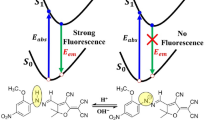
Abstract
Photophysical and TPA properties of series of push—pull aryl(bi)thiophene chromophores bearing electron-donating (D) and electron-withdrawing (A) end-groups of increasing strength are presented. All compounds show an intense intramolecular charge transfer (ICT) absorption band in the visible region. Increasing the D and/or A strength as well as the length of the conjugated path induces bathochromic and hyperchromic shifts of the absorption band as reported for analogous push—pull polyenes. Yet, in contrast with corresponding push—pull polyenes, a significant increase in fluorescence is observed. In particular, chromophores built from a phenyl—bithienyl conjugated path and bearing strong D and A end-groups were found to combine very large one and two-photon brightness as well as strong emission in the red/NIR region. These molecules hold promise as biphotonic fluorescent probes for bioimaging.
Similar content being viewed by others
References
J. Roncali, Synthetic principles for bandgap control in linear pi-conjugated systems, Chem. Rev. 1997, 97, 173–205.
A. Mishra, C.-Q. Ma and P. Baeuerle, Functional oligothiophenes: molecular design for multidimensional nanoarchitectures and their applications, Chem. Rev. 2009, 109, 1141–1276.
Handbook of Thiophene-Based Materials: Applications in Organic electronics and Photonics, ed. F. Perepichka and D. F. Perepichka, Wiley, New York, 2009.
G. Barbarella, M. Melucci and G. Sotgiu, The versatile thiophene: an overview of recent research on thiophene-based materials, Adv. Mater. 2005, 17, 1581–1593.
A. Pron, P. Gawrys, M. Zagorska, D. Djurado and R. Demadrille, Electroactive materials for organic electronics: preparation strategies, structural aspects and characterization techniques, Chem. Soc. Rev. 2010, 39, 2577–2632.
P. M. Beaujuge, J. M. J. Fréchet, Molecular design and ordering effects in p-functional materials for transistor and solar cell applications, J. Am. Chem. Soc. 2011, 133, 20009–20029.
A. R. Murphy, J. M. J. Fréchet, Organic semiconducting oligomers for use in thin film transistors, Chem. Rev. 2007, 107, 1066–1096.
C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Semiconducting p-conjugated systems in field-effect transistors: a material odyssey of organic electronics, Chem. Rev. 2011, 112, 2208–2267.
J. Roncali, Linear pi-conjugated systems derivatized with C-60-fullerene as molecular heterojunctions for organic photovoltaics, Chem. Soc. Rev. 2005, 34, 483–495.
J. Pina, S. S. de Melo, H. D. Burrows, R. M. F. Batista, S. P. G. Costa and M. M. M. Raposo, Spectral and photophysical characterization of donor-pi-acceptor arylthienyl- and bithienyl-benzothiazole derivatives in solution and solid state, J. Phys. Chem. A 2007, 111, 8574–8578.
R. M. F. Batista, S. P. G. Costa, M. Belsley and M. M. M. Raposo, Synthesis and optical properties of novel, thermally stable phenanthrolines bearing an arylthienyl-imidazo conjugation pathway, Dyes Pigm. 2009, 80, 329–336.
F. Mariano, M. Mazzeo, Y. Duan, G. Barbarella, L. Favaretto, S. Carallo, R. Cingolani and G. Gigli, Very low voltage and stable p-i-n organic light-emitting diodes using a linear S, S-dioxide oligothiophene as emitting layer, Appl. Phys. Lett. 2009, 94, 063510.
X. H. Zhu, J. B. Peng, Y. Caoa and J. Roncali, Solution-processable single-material molecular emitters for organic light-emitting devices, Chem. Soc. Rev. 2011, 40, 3509–3524.
F. Zhang, D. Wu, Y. Xu and X. Feng, Thiophene-based conjugated oligomers for organic solar cells, J. Mater. Chem. 2011, 21, 17590–17600.
