A correlation of substituent effects with the acidity of aromatic tetrazolic acids
Abstract
The values of thermodynamic dissociation constants, Ka, have been determined for ortho-, meta-, and para-substituted 5-phenyltetrazoles (referred to as tetrazolic acids) and the effect of the nature of the substituent on the strength of the acids are discussed. The pKa values, determined in 50%(v/v) ethanol–water and in 75%(v/v) dimethyl sulphoxide–water are correlated with the substituent constants σm and σp, which are equal to 0.64 and 0.56 respectively for the tetrazolyl substituent. To evaluate the contribution of the field and resonance effects, the Ka values were correlated with the î…� and 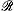 constants based on the Swain–Lupton equation. The correlation, accomplished by using a two-parameter linear regression, gave î…�= 1.17 and
constants based on the Swain–Lupton equation. The correlation, accomplished by using a two-parameter linear regression, gave î…�= 1.17 and 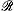 =–0.13.
=–0.13.

 Please wait while we load your content...
Please wait while we load your content...