Abstract
DNA methylation of the Fragile X mental retardation 1 (FMR1) exon 1/intron 1 boundary has been associated with executive dysfunction in female carriers of a FMR1 premutation (PM: 55–199 CGG repeats), whereas neuroanatomical changes have been associated with executive dysfunction in PM males. To our knowledge, this study for the first time examined the inter-relationships between executive function, neuroanatomical structure and molecular measures (DNA methylation and FMR1 mRNA levels in blood) in PM and control (<44 CGG repeats) females. In the PM group, FMR1 intron 1 methylation was positively associated with executive function and cortical thickness in middle and superior frontal gyri, and left inferior parietal gyrus. By contrast, in the control group, FMR1 intron 1 methylation was negatively associated with cortical thickness of the left middle frontal gyrus and superior frontal gyri. No significant associations were revealed for either group between FMR1 mRNA and neuroanatomical structure or executive function. In the PM group, the lack of any significant association between FMR1 mRNA levels and phenotypic measures found in this study suggests that either FMR1 expression is not well conserved between tissues, or that FMR1 intron 1 methylation is linked to neuroanatomical and cognitive phenotype in PM females via a different mechanism.
Similar content being viewed by others
Introduction
Trinucleotide CGG repeat expansions of the Fragile X mental retardation 1 (FMR1) gene are related to a number of Fragile X-associated disorders. Full mutation alleles (FM: greater than 200 CGG repeats) are associated with silencing of FMR1 through methylation of the promoter region located in the 5′ untranslated region,1 resulting in a neurodevelopmental disorder known as Fragile X syndrome. The prevalence of Fragile X syndrome in the general population is ~1 in 4000.2 The more common FMR1 premutation (PM) expansion (55–199 CGG repeats), which is found in ~1 in 209 females and 1 in 430 males,3 confers the risk of developing Fragile X-associated tremor/ataxia syndrome (FXTAS). FXTAS is a progressive neurodegenerative disorder, thought to result, in part, from elevated levels of FMR1 mRNA, leading to protein aggregation (ubiquitin-positive intracellular inclusion bodies likely due to repeat-associated non-AUG-initiated translation) and reduced neuronal cell function.4, 5, 6 FXTAS manifests in a range of neurological and clinical symptoms as well as executive dysfunction.7 Executive dysfunction, specifically pertaining to working memory and response inhibition processes, has been reported in both PM males8, 9, 10 and females without FXTAS,11, 12, 13, 14, 15 and may represent either an independent PM phenotype or a precursor to FXTAS.
Significant associations between neuroanatomical structure (white and grey matter) and measures of cognition, including executive function, have been reported in PM males and females.16, 17, 18, 19, 20, 21 More recently, a link has also been demonstrated between molecular changes and the risk of developing executive dysfunction in PM females; specifically, methylation changes at the FMR1 exon 1/intron 1 boundary measured in blood DNA—a region also known as Fragile X-related epigenetic element 2 (FREE2).22 To our knowledge, this study for the first time examined whether CGG repeat length, FMR1 mRNA levels and methylation levels of the CpG island (or the activation ratio, AR) and FREE2 region correlate significantly with altered neuroanatomy in PM females without FXTAS. It also examined the relationships between these molecular and neural measures and cognitive performance; specifically, changes in executive function based on an ocular motor switch task.
Materials and methods
Participants
CGG repeat lengths were determined for 36 females aged between 22 and 54 years. Of these, 19 exhibited PM alleles with a CGG repeat length between 55 and 199, and 17 exhibited normal alleles with CGG repeat length <44 (thus providing control data). All were recruited from support groups and population-based Fragile X carrier screening studies,23 as well as local networks and via online advertisements.
All participants were English-speaking, had normal (or corrected) vision and hearing, and had no history of any serious neurological damage/disease (including FXTAS). Exclusion criteria extended to those who thought they may be pregnant, as well as those with any magnetic resonance imaging (MRI) contraindication. Ethics approval for this study was granted by the Monash University and Southern Health Human Research Committees (Project Number 10147B); all participants gave their informed consent before inclusion in the study in accordance with the Declaration of Helsinki.