A. Mishra, P. Bäuerle, Small molecule organic semiconductors on the move: promises for future solar energy technology, Angew. Chem., Int. Ed. 2012, 51, 2020–2067.
S. Ko, E. Verploegen, S. Hong, R. Mondal, E. T. Hoke, M. F. Toney, M. D. McGehee and Z. Bao, 3,4-Disubstituted polyalkylthiophenes for high-performance thin-film transistors and photovoltaics, J. Am. Chem. Soc. 2011, 133, 16722–16725.
C. Mallet, G. Savitha, M. Allain, V. Kozmik, J. Svoboda, P. Frere and J. Roncali, Synthesis and electronic properties of D–A–D triads based on 3-alkoxy-4-cyanothiophene and benzothienothiophene blocks, J. Org. Chem. 2012, 77, 2041–2046.
Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Synthesis of conjugated polymers for organic solar cell applications, Chem. Rev. 2009, 109, 5868–5923.
R. Schueppel, K. Schmidt, C. Uhrich, K. Schulze, D. Wynands, J. L. Bredas, E. Brier, E. Reinold, H. B. Bu, P. Baeuerle, B. Maennig, M. Pfeiffer and K. Leo, Optimizing organic photovoltaics using tailored heterojunctions: A photoinduced absorption study of oligothiophenes with low band gaps, Phys. Rev. B 2008, 77, 085311.
I. D. W. Samuel and G. A. Turnbull, Organic semiconductor lasers, Chem. Rev. 2007, 107, 1272–1295.
J.-i. Nishida, T. Miyagawa and Y. Yamashita, Novel thiophene oligomers containing a redox active hexaarylethane unit, Org. Lett. 2004, 6, 2523–2526.
S. R. Marder, C. B. Gorman, B. G. Tiemann and L. T. Cheng, Stronger acceptors can diminish nonlinear optical-response in simple donor–acceptor polyenes, J. Am. Chem. Soc. 1993, 115, 3006–3007.
S. R. Marder, L. T. Cheng, B. G. Tiemann, A. C. Friedli, M. Blanchard-Desce, J. W. Perry and J. Skindhoj, Large 1st hyperpolarizabilities in push–pull polyenes by tuning of the bond-length alternation and aromaticity, Science 1994, 263, 511–514.
S. P. G. Costa, R. M. F. Batista, P. Cardoso, M. Belsley and M. M. M. Raposo, 2-arylthienyl-substituted 1,3-benzothiazoles as new nonlinear optical chromophores, Eur. J. Org. Chem. 2006 3938–3946.
R. M. F. Batista, S. P. G. Costa, M. Belsley, C. Lodeiro and M. M. M. Raposo, Synthesis and characterization of novel (oligo)thienyl-imidazo-phenanthrolines as versatile pi-conjugated systems for several optical applications, Tetrahedron 2008, 64, 9230–9238.
M. M. M. Raposo, A. Ferreira, M. Belsley and J. Moura, 5’-Alkoxy-2,2’-bithiophene azo dyes: a novel promising series of NLO-chromophores, Tetrahedron 2008, 64, 5878–5884.
C. Herbivo, A. Comel, G. Kirsch, A. M. C. Fonseca, M. Belsley and M. M. M. Raposo, Synthesis and characterization of novel, thermally stable 2-aryl-5-dicyanovinylthiophenes and 5-aryl-5’-dicyanovinyl-2,2’-bithiophenes as potentially promising non-linear optical materials, Dyes Pigm. 2010, 86, 217–226.
M. Blanchard-Desce, V. Alain, P. V. Bedworth, S. R. Marder, A. Fort, C. Runser, M. Barzoukas, S. Lebus and R. Wortmann, Large quadratic hyperpolarizabilities with donor–acceptor polyenes exhibiting optimum bond length alternation: correlation between structure and hyperpolarizability, Chem.–Eur. J. 1997, 3, 1091–1104.
