Experimental and computational studies on ruthenium(ii) bis-diimine complexes of N,N′-chelate ligands: the origin of changes in absorption spectra upon oxidation and reduction†
Abstract
This work presents an interpretation of the origin of changes in absorption spectra upon one-electron oxidation and reduction of two ruthenium polypyridyl complexes based on a combination of UV-Vis spectroelectrochemical experiments and theoretical calculations using the Gaussian 09 program. A bis-chelating ligand containing a p-bromobenzoylthiourea unit connected to 1,10-phenanthroline (phen-p-BrBT) has been prepared. Complexation of phen-p-BrBT to ruthenium bis-diimine centres, Ru(N-N)2 [N-N = 2,2′-bipyridine (bpy) or 1,10-phenanthroline (phen)], affords octahedral Ru(II) tris–diimine complexes that are synthesised and structurally characterised. The two complexes exhibit similar MLCT bands and electronic energy levels owing to the similar electronic structures of the bpy and phen ligands. However, [Ru(phen)2(phen-p-BrBT)]2+ exhibits a slightly broader visible region 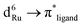 MLCT (metal-to-ligand-charge transfer) band than [Ru(bpy)2(phen-p-BrBT)]2+ as expected from a slightly more delocalised π-electron system in the phen diimine ligands. In addition, the π → π* absorption in the UV is blue-shifted for [Ru(phen)2(phen-p-BrBT)]2+ relative to that for [Ru(bpy)2(phen-p-BrBT)]2+, because of greater stabilisation of the bpy HOMO relative to that of phen. The extra C–C bond in phen produces greater delocalisation of electron density leading to a blue-shift in the π → π* transition. The MLCT band is blue-shifted and diminished in intensity upon oxidation due to stabilisation of the Ru d-orbitals by removal of one electron. A new broad absorption band appears in the UV region upon reduction. The new transition is attributed to a blue-shift of the first MLCT transition for [Ru(bpy)2(phen-p-BrBT)]2+ and a red-shift of the second MLCT transition for [Ru(phen)2(phen-p-BrBT)]2+. The new transitions originate from destabilisation or stabilisation of the ligand LUMO orbitals relative to the Ru d-orbitals. A red-shift of the UV band in the initial complex also contributes to the new band produced upon reduction of [Ru(bpy)2(phen-p-BrBT)]2+. The new band does not involve an n(C
MLCT (metal-to-ligand-charge transfer) band than [Ru(bpy)2(phen-p-BrBT)]2+ as expected from a slightly more delocalised π-electron system in the phen diimine ligands. In addition, the π → π* absorption in the UV is blue-shifted for [Ru(phen)2(phen-p-BrBT)]2+ relative to that for [Ru(bpy)2(phen-p-BrBT)]2+, because of greater stabilisation of the bpy HOMO relative to that of phen. The extra C–C bond in phen produces greater delocalisation of electron density leading to a blue-shift in the π → π* transition. The MLCT band is blue-shifted and diminished in intensity upon oxidation due to stabilisation of the Ru d-orbitals by removal of one electron. A new broad absorption band appears in the UV region upon reduction. The new transition is attributed to a blue-shift of the first MLCT transition for [Ru(bpy)2(phen-p-BrBT)]2+ and a red-shift of the second MLCT transition for [Ru(phen)2(phen-p-BrBT)]2+. The new transitions originate from destabilisation or stabilisation of the ligand LUMO orbitals relative to the Ru d-orbitals. A red-shift of the UV band in the initial complex also contributes to the new band produced upon reduction of [Ru(bpy)2(phen-p-BrBT)]2+. The new band does not involve an n(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) → π* transition. Although both complexes show subtle differences in behaviour, their spectral changes are distinct, and the origin of changes in their absorption spectra upon oxidation and reduction is successfully interpreted.
S) → π* transition. Although both complexes show subtle differences in behaviour, their spectral changes are distinct, and the origin of changes in their absorption spectra upon oxidation and reduction is successfully interpreted.



 Please wait while we load your content...
Please wait while we load your content...