Molecular analyses
DNA was extracted from whole blood for CGG sizing and methylation analysis. The AmplideX FMR1 PCR Kit was used for CGG sizing, as per the manufacturer’s instructions (Asuragen, Austin, TX, USA). RNA was extracted from peripheral blood mononuclear cells, followed by cDNA synthesis and real-time PCR gene expression analysis performed on a ViiA 7 Real-Time PCR System (Life Technologies, Global). The relative standard curve method was utilised for FMR1 5′ and 3′ mRNA quantification normalised to mRNA levels of two internal control genes (SDHA and EIF4A2), as previously described.24 AR was determined using methylation-sensitive Southern blot targeting a NruI restriction site within the FMR1 CpG island, as previously described.22 The EpiTYPER system was used to analyse FREE2 methylation in the blood, consisting of five CpG unit outputs (targeting nine CpG sites) per sample tested.25 Blood DNA from each participant was bisulfite-converted in duplicate, with each conversion analysed twice using the EpiTYPER system. A summary measure for each CpG unit was determined as the mean of the four methylation output ratio measurements per sample. These procedures resulted in a total of eight molecular measures: CGG, AR, FMR1 mRNA, FMR1 exon 1 (CpG 1 and CpG 2) and intron 1 (CpG 6/7, CpG 8/9 and CpG 10–12) methylation markers.
Assessment and analysis of executive function
Haylings Sentence Completion Test
The Haylings Sentence Completion Test,26 a test of response inhibition, required participants to respond to 15 sentences with the last word omitted, by providing a word that was unconnected to the sentence. Responses were classified as either correct, a Category A error (word plausibly finished the sentence) or Category B error (word was somewhat connected to the sentence)—both of which measure inhibitory processing. The total number of Category A and Category B errors were recorded, with larger error numbers indicating impaired response inhibition processes.
Ocular motor switch task
The ocular motor switch task27 assesses attention, response inhibition and working memory processes. It required participants to move their eye either towards (prosaccade trial) or away (antisaccade) from a target as quickly and as accurately as possible depending on a central colour cue given at the start of each trial (Supplementary Note 1 for more details). As this study was interested in executive dysfunction, antisaccade data were removed from this analysis to avoid any contamination of the paradoxical ‘benefit’ that is commonly seen for antisaccade trials following a prosaccade trial (antisaccade switch trials).28, 29, 30 This yielded a total of seven prosaccade variables: correct latency (ms), error latency (ms), time to correct (ms), switch/non-switch directional error percentage and switch/non-switch anticipatory error percentage.
MRI acquisition and analysis
Structural MRIs were acquired on a 3 T Siemens Magneto Skyra scanner using a 20-channel head coil using a T1-weighted three-dimensional MPRAGE scan (208 sagittal slices of 1 mm thickness (no gap), repetition time=1540 ms, echo time=2.55 ms, inversion time=900 ms, a flip angle of 9°, field of view=256 × 256 mm2, yielding a standard voxel size=1 × 1 × 1 mm3).
T1-weighted three-dimensional MPRAGE data were analysed using FreeSurfer version 5.1.0 (http://surfer.nmr.mgh.harvard.edu) with technical details previously described.31, 32, 33 Automated anatomic segmentation procedure was used to measure volume of T1 white matter hypointensities,32, 34 whereas regional cortical thickness measures were obtained from the automated anatomic parcellation procedure34 for each participant.
Regional cortical thickness from the middle and superior frontal gyri (representing the dorsolateral prefrontal cortex) and inferior parietal gyrus from both left and right hemispheres were selected as they are pivotally involved in the control of saccades.35, 36, 37
Statistical analysis
Composite cognitive scores
To reduce the number of executive function variables, separate principal component analyses, using oblique direct rotation with one fixed factor, were hypothesised and tested using the IBM SPSS Statistics software (version 21, IBM, Armonk, NY, USA). This resulted in the creation of three composite cognitive scores: (1) prosaccade response time, (2) prosaccade error score and (3) executive function score (Supplementary Note 2 for more details).
Between-group differences
The Stata statistical software (version 14, StataCorp, College Station, TX, USA), was used for all further statistical analyses. Comparisons of demographic information, molecular, composite cognitive scores and neuroanatomical measures between PM and control females were conducted using independent samples t-tests (for equal or unequal variances) or Mann–Whitney U (when violations of the assumption of normality occurred). The generalised estimating equation was not employed, as correlations within a family were not seen to be significant.
Regression models
To assess the inter-relationships between molecular variables, neuroanatomical measures and composite cognitive scores for both PM and control groups, we performed least squares or robust regression analyses (which downweighs the effect of outliers when present). The following models were examined: (I) molecular markers (predictor) and composite cognitive scores (outcome), (II) molecular markers (predictor) and neuroanatomical measures (outcome) and (III) neuroanatomical measures (predictor) and composite cognitive scores (outcome). The goodness of fit was assessed for each regression analysis using the coefficient of determination (r2). Further, the interaction effect of group by (i) composite cognitive score and (ii) neuroanatomical measures was assessed using a general linear model in the IBM SPSS Statistics 21.0. The relationships between FMR1 mRNA levels and FMR1 methylation (AR and FREE2 methylation) in both groups were examined using regression analyses.