G. Ridolfi, N. Camaioni, P. Samori, M. Gazzano, G. Accorsi, N. Armaroli, L. Favaretto and G. Barbarella, All-thiophene donor–acceptor blends: photophysics, morphology and photoresponse, J. Mater. Chem. 2005, 15, 895–901.
I. Palama, F. Di Maria, I. Viola, E. Fabiano, G. Gigli, C. Bettini and G. Barbarella, Live-cell-permeant thiophene fluorophores and cell-mediated formation of fluorescent fibrils, J. Am. Chem. Soc. 2011, 133, 17777–17785.
M. L. Capobianco, G. Barbarella and A. Manetto, Oligothiophenes as fluorescent markers for biological applications, Molecules 2012, 17, 910–933.
M. Duca, B. Dozza, E. Lucarelli, S. Santi, A. Di Giorgio and G. Barbarella, Fluorescent labeling of human mesenchymal stem cells by thiophene fluorophores conjugated to a lipophilic carrier, Chem. Commun. 2010, 46, 7948–7950.
G. Sotgiu, M. Zambianchi, G. Barbarella, F. Aruffo, F. Cipriani and A. Ventola, Rigid-core fluorescent oligothiophene- S, S-dioxide isothiocyanates. Synthesis, optical characterization, and conjugation to monoclonal antibodies, J. Org. Chem. 2003, 68, 1512–1520.
G. Barbarella, M. Zambianchi, O. Pudova, V. Paladini, A. Ventola, F. Cipriani, G. Gigli, R. Cingolani and G. Citro, Oligothiophene isothiocyanates as a new class of fluorescent markers for biopolymers, J. Am. Chem. Soc. 2001, 123, 11600–11607.
M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Two-photon absorption and the design of two-photon dyes, Angew. Chem., Int. Ed. 2009, 48, 3244–3266.
F. Terenziani, C. Katan, E. Badaeva, S. Tretiak, M. Blanchard-Desce, Enhanced two-photon absorption of organic chromophores: theoretical and experimental assessments, Adv. Mater. 2008, 20, 4641–4678.
G. S. He, L.-S. Tan, Q. Zheng and P. N. Prasad, multiphoton absorbing materials: molecular designs, characterizations, and applications, Chem. Rev. 2008, 108, 1245–1330.
H. M. Kim and B. R. Cho, Two-photon probes for intracellular free metal ions, acidic vesicles, and lipid rafts in live tissues, Acc. Chem. Res. 2009, 42, 863–872.
H. Myung Kim, B. Rae Cho, Two-photon materials with large two-photon cross sections. Structure-property relationship, Chem. Commun. 2009 153–164.
M. Blanchard-Desce, Molecular engineering of NLO-phores for new NLO microscopies, C. R. Phys. 2002, 3, 439–448.
M. Drobizhev, N. S. Makarov, S. E. Tillo, T. E. Hughes and A. Rebane, Describing two-photon absorptivity of fluorescent proteins with a new vibronic coupling mechanism, J. Phys. Chem. B 2012, 116, 1736–1744.
M. Drobizhev, N. S. Makarov, S. E. Tillo, T. E. Hughes and A. Rebane, Two-photon absorption properties of fluorescent proteins, Nat. Methods 2011, 8, 393–399.
W. Denk, J. H. Strickler and W. W. Webb, Two-photon laser scanning fluorescence microscopy, Science 1990, 248, 73–76.
K. Svoboda, W. Denk, D. Kleinfeld and D. W. Tank, In vivo dendritic calcium dynamics in neocortical pyramidal neurons, Nature 1997, 385, 161–165.
H. Meier, Conjugated oligomers with terminal donor–acceptor substitution, Angew. Chem., Int. Ed. 2005, 44, 2482–2506.
S. Yao, H.-Y. Ahn, X. Wang, J. Fu, E. W. Van Stryland, D. J. Hagan and K. D. Belfield, Donor–Acceptor–Donor Fluorene Derivatives for Two-Photon Fluorescence Lysosomal Imaging, J. Org. Chem. 2010, 75, 3965–3974.
