Summary
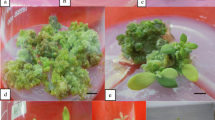
Direct plant regeneration from flowering plant-derived lamina explants of Anthurium andraeanum Hort. cultivars Tinora Red and Senator was established on modified Murashige and Skoog (MS) medium. Cultivar difference, stage of source lamina and the position of explant in lamina, medium pH, and type of growth regulators significantly influenced direct plant regeneration. Explants from young brown lamina were superior to young green lamina. The half-strength MS medium containing 1.11 μM N6-benzyladenine (BA), 1.14 μM indole-3-acetic acid, and 0.46 μM kinetin at pH 5.5 was most effective for induction of shoot formation. Explants from the proximal end of the source lamina gave rise to a higher number of shoots compared to the mid and distal regions. Cultivar Tinora Red was more regenerative than Senator in terms of number of shoots per explant. The use of a lower BA concentration (0.44 μM) was essential for callus-free shoot multiplication during subculture. Regenerated shoots could be induced to form roots on half-strength MS medium supplemented with 0.54 μM α-naphthaleneacetic acid and 0.93 μM kinetin. More than 300 plantlets of each eultivar were harvested from a single source lamina within 200 d of culture. Most plantlets (95%) survived after acclimation in soil.
Similar content being viewed by others
References
Atta-Alla, H.; McAlister, B.; van Staden, J. In vitro culture and establishment of Anthurium parvispathum. South African J. Bot. 64: 296–298; 1998.
Carelli, B. P.; Echeverrigaray, S. An improved system for the in vitro propagation of rose cultivars. Sci. Hort. 91: 69–74; 2001.
Duncan, D. B. Multiple range and multiple F-tests. Biometrics 11: 1–42; 1955.
Eapen, S.; Rao, P. S. Regeneration of plant from Anthurium patulum. Curr. Sci. 54: 284–286; 1985.
Joseph, D.; Martin, K. P.; Madassery, J.; Philip, V. J. In vitro propagation of three commercial cut flower cultivars of Anthurium andraeanum Hort. Indian J. Exp. Biol. 41: 154–159; 2003.
Kuehnle, A. R.; Sugii, N. Callus