Results
Clinical and molecular intergroup comparisons
PM and control groups were well matched for age, education and full-scale intelligence quotient (assessed via the Wechsler Abbreviated Scale of Intelligence)38 (Supplementary Table S1).
Significant group differences were found for FMR1 mRNA levels; PM females had a 1.31 mean fold increase in FMR1 mRNA levels compared with controls (Supplementary Table S1). The mean methylation levels of exon 1 CpG sites 1 and 2; intron 1 CpG sites 6/7, 8/9 and 10–12 and of the CpG island (AR; CpG locations indicated in Figure 1a) were not significantly different between PM and control groups. Further, FMR1 mRNA levels were not found to be significantly correlated with any FMR1 methylation measure for either the PM or control group (Supplementary Table S2).
FMR1 methylation sites and associations with the left middle frontal gyrus in PM and control groups. (a) Organisation of the Xq27.3 sequence encompassing specific FREE2 CpG sites (GenBank L29074 L38501) targeted by FREE2 EpiTYPER system. The CTCF box indicates 5′ CTCF-binding sites from UCSF Chip-Seq, which overlap with FREE2 CpG 10-12; the RNA:DNA hybrid box indicates locations of forward and reverse primers used in ChiRP to show formation of RNA:DNA hybrids denoted as fP(200–400) (Colak et al.39 Figure 4, Supplementary Figure S16 and Supplementary Table S1). Associations between biomarker methylation within FMR1 CpG island (represented by AR), exon 1 and intron 1 and left middle frontal gyrus thickness (assessed using structural magnetic resonance imaging Model II; unstandardised values) in PM (b) and control (c) groups. β represents standardized coefficients from least or robust (downweighs outliers) regression analysis. AR, activation ratio; FMR1, Fragile X mental retardation 1; FREE2, Fragile X-related epigenetic element 2; PM, premutation.
Higher prosaccade error and executive function scores were found for PM females compared with controls, indicating executive dysfunction. No significant differences were found between PM and control groups for prosaccade response time, white matter hypointensities or any cortical thickness measure (Supplementary Table S3).
Epigenotype–phenotype relationships in PM and control groups
FREE2 methylation levels of FMR1 intron 1 CpG sites showed the greatest number of significant relationships with composite cognitive scores in the PM group compared with CGG size, AR, exon 1 methylation or FMR1 mRNA levels in the blood (Table 1). Significant molecular–composite cognitive score relationships were completely absent from the control group (Table 2).
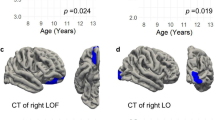
Again, FMR1 intron 1 methylation levels showed the greatest number of significant relationships with neuroanatomical measures for both the PM and control groups compared with CGG size, AR, exon 1 methylation or FMR1 mRNA levels in the blood (Figure 1 and Table 1). Methylation of FMR1 intron 1 CpG sites correlated positively with MRI measures in the PM group (Figure 1b, Figure 2 and Table 1). Conversely, for controls, increased methylation of FMR1 CpG 2, 6/7, 10–12 was associated with decreased cortical thickness in frontal lobe regions. No significant CpG 8/9–neuroanatomical relationships were found for the control group (Figure 1c, Figure 2 and Table 2). Interaction analysis revealed that significant group differences in the relationships between FMR1 intron 1 methylation and middle frontal, superior frontal and inferior parietal thickness were evident (Table 3).
Associations between neuroanatomical cortical thickness and CpG 8/9 methylation in PM and control groups. P-values represent results from individual least squares regression analyses assessing the relationship between CpG 8/9 and cortical thickness (unstandardised values), and are presented only for values reaching significance of P<0.05. PM, premutation.
Neuroanatomical measures were related to executive function measures for both PM and control groups. The three significant relationships for the PM group suggest that executive function deficits, denoted by composite cognitive scores, were related to increased white matter hypointensities (prosaccade response time: coefficient (β)=0.491, s.e.=0.211, P=0.033, r2=0.241) and decreased cortical volume in the left middle frontal gyrus (prosaccade error score: β=−0.495, s.e.=0.211, P=0.031, r2=0.245), and left inferior parietal gyrus (executive function score: β=−0.547, s.e.=0.203, P=0.015, r2=0.299). Conversely, increased bilateral inferior parietal gyrus thickness was positively associated with greater prosaccade error scores in controls (left: β=0.439, s.e.=0.166, P=0.018, r2=0.319: β=0.490, s.e.=0.225, P=0.046, r2=0.240).