C. D. Andrade, C. O. Yanez, L. Rodriguez and K. D. Belfield, A series of fluorene-based two-photon absorbing molecules: synthesis, linear and nonlinear characterization, and bioimaging, J. Org. Chem. 2010, 75, 3975–3982.
D. R. Larson, W. R. Zipfel, R. M. Williams, S. W. Clark, M. P. Bruchez, F. W. Wise and W. W. Webb, Water-soluble quantum dots for multiphoton fluorescence imaging in vivo, Science 2003, 300, 1434–1436.
L. Ventelon, S. Charier, L. Moreaux, J. Mertz, M. Blanchard-Desce, Nanoscale push–push dihydrophenanthrene derivatives as novel fluorophores for two-photon-excited fluorescence, Angew. Chem., Int. Ed. 2001, 40, 2098–2101.
T. R. Krishna, M. Parent, M. H. V. Werts, L. Moreaux, S. Gmouh, S. Charpak, A.-M. Caminade, J.-P. Majoral, M. Blanchard-Desce, Water-soluble dendrimeric two-photon tracers for in vivo imaging, Angew. Chem., Int. Ed. 2006, 45, 4645–4648.
O. Mongin, T. R. Krishna, M. H. V. Werts, A.-M. Caminade, J.-P. Majoral, M. Blanchard-Desce, A modular approach to two-photon absorbing organic nanodots: brilliant dendrimers as an alternative to semiconductor quantum dots?, Chem. Commun. 2006 915–917.
V. Parthasarathy, S. Fery-Forgues, E. Campioli, G. Recher, F. Terenziani, M. Blanchard-Desce, Dipolar versus octupolar triphenylamine-based fluorescent organic nanoparticles as brilliant one- and two-photon emitters for (Bio)imaging, Small 2011, 7, 3219–3229.
S. Kim, T. Y. Ohulchanskyy, H. E. Pudavar, R. K. Pandey and P. N. Prasad, Organically Modified Silica Nanoparticles Co-encapsulating photosensitizing drug and aggregation-enhanced two-photon absorbing fluorescent dye aggregates for two-photon photodynamic therapy, J. Am. Chem. Soc. 2007, 129, 2669–2675.
K. Ogawa and Y. Kobuke, Design of two-photon absorbing materials for molecular optical memory and photodynamic therapy, Org. Biomol. Chem. 2009, 7, 2241–2246.
C. B. Nielsen, J. Arnbjerg, M. Johnsen, M. Jørgensen and P. R. Ogilby, Molecular tuning of phenylene-vinylene derivatives for two-photon photosensitized singlet oxygen production, J. Org. Chem. 2009, 74, 9094–9104.
J. R. Starkey, A. K. Rebane, M. A. Drobizhev, F. Meng, A. Gong, A. Elliott, K. McInnerney and C. W. Spangler, New two-photon activated photodynamic therapy sensitizers induce xenograft tumor regressions after near-IR laser treatment through the body of the host mouse, Clin. Cancer Res. 2008, 14, 6564–6573.
H. A. Collins, M. Khurana, E. H. Moriyama, A. Mariampillai, E. Dahlstedt, M. Balaz, M. K. Kuimova, M. Drobizhev, V. X. D. Yang, D. Phillips, A. Rebane, B. C. Wilson and H. L. Anderson, Blood-vessel closure using photosensitizers engineered for two-photon excitation, Nat. Photonics 2008, 2, 420–424.
M. Gary-Bobo, Y. Mir, C. Rouxel, D. Brevet, I. Basile, M. Maynadier, O. Vaillant, O. Mongin, M. Blanchard-Desce, A. Morère, M. Garcia, J.-O. Durand and L. Raehm, Mannose-functionalized mesoporous silica nanoparticles for efficient two-photon photodynamic therapy of solid tumors, Angew. Chem., Int. Ed. 2011, 50, 11425–11429.
