikh V, Waller JL, Evans DR, Mahadik SP . Differential effects of haloperidol, risperidone, and clozapine exposure on cholinergic markers and spatial learning performance in rats. Neuropsychopharmacology 2003; 28: 300–309.
Rosengarten H, Quartermain D . The effect of chronic treatment with typical and atypical antipsychotics on working memory and jaw movements in three- and eighteen-month-old rats. Prog Neuropsychopharmacol Biol Psychiatry 2002; 26: 1047–1054.
Amar S, Ovadia O, Maier W, Ebstein R, Belmaker RH, Mishmar D et al. Copy number variation of the SELENBP1 gene in schizophrenia. Behav Brain Funct 2010; 6: 40.
Buxbaum AR, Haimovich G, Singer RH . In the right place at the right time: visualizing and understanding mRNA localization. Nat Rev Mol Cell Biol 2015; 16: 95–109.
Huber KM, Kayser MS, Bear MF . Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 2000; 288: 1254–1257.
Prabakaran S, Wengenroth M, Lockstone HE, Lilley K, Leweke FM, Bahn S . 2-D DIGE analysis of liver and red blood cells provides further evidence for oxidative stress in schizophrenia. J Proteom Res 2007; 6: 141–149.
Kekesi KA, Juhasz G, Simor A, Gulyassy P, Szego EM, Hunyadi-Gulyas E et al. Altered functional protein networks in the prefrontal cortex and amygdala of victims of suicide. PloS One 2012; 7: e50532.
Weir L, Robertson D, Leigh IM, Vass JK, Panteleyev AA . Hypoxia-mediated control of HIF/ARNT machinery in epidermal keratinocytes. Biochim Biophys Acta 2011; 1813: 60–72.
Bansal MP, Mukhopadhyay T, Scott J, Cook RG, Mukhopadhyay R, Medina D . DNA sequencing of a mouse liver protein that binds selenium: implications for selenium's mechanism of action in cancer prevention. Carcinogenesis 1990; 11: 2071–2073.
Chang PW, Tsui SK, Liew C, Lee CC, Waye MM, Fung KP . Isolation, characterization, and chromosomal mapping of a novel cDNA clone encoding human selenium binding protein. J Cell Biochem 1997; 64: 217–224.
Dalla Puppa L, Savaskan NE, Brauer AU, Behne D, Kyriakopoulos A . The role of selenite on microglial migration. Ann N Y Acad Sci 2007; 1096: 179–183.
Imai H, Nakagawa Y . Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 2003; 34: 145–169.
Ogawa A, Ogawa I, Obayashi R, Umezu K, Doi M, Hirao T . Highly selective thioselenation of vinylcyclopropanes with a (PhS)(2)-(PhSe)(2) binary system and its application to thiotelluration. J Org Chem 1999; 64: 86–92.
Porciuncula LO, Rocha JB, Boeck CR, Vendite D, Souza DO . Ebselen prevents excitotoxicity provoked by glutamate in rat cerebellar granule neurons. Neurosci Lett 2001; 299: 217–220.
Saito Y, Hashimoto T, Sasaki M, Hanaoka S, Sugai K . Effect of selenium deficiency on cardiac function of individuals with severe disabilities under long-term tube feeding. Dev Med Child Neurol 1998; 40: 743–748.
Yeo JE, Kang SK . Selenium effectively inhibits ROS-mediated apoptotic neural precursor cell death in vitro and in vivo in traumatic brain injury. Biochim Biophys Acta 2007; 1772: 1199–1210.
Brown JS Jr . Role of selenium and other trace elements in the geography of schizophrenia. Schizophr Bull 1994; 20: 387–398.
Joyce PR . Changing trends in first admissions and readmissions for mania and schizophrenia in New Zealand, 1974 to 1984. Aust N Z J Psychiatry 1987; 21: 82–86.
Vaddadi KS, Soosai E, Vaddadi G . Low blood selenium concentrations in schizophrenic patients on clozapine. Br J Clin Pharmacol 2003; 55: 307–309.
Arinola O, Idonije O . Status of plasma nitric oxide and non-enzymatic antioxidants before and after antipsychotic treatment in Nigerian patients with schizophrenia. J Res Med Sci 2009; 14: 37–42.
Alertsen AR, Aukrust A, Skaug OE . Selenium concentrations in blood and serum from patients with mental diseases. Acta Psychiatr Scand 1986; 74: 217–219.
Yanik M, Kocyigit A, Tutkun H, Vural H, Herken H . Plasma manganese, selenium, zinc, copper, and iron concentrations in patients with schizophrenia. Biol Trace Element Res 2004; 98: 109–117.
Huang C, Ding G, Gu C, Zhou J, Kuang M, Ji Y et al. Decreased selenium-binding protein 1 enhances glutathione peroxidase 1 activity and downregulates HIF-1alpha to promote hepatocellular carcinoma invasiveness. Clin Cancer Res 2012; 18: 3042–3053.
Jeong JY, Zhou JR, Gao C, Feldman L, Sytkowski AJ . Human selenium binding protein-1 (hSP56) is a negative regulator of HIF-1alpha and suppresses the malignant characteristics of prostate cancer cells. BMB Rep 2014; 47: 411–416.
Weinberger DR . Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44: 660–669.
Shenton ME, Dickey CC, Frumin M, McCarley RW . A review of MRI findings in schizophrenia. Schizophr Res 2001; 49: 1–52.
Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ et al. Progressive brain structural changes mapped as psychosis develops in 'at risk' individuals. Schizophr Res 2009; 108: 85–92.
Acknowledgements
We gratefully acknowledge the assistance of Geoffrey Pavey for the preparation of post-mortem tissue, Chad Bousman for the ancestry marker and genotyping data collection, and Aradhana Upadhyay and Gayathri Perera for their assistance with sample preparation. This project was supported by the NHMRC (project grant 566967, Centre for Research Excellence Grant 1001216 (TTM), Senior Research Fellowship no. APP1002240 (BD)), the Australian Research Council (Future Fellowship FT100100689 to ES), as well as Operational Infrastructure Support from the Victorian State Government, the Rebecca L. Cooper Medical Research Foundation and the Cooperative Research Centre for Mental Health.
Disclaimer
The Cooperative Research Centre programme is an Australian Government Initiative. None of these funding sources had any role in the study design, collection, analysis or interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Udawela, M., Money, T., Neo, J. et al. SELENBP1 expression in the prefrontal cortex of subjects with schizophrenia. Transl Psychiatry 5, e615 (2015). https://doi.org/10.1038/tp.2015.108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2015.108
This article is cited by
-
Evolutionary Aspects of Selenium Binding Protein (SBP)
Journal of Molecular Evolution (2023)
-
Knocking out Selenium Binding Protein 1 Induces Depressive-Like Behavior in Mice
Biological Trace Element Research (2023)
-
Androgens increase excitatory neurogenic potential in human brain organoids
Nature (2022)
-
Are Essential Trace Elements Effective in Modulation of Mental Disorders? Update and Perspectives
Biological Trace Element Research (2022)
-
Changes in cortical gene expression in the muscarinic M1 receptor knockout mouse: potential relevance to schizophrenia, Alzheimer’s disease and cognition
npj Schizophrenia (2021)