Discussion
Understanding the disorder-specific role of intragenic DNA methylation is critically important,40, 41 providing a unique opportunity to investigate gene/environment interactions of clinical significance.42 In this study, highly significant relationships were found between the intragenic methylation within the 5′ end of the FMR1 intron 1 and phenotype measures of executive function, volume of white matter hypointensities and regional cortical thickness in the frontal and parietal cortices of PM females without FXTAS. The differences in the relationships between methylation markers CpG 6/7 and CpG 8/9 and cortical thickness between PM and control females suggest that in normal neurobiology, FMR1 methylation (potentially X chromosome inactivation (XCI)) is related to thickness of specific cortical regions and volume of white matter hypointensities, which are disrupted in PM females without FXTAS through a currently unknown mechanism that modifies the observed associations.
FMR1 intron 1 methylation, but not FMR1 mRNA, predicts executive dysfunction in PM females
In PM females without FXTAS, decreased methylation of both FMR1 promotor (AR) and FMR1 intron 1 regions was found to relate to executive dysfunction. This relationship was absent in controls entirely. Further, the strongest relationships for each composite cognitive score were seen within the 5′ end of FMR1 intron 1, as compared with methylation of exon 1 or AR. This is consistent with the study by Cornish and colleagues,22 supporting the prior hypothesis that methylation of FMR1 intron 1 CpG sites is a good predictor of deficits within the executive function phenotype of PM and FM females.22, 43, 44, 45, 46, 47
Unlike previous ocular motor studies, FMR1 mRNA levels were not correlated with executive function scores in this cohort of PM females without FXTAS.48 Conversely, FMR1 intron 1 methylation correlated with both executive function and neuroanatomical structure in the PM group. We also found no significant relationships between any methylation measure (AR and FREE2 methylation) and FMR1 mRNA for PM or control groups. This suggests that, in PM females without FXTAS, FMR1 intron 1 methylation has clinical significance involving a different mode or pathway of action that does not directly involve overexpression of FMR1 mRNA.
It is important to note that in this study FMR1 mRNA was normalised to two control genes (SDHA and EIF4A2) and not beta-glucuronidase (GUS), as in previous observations assessing differing aspects of executive function.22, 48, 49 GUS is a commonly used reference gene or internal control for transcript quantification with PCR. In a study of FM males where FMR1 mRNA was normalised to actin B and GUS, a positive linear relationship between FMR1 mRNA and methylation of the FMR1 promotor region was found,50 which was not evident in this study. This difference could have several explanations including that (a) the Brasa et al. study50 performed correlation analyses for different CpG sites, (b) used FM males as opposed to PM females without FXTAS, (c) had a much smaller sample size of only seven individuals (susceptible to the effects of outliers) or most likely (d) used a different normalisation strategy of FMR1 mRNA. In relation to the last potential explanation, it is important to note that variability in gene expression of internal control genes has been well documented to have an impact on target gene real-time PCR outputs,51 which we have recently shown to apply in PM females without FXTAS.52
FMR1 intron 1 differently predicts neuroanatomical structure between PM and control groups
Juxtaposing associations were found between increased FREE2 methylation and cortical thickness in our PM and control groups: increased cortical thickness for the PM group and decreased cortical thickness for the control group. This was most evidenced when assessing the FREE2 methylation relationships with cortical thickness of the left middle frontal gyrus, where there was a trend towards increased cortical thickness for the PM group compared with controls (P=0.058). The clear dissociation between FMR1 intron 1 methylation and cortical thickness of the left middle frontal gyrus, as well as other FMR1 intron 1 methylation–frontal and inferior parietal relationships, between groups, suggests a possible involvement for XCI skewing in regulating the thickness of this region as part of normal biology. This also suggests that FMR1 intron 1 methylation in peripheral blood is important when considering XCI in neurological disorders without a PM expansion. This is reinforced by the absence of significant associations between methylation of FREE2 CpG 8/9 and cortical thickness in the control group, compared with the highly significant relationships seen for the PM group. Not only does this study show that methylation of FMR1 intron 1 CpG sites is a useful biomarker of cortical thickness in PM females without FXTAS, but it also opens up the broader possibility that this may be the case for other disorders involving cortical thickness disruption, such as Alzheimer’s (PSEN1 mutations),53 Parkinson’s,54, 55 major depressive disorder56 and social anxiety disorder.57
Multiple neuroanatomical correlates of executive function found in the PM group
Each of the composite cognitive scores was found to be associated with either regional cortical thickness or volume of white matter hypointensities within the frontoparietal executive processing network (Model III) for PM females without FXTAS, whereas only inferior parietal thickness related to the prosaccade error score in the control group. Specifically, a positive relationship was found between white matter hypointensities and prosaccade reaction time in PM females without FXTAS, which is consistent with the hypothesis that reduced white matter integrity results in increased response times in cognitive tasks generally.58
Similarly to our findings of an association between left middle frontal gyrus thickness and prosaccade error scores, decreased cortical thickness in the middle frontal cortex has been linked to executive dysfunction.59 Equally, we also reveal that decreased cortical thickness of the left inferior parietal gyrus related to impaired executive function scores in PM females without FXTAS. Collectively, these findings are in direct contrast to a previous Fragile X syndrome study, where increased cortical thickness was associated with poorer performance on multiple domains of the Stanford-Binet Intelligence Scale.60 In that study, the Fragile X syndrome findings were hypothesised to reflect inefficient synaptic pruning due to FMRP deficiencies.60 As such, other mechanism(s) and pathways discussed below are likely to underlie these neuroanatomical–executive function relationships in PM females without FXTAS.