M. Gary-Bobo, Y. Mir, C. Rouxel, D. Brevet, O. Hocine, M. Maynadier, A. Gallud, A. Da Silva, O. Mongin, M. Blanchard-Desce, S. Richeter, B. Loock, P. Maillard, A. Morere, M. Garcia, L. Raehm and J. O. Durand, Multifunctionalized mesoporous silica nanoparticles for the in vitro treatment of retinoblastoma: drug delivery, one and two-photon photodynamic therapy, Int. J. Pharm. 2012, 432, 99–104.
L. Donato, A. Mourot, C. M. Davenport, C. Herbivo, D. Warther, J. Léonard, F. Bolze, J.-F. Nicoud, R. H. Kramer, M. Goeldner and A. Specht, Water-soluble, donor–acceptor biphenyl derivatives in the 2-(o-Nitrophenyl)propyl series: highly efficient two-photon uncaging of the neurotransmitter ?-aminobutyric acid at ? = 800 nm, Angew. Chem., Int. Ed. 2012, 51, 1840–1843.
D. Warther, S. Gug, A. Specht, F. Bolze, J. F. Nicoud, A. Mourot and M. Goeldner, Two-photon uncaging: new prospects in neuroscience and cellular biology, Bioorg. Med. Chem. 2010, 18, 7753–7758.
G. C. R. Ellis-Davies, Two-photon microscopy for chemical neuroscience, ACS Chem. Neurosci. 2011, 2, 185–197.
G. C. R. Ellis-Davies, Caged compounds: photorelease technology for control of cellular chemistry and physiology, Nat. Methods 2007, 4, 619–628.
D. A. Parthenopoulos and P. M. Rentzepis, Three-dimensional optical storage memory, Science 1989, 245, 843–845.
C. C. Corredor, Z.-L. Huang, K. D. Belfield, A. R. Morales and M. V. Bondar, Photochromic polymer composites for two-photon 3D optical data storage, Chem. Mater. 2007, 19, 5165–5173.
P.-A. Bouit, G. Wetzel, G. Berginc, B. Loiseaux, L. Toupet, P. Feneyrou, Y. Bretonnière, K. Kamada, O. Maury and C. Andraud, Near IR nonlinear absorbing chromophores with optical limiting properties at telecommunication wavelengths, Chem. Mater. 2007, 19, 5325–5335.
Q. Zheng, G. S. He and P. N. Prasad, A novel near IR two-photon absorbing chromophore: optical limiting and stabilization performances at an optical communication wavelength, Chem. Phys. Lett. 2009, 475, 250–255.
G. S. He, G. C. Xu, P. N. Prasad, B. A. Reinhardt, J. C. Bhatt, R. McKellar and A. G. Dillard, Two-photon absorption and optical-limiting properties of novel organic compounds, Opt. Lett. 1995, 20, 435–437.
J. E. Ehrlich, X. L. Wu, I. Y. S. Lee, Z. Y. Hu, H. Röckel, S. R. Marder and J. W. Perry, Two-photon absorption and broadband optical limiting with bis-donor stilbenes, Opt. Lett. 1997, 22, 1843–1845.
M. Charlot, N. Izard, O. Mongin, D. Riehl, M. Blanchard-Desce, Optical limiting with soluble two-photon absorbing quadrupoles: structure-property relationships, Chem. Phys. Lett. 2006, 417, 297–302.
G. Lemercier, J.-C. Mulatier, C. Martineau, R. Anémian, C. Andraud, I. Wang, O. Stéphan, N. Amari and P. Baldeck, Two-photon absorption: from optical power limiting to 3D microfabrication, C. R. Chim. 2005, 8, 1308–1316.
S. Maruo, O. Nakamura and S. Kawata, Three-dimensional microfabrication with two-photon-absorbed photopolymerization, Opt. Lett. 1997, 22, 132–134.