Alternative explanations to the observed relationships
The process of XCI, where only one of the two X chromosomes becomes inactivated in females, is complex and relies on a number of factors including DNA methylation, non-coding RNAs and nuclear protein. DNA methylation is an important process in the regulation of XCI and gene expression. DNA hydroxymethylation (5-hydroxymethylcytosine (5hmC)), is thought to be an epigenetic modifier and a possible intermediate product within an active DNA demethylation pathway, potentially having a role in both neurodevelopmental and neurodegenerative diseases/disorders.61, 62, 63 In a FXTAS mouse model, 5hmC levels were found to be reduced compared to wild-type littermates, suggesting that for PM individuals, 5hmC may have a neurodegenerative role.64 Moreover, non-coding RNAs are most commonly derived from intragenic DNA regions.65 Specifically, RNA:DNA hybrids are thought to form at the location of FMR1 intron 1 CpG sites39 and may also have a role in XCI. Further, overexpression of ASFMR1 and long non-coding RNA have previously been reported in PM individuals,66 and have also been associated with parkinsonism and mitochondrial dysfunction.24 Future studies should explore the contribution of the aforementioned pathways as alternative explanations for the relationships observed in this study between FMR1 intron 1 methylation and phenotype measures.
Conclusion
Overall, understanding how epigenetic changes influence neuroanatomy, executive function and clinical outcomes is highly important for both FMR1 PM- and FM-related disorders, and broader neurological disorders influenced by abnormal XCI. Although preliminary, this is, to our knowledge, the first study to link FMR1 intron 1 methylation and neuroanatomical structure in PM and control females. Second, FMR1 intron 1 methylation produced the greatest number of associations (for both phenotype measures), compared with FMR1 exon 1 methylation, AR, CGG repeat size and FMR1 mRNA levels in the blood, confirming our previous observation.22 Frontal and parietal cortical thickness, as well as white matter hypointensities, in brain regions that support executive function, also negatively related to composite cognitive scores. Importantly, differences in the relationships between FMR1 intron 1 methylation and left middle frontal gyrus thickness, and between CpG site 8/9 and frontal and parietal cortical thickness, suggest that XCI skewing in controls may be critical when assessing changes in cortical thickness in females with other neurological diseases. Whereas we provide specific hypotheses regarding the mechanisms underlying such relationships, further confirmatory analysis of the molecular pathways that link FMR1 intron 1 methylation to neuroanatomical structure and executive dysfunction are needed to support these assertions for the PM neurocognitive phenotype and in normal neurobiology. Importantly, together with our previous studies, the utility of FREE2 methylation, particularly methylation of the 5′ FMR1 intron 1 region, as a sensitive measure that relates to both neuroanatomical structure and executive dysfunction in PM females without FXTAS, has been now confirmed.
References
Godler DE, Tassone F, Loesch DZ, Taylor AK, Gehling F, Hagerman RJ et al. Methylation of novel markers of fragile X alleles is inversely correlated with FMRP expression and FMR1 activation ratio. Hum Mol Genet 2010; 75: 255–260.
Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL et al. Incidence of Fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet 2009; 85: 503–514.
Tassone F, Long KP, Tong TH, Lo J, Gane LW, Berry-Kravis E et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med 2012; 4: 100–113.
Arocena DG, Iwahashi CK, Won N, Beilina A, Ludwig AL, Tassone F et al. Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG-repeat RNA in human cultured neural cells. Hum Mol Genet 2005; 14: 3661–3671.
Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen L-A, W. F M et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet 2003; 12: 949–959.
Todd PK, Oh SY, Krans A, He F, Sellier C, Frazer M et al. CGG repeat associated translation mediates neurodegeneration in Fragile X tremor ataxia syndrome. Neuron 2013; 78: 440–455.
Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J et al. Intention tremor, parkinsonism, and generalised brain atrophy in male carriers of fragile X. Neurology 2001; 57: 127–130.
Cornish KM, Hocking DR, Moss SA, Kogan CS . Selective executive markers of at-risk profiles associated with the fragile X premutation. Neurology 2011; 77: 618–622.
Cornish KM, Kogan CS, Li L, Turk J, Jacquemont S, Hagerman RJ . Lifespan changes in working memory in fragile X premutation males. Brain Cogn 2009; 69: 551–558.
Cornish KM, Li L, Kogan CS, Jacquemont S, Turk J, Dalton A et al. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex 2008; 44: 628–636.
Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Srivastava S, Tassone F, Harvey D et al. Young adult female fragile X premutation carriers show age- and genetically-modulated cognitive impairments. Brain Cogn 2011; 75: 255–260.
Semenza C, Bonollo S, Polli R, Busana C, Pignatti R, Iuculano T et al. Genetics and mathematics: FMR1 premutation female carriers. Neuropsychologia 2012; 50: 3757–3763.
Kraan CM, Hocking DR, Georgiou-Karistianis N, Metcalfe SA, Archibald AD, Fielding J et al. Impaired response inhibition is associated with self-reported symptoms of depression, anxiety, and ADHD in female FMR1 premutation carriers. Am J Med Genet 2014; 165: 41–51.
Kraan CM, Hocking DR, Georgiou-Karistianis N, Metcalfe SA, Archibald AD, Fielding J et al. Cognitive-motor interference during postural control indicates at-risk cerebellar profiles in females with the FMR1 premutation. Behav Brain Res 2013; 253: 329–336.
Shelton AL, Cornish K, Kraan C, Georgiou-Karistianis N, Metcalfe SA, Bradshaw JL et al. Exploring inhibitory deficits in female premutation carriers of fragile X syndrome: through eye movements. Brain Cogn 2014; 85: 201–208.
Cohen S, Masyn K, Adams J, Hessl D, Rivera S, Tassone F et al. Molecular and imaging correlates of the fragile X-associated tremor ataxia syndrome. Neurology 2006; 67: 1426–1431.
Filley CM, Brown MS, Onderko K, Ray M, Bennett RE, Berry-Kravis E et al. White matter disease and cognitive impairment in FMR1 premutation carriers. Neurology 2015; 84: 2146–2152.
Hippolyte L, Battistell G, Perrin AG, Fornari E, Cornish KM, Beckmann JS et al. Investigation of memory, executive functions and anatomical correlates in asymptomatic FMR1 premutation carriers. Neurobiol Aging 2014; 35: 1939–1946.
Jäkälä P, Hänninen T, Ryynänen M, Laakso M, Partanen K, Mannermaa A et al. Fragile-X: Neuropsychological test performance, CCG triplet repeat lengths, and hippocampal volumes. J Clin Invset 1997; 100: 331–338.
Wang JY, Hessl D, Schneider A, Tassone F, Hagerman RJ, Rivera SM . Fragile X-associated tremor/ataxia syndrome: influence of the FMR1 gene on motor fiber tracts in males with normal and premutation alleles. JAMA Neurol 2013; 70: 1022–1029.
Hashimoto R, Javan AK, Tassone F, Hagerman RJ, Rivera SM . A voxel-based morphometry study of grey matter loss in fragile X-assocaited tremor/ataxia syndrome. Brain 2011; 134: 863–878.
Cornish KM, Kraan CM, Bui MQ, Bellgrove MA, Metcalfe SA, Troller JN et al. Novel methylation markers of the dysexecutive-psychiatric phenotype in FMR1 premutation women. Neurology 2015; 84: 1631–1638.
Metcalfe S, Jacques A, Archibald A, Burgess T, Collins V, Henry A et al. A model for offering carrier screening for fragile X syndrome to nonpregnant women: results from a pilot study. Genet Med 2008; 10: 525–535.
Loesch D, Godler DE, Evans A, Bui QM, Gehling F, Kotschet K et al. Evidence for the toxicity of bidirectional transcripts and mitochondrial dysfunction in blood associated with small CGG expansions in the FMR1 gene in patients with parkinsonism. Genet Med 2011; 13: 392–399.