S. Kawata, H.-B. Sun, T. Tanaka and K. Takada, Finer features for functional microdevices, Nature 2001, 412, 697–698.
W. Zhou, S. M. Kuebler, K. L. Braun, T. Yu, J. K. Cammack, C. K. Ober, J. W. Perry and S. R. Marder, An efficient two-photon-generated photoacid applied to positive-tone 3D microfabrication, Science 2002, 296, 1106–1109.
F. Claeyssens, E. A. Hasan, A. Gaidukeviciute, D. S. Achilleos, A. Ranella, C. Reinhardt, A. Ovsianikov, X. Shizhou, C. Fotakis, M. Vamvakaki, B. N. Chichkov and M. Farsari, Three-dimensional biodegradable structures fabricated by two-photon polymerization, Langmuir 2009, 25, 3219–3223.
I. Sakellari, E. Kabouraki, D. Gray, V. Purlys, C. Fotakis, A. Pikulin, N. Bityurin, M. Vamvakaki and M. Farsari, Diffusion-assisted high-resolution direct femtosecond laser writing, ACS Nano 2012, 6, 2302–2311.
L. Ventelon, L. Moreaux, J. Mertz, M. Blanchard-Desce, New quadrupolar fluorophores with high two-photon excited fluorescence, Chem. Commun. 1999 2055–2056.
O. Mongin, L. Porrès, L. Moreaux, J. Mertz, M. Blanchard-Desce, Synthesis and photophysical properties of new conjugated fluorophores designed for two-photon-excited fluorescence, Org. Lett. 2002, 4, 719–722.
L. Porrès, O. Mongin, C. Katan, M. Charlot, T. Pons, J. Mertz, M. Blanchard-Desce, Enhanced two-photon absorption with novel octupolar propeller-shaped fluorophores derived from triphenylamine, Org. Lett. 2004, 6, 47–50.
C. Le Droumaguet, O. Mongin, M. H. V. Werts, M. Blanchard-Desce, Towards “smart” multiphoton fluorophores: strongly solvatochromic probes for two-photon sensing of micropolarity, Chem. Commun. 2005 2802–2804.
M. H. V. Werts, S. Gmouh, O. Mongin, T. Pons, M. Blanchard-Desce, Strong modulation of two-photon excited fluorescence of quadripolar dyes by (De)protonation, J. Am. Chem. Soc. 2004, 126, 16294–16295.
M. G. Silly, L. Porrès, O. Mongin, P.-A. Chollet, M. Blanchard-Desce, Optical limiting in the red-NIR range with soluble two-photon absorbing molecules, Chem. Phys. Lett. 2003, 379, 74–80.
O. Mongin, L. Porrès, M. Charlot, C. Katan, M. Blanchard-Desce, Synthesis, fluorescence, and two-photon absorption of a series of elongated rodlike and banana-shaped quadrupolar fluorophores: a comprehensive study of structure-property relationships, Chem.–Eur. J. 2007, 13, 1481–1498.
O. Mongin, A. Pla-Quintana, F. Terenziani, D. Drouin, C. Le Droumaguet, A.-M. Caminade, J.-P. Majoral, M. Blanchard-Desce, Organic nanodots for multiphotonics: synthesis and photophysical studies, New J. Chem. 2007, 31, 1354–1367.
O. Mongin, C. Rouxel, A.-C. Robin, A. Pla-Quintana, T. Rama Krishna, G. Recher, F. Tiaho, A.-M. Caminade, J.-P. Majoral, M. Blanchard-Desce, Brilliant organic nanodots: novel nano-objects for bionanophotonics, Proc. SPIE 2008, 7040, 704006.
M. Guo, O. Varnavski, A. Narayanan, O. Mongin, J.-P. Majoral, M. Blanchard-Desce and T. Goodson, Investigations of energy migration in an organic dendrimer macromolecule for sensory signal amplification, J. Phys. Chem. A 2009, 113, 4763–4771.
O. Mongin, C. Rouxel, J.-M. Vabre, Y. Mir, A. Pla-Quintana, Y. Wei, A.-M. Caminade, J.-P. Majoral, M. Blanchard-Desce, Customized multiphotonics nanotools for bioapplications: soft organic nanodots as an eco-friendly alternative to quantum dots, Proc. SPIE 2009, 7403, 740303.
A. Rebane, M. Drobizhev, N. S. Makarov, E. Beuerman, J. E. Haley, M. K. Douglas, A. R. Burke, J. L. Flikkema and T. M. Cooper, Relation between two-photon absorption and dipolar properties in a series of fluorenyl-based chromophores with electron donating or electron withdrawing substituents, J. Phys. Chem. A 2011, 115, 4255–4262.
C. Herbivo, A. Comel, G. Kirsch and M. M. M. Raposo, Synthesis of 5-aryl-5’-formyl-2,2’-bithiophenes as new precursors for nonlinear optical (NLO) materials, Tetrahedron 2009, 65, 2079–2086.
M. M. M. Raposo, M. C. R. Castro, M. Belsley and A. M. C. Fonseca, Push pull bithiophene azo-chromophores bearing thiazole and benzothiazole acceptor moieties: Synthesis and evaluation of their redox and nonlinear optical properties, Dyes Pigm. 2011, 91, 454–465.
Y.-S. Yen, W.-T. Chen, C.-Y. Hsu, H.-H. Chou, J. T. Lin, M.-C. P. Yeh, Arylamine-based dyes for p-type dye-sensitized solar cells, Org. Lett. 2011, 13, 4930–4933.
F. Zhang, Y.-h. Luo, J.-s. Song, X.-z. Guo, W.-l. Liu, C.-p. Ma, Y. Huang, M.-f. Ge, Z. Bo and Q.-B. Meng, Triphenylamine-based dyes for dye-sensitized solar cells, Dyes Pigm. 2009, 81, 224–230.
E. Ripaud, Y. Olivier, P. Leriche, J. Cornil and J. Roncali, Polarizability and internal charge transfer in thiophene-triphenylamine hybrid pi-conjugated systems, J. Phys. Chem. B 2011, 115, 9379–9386.
P. Leriche, P. Frere, A. Cravino, O. Aleveque and J. Roncali, Molecular engineering of the internal charge transfer in thiophene-triphenylamine hybrid pi-conjugated systems, J. Org. Chem. 2007, 72, 8332–8336.
A. Leliège, P. Blanchard, T. o. Rousseau and J. Roncali, Triphenylamine/tetracyanobutadiene-based D–A–D p-conjugated systems as molecular donors for organic solar cells, Org. Lett. 2011, 13, 3098–3101.
Q. Bricaud, A. Cravino, P. Leriche and J. Roncali, Terthiophene-cyanovinylene p-conjugated polymers as donor material for organic solar cells, Synth. Met. 2009, 159, 2534–2538.
T. Narita, M. Takase, T. Nishinaga, M. Iyoda, K. Kamada and K. Ohta, Star-shaped oligothiophenes with unique photophysical properties and nanostructured polymorphs, Chem.–Eur. J. 2010, 16, 12108–12113.
S. Ellinger, K. R. Graham, P. Shi, R. T. Farley, T. T. Steckler, R. N. Brookins, P. Taranekar, J. Mei, L. A. Padilha, T. R. Ensley, H. Hu, S. Webster, D. J. Hagan, E. W. Van Stryland, K. S. Schanze and J. R. Reynolds, Donor–acceptor–donor-based p-conjugated oligomers for nonlinear optics and near-IR emission, Chem. Mater. 2011, 23, 3805–3817.
X. J. Feng, P. L. Wu, H. L. Tam, K. F. Li, M. S. Wong and K. W. Cheah, Fluorene-based p-conjugated oligomers for efficient three-photon excited photoluminescence and lasing, Chem.–Eur. J. 2009, 15, 11681–11691.
V. Alain, L. Thouin, M. Blanchard-Desce, U. Gubler, C. Bosshard, P. Günter, J. Muller, A. Fort and M. Barzoukas, Molecular engineering of push–pull phenylpolyenes for nonlinear optics: improved solubility, stability, and nonlinearities, Adv. Mater. 1999, 11, 1210–1214.
M. Blanchard-Desce, V. Alain, L. Midrier, R. Wortmann, S. Lebus, C. Glania, P. Krämer, A. Fort, J. Muller and M. Barzoukas, Intramolecular charge transfer and enhanced quadratic optical non-linearities in push pull polyenes, J. Photochem. Photobiol., A 1997, 105, 115–121.
W. Akemann, D. Laage, P. Plaza, M. M. Martin, M. Blanchard-Desce, Photoinduced Intramolecular charge transfer in push–pull polyenes: effects of solvation, electron-donor group, and polyenic chain length, J. Phys. Chem. B 2008, 112, 358–368.
M. Blanchard-Desce, R. Wortmann, S. Lebus, J.-M. Lehn, P. Krämer, Intramolecular charge transfer in elongated donor–acceptor conjugated polyenes, Chem. Phys. Lett. 1995, 243, 526–532.
D. Laage, P. Plaza, M. Blanchard-Desce and M. M. Martin, Multiple relaxation pathways in push–pull polyenes, Photochem. Photobiol. Sci. 2002, 1, 526–535.
M. Barzoukas, C. Runser, A. Fort, M. Blanchard-Desce, A two-state description of (hyper) polarizabilities of push–pull molecules based on a two-form model, Chem. Phys. Lett. 1996, 257, 531.
M. Barzoukas, M. Blanchard-Desce, Molecular engineering of push–pull dipolar and quadrupolar molecules for two-photon absorption: a multivalence-bond states approach, J. Chem. Phys. 2000, 113, 3951–3959.
S. J. Strickler and R. A. Berg, Relation between absorption intensity and fluorescence lifetime of molecules, J. Chem. Phys. 1962, 37, 814–822.
C. Xu and W. W. Webb, Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm, J. Opt. Soc. Am. B 1996, 13, 481–491.
M. A. Albota, C. Xu and W. W. Webb, Two-photon fluorescence excitation cross sections of biomolecular probes from 690 to 960 nm, Appl. Opt. 1998, 37, 7352–7356.
A. Rebane, M. A. Drobizhev, N. S. Makarov, E. Beuerman, C. Nacke and J. Pahapill, Modeling non-Lorentzian two-photon absorption line shape in dipolar chromophores, J. Lumin. 2010, 130, 1055–1059.
Y. M. Poronik, V. Hugues, M. Blanchard-Desce and D. T. Gryko, Octupolar merocyanine dyes: a new class of nonlinear optical chromophores, Chem.–Eur. J. 2012, 18, 9258–9266.
A. M. Brouwer, Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report), Pure Appl. Chem. 2011, 83, 2213–2228.
M. H. V. Werts, N. Nerambourg, D. Pélégry, Y. Le Grand, M. Blanchard-Desce, Action cross sections of two-photon excited luminescence of some Eu(iii) and Tb(iii) complexes, Photochem. Photobiol. Sci. 2005, 4, 531–538.
C. Katan, S. Tretiak, M. H. V. Werts, A. J. Bain, R. J. Marsh, N. Leonczek, N. Nicolaou, E. Badaeva, O. Mongin, M. Blanchard-Desce, Two-photon transitions in quadrupolar and branched chromophores: experiment and theory, J. Phys. Chem. B 2007, 111, 9468–9483.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of a themed issue in honour of Jean-Pierre Desvergne on the occasion of his 65th birthday.
Electronic supplementary information (ESI) available. See DOI: 10.1039/c2pp25258a
Rights and permissions
About this article
Cite this article
Genin, E., Hugues, V., Clermont, G. et al. Fluorescence and two-photon absorption of push—pull aryl(bi)thiophenes: structure—property relationships. Photochem Photobiol Sci 11, 1756–1766 (2012). https://doi.org/10.1039/c2pp25258a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c2pp25258a