Godler DE, Slater HR, Bui QM, Ono M, Gehling F, Francis D et al. FMR1 intron 1 methylation predicts FMRP expression in blood of female carriers of expanded FMR1 alleles. J Mol Diagn 2011; 13: 528–536.
Burgess PW, Shallice T . The Hayling and Brixton Tests. Thames Valley Test Company Limited: Edmunds, England, 1997.
Jamadar S, Johnson BP, Clough M, Egan GF, Fielding J . Behavioural and neural plasticity of ocular motor control: changes in performance and fMRI activity following antisaccade training. Front Hum Neurosci 2015; 9.
Barton JJS, Greenzang C, Hefter R, Edelman JA, Manoach DS . Switching, plasticity, and prediction in a saccade task-switch paradigm. Exp Brain Res 2006; 168: 76–87.
Chan JL, DeSouza JFX . The effects of attentional load on saccadic task switching. Exp Brain Res 2013; 227: 301–309.
DeSimone K, Weiler J, Aber GS, Heath M . The unidirectional prosaccade switch-cost: Correct and error antisaccades differentially influence the planning times for subsequent prosaccades. Vis Res 2014; 96: 17–24.
Fischl B, Dale AM . Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97: 11050–11055.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 2002 33: 341–355.
Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage 2004; 23: S69–S84.
Fischl B . FreeSurfer. NeuroImage 2012; 62: 774–781.
McDowell JE, Dyckman KA, Austin BP, Clementz BA . Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn 2008; 68: 255–270.
Munoz DP, Everling S . Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosc 2004; 5: 218–228.
Jamadar S, Fielding J, Egan G . Quantitative meta-analysis reveals consistent fMRI activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Front Cogn 2013; 4: 749.
Wechsler D . Wechsler abbreviated scale of intelligence (WASI). Pearson Inc San Antonio 1999.
Colak D, Zaninovic N, Cohen MS, Rosenwaks Z, Yang WY, Gerhardt J et al. Promoter-bound trinucleotide repeat mRNA drives epigenetic silencing in fragile X syndrome. Science 2014; 343: 1002–1005.
Robertson KD . DNA methylation and human disease. Nat Rev Genet 2005; 6: 597–610.
Neidhart M. Chapter 3 - DNA methylation and epigenetic biomarkers in non-neoplastic diseases. In: DNA Methylation and Complex Human Disease. Academic Press: Oxford, UK, 2016 pp 29–43.
Zannas AS, West AE . Epigenetics and the regulation of stress vulnerability and resilience. Neuroscience 2014; 264: 157–170.
Godler DE, Slater HR, Amor D, Loesch DZ . Methylation analysis of fragile X-related epigenetic elements may provide newborn screening test for fragile X syndrome. Genet Med 2010; 12: 595.
Godler DE, Inaba Y, Shi EZ, Skinner C, Bui QM, Francis D et al. Relationships between age and epi-genotype of the FMR1 exon 1/intro 1 boundary are consistent with non-random X-chromosome inactivation in FM individuals, with the selection for the unmethylated state being most significant between birth and puberty. Hum Mol Genet 2013; 22: 1516-1524.
Godler DE, Slater HR, Bui QM, Storey E, Ono MY, Gehling F et al. Fragile X mental retardation (FMR1) intro 1 methylation in blood predicts verbal cognitive impairment in female carriers of expanded FMR1 alleles: evidence from a pilot study. Clin Chem 2012; 58: 590–598.
Inaba Y, Herlihy AS, Schwartz CE, Skinner C, Bui QM, Cobb J et al. Fragile X-related element 2 methylation analysis may provide a suitable option for inclusion of fragile X syndrome and/or sex chromosome aneuploidy into newborn screening: a technical validation study. Genet Med 2013; 15: 290–298.
Pastori C, Peschansky VJ, Barbouth D, Mehta A, Silva JP, Wahlestedt C . Comprehensive analysis of the transcriptional landscape of the human FMR1 gene reveals two new long noncoding RNAs differentially expressed in Fragile X syndrome and Fragile X-associated tremor/ataxia syndrome. Hum Genet 2014; 133: 59–67.
Shelton AL, Cornish KM, Godler DE, Clough M, Kraan C, Bui MQ et al. Delineation of the working memory profile in female FMR1 premutation carriers: the effect of cognitive load on ocular motor responses. Behav Brain Res 2015; 282: 194–200.
Hocking DR, Kraan CM, Godler DE, Bui QM, Li X, Bradshaw JL et al. Evidence linking FMR1 mRNA and attentional demands of stepping and postural control in women with the premutation. Neurobiol Aging 2015; 36: 1400–1408.
Brasa S, Mueller A, Jacquemont S, Hahne F, Rozenberg I, Peters T et al. Reciprocal changes in DNA methylation and hydroxymethylation and a broad repressive epigenetic switch characterize FMR1 transcriptional silencing in fragile X syndrome. Clin Epigenet 2016; 8: 1–15.
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J . qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 2007; 8: R19–R19.
Kraan CM, Cornish KM, Bui QM, Li X, Slater HR, Godler DE . Beta-glucuronidase mRNA levels are correlated with gait and working memory in premutation females: understanding the role of FMR1 premutation alleles. Sci Rep 2016; 6: 29366.
Fortea J, Sala-Llonch R, Bartres-Faz D, Bosch B, Llado A, Bargallo N et al. Increased cortical thickness and caudate volume precede atrophy in PSEN1 mutation carriers. J Alzheim Dis 2010; 22: 909–922.
Madhyastha TM, Askren MK, Boord P, Zhang J, Leverenz JB, Grabowski TJ . Cerebral perfusion and cortical thickness indicate cortical involvement in mild Parkinson's disease. Mov Disord 2015; 30: 1893–1900.
Jubault T, Gagnon JF, Karama S, Ptito A, Lafontaine AL, Evans AC et al. Patterns of cortical thickness and surface area in early Parkinson's disease. Neuroimage 2011; 55: 462–467.
Qiu L, Lui S, Kuang W, Huang X, Li J, Li J et al. Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Transl Psychiatry 2014; 4: e378.
Brüuhl AB, Hänggi J, Baur V, Rufer M, Delsignore A, Weidt S et al. Increased cortical thickness in a frontoparietal network in social anxiety disorder. Hum Brain Mapp 2013; 35: 2966–2977.
Gunning-Dixon FM, Raz N . The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology 2000; 14: 224–234.
Alahyane N, Salemme R, Urquizar C, Cotti J, Guillaume A, Vercher JL et al. Oculomotor plasticity: are mechanisms of adaptation for reactive and voluntary saccades separate? Brain Res 2007; 1135: 107–121.
Meguid NA, Fahim C, Sami R, Nashaat NH, Yoon U, Anwar M et al. Cognition and lobar morphology in full mutation boys with fragile X syndrome. Brain Cogn 2012; 78: 74–84.
Branco MR, Ficz G, Reik W . Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet 2012; 13: 7–13.
Cheng Y, Bernstein A, Chen D, Jin P . 5-Hyroxymethylcytosine: a new player in brain disorders? Exp Neurol 2015; 268: 3–9.
Al-Mahdawi S, Virmouni SA, Pook MA . The emerging role of 5-hydroxymethylcytosine in neurodegenerative diseases. Front Neurosci 2014; 8: 397.
Yao B, Lin L, Street RC, Zalewski ZA, Galloway JN, Wu H et al. Genome-wide alteration of 5-hydroxymethylcytosine in a mouse model of fragile X-associated tremor/ataxia syndrome. Hum Mol Genet 2014; 23: 1095–1107.
St Laurent G, Shtokalo D, Tackett MR, Yang Z, Eremina T, Wahlestedt C et al. Intronic RNAs constitute the major fraction of the non-coding RNA in mammalian cells. BMC Genomics 2012; 13: 1–23.
Ladd PD, Smith LE, Rabaia NA, Moore JM, Georges SA, Hansen RS et al. An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum Mol Genet 2007; 16: 3174–3187.
Acknowledgements
We express our thanks to the Fragile X Association of Australia and Fragile X Alliance for their support in recruitment. We also thank Jonathan Whitty from Healthscope Pathology and Erin Turbitt from the Murdoch Childrens Research Institute for their assistance on the molecular procedures. Finally, we are indebted to all the families who participated in this research. This work was funded by Australian Research Council (ARC) Discovery grant (DP110103346) to K Cornish and J Fielding and Australian Postgraduate Award to A Shelton. Salary for the molecular component, in part, was supported by the National Health and Medical Research Council (Project Grants 104299 and 1103389) to D Godler.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D Golder is an inventor of the following patents, PCT/AU2010/001134; filing no. AU2010/903595; filing no. AU2011/902500; filing no. 2013/900227; related to the technology described in this publication. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Shelton, A., Cornish, K., Kolbe, S. et al. Brain structure and intragenic DNA methylation are correlated, and predict executive dysfunction in fragile X premutation females. Transl Psychiatry 6, e984 (2016). https://doi.org/10.1038/tp.2016.250
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2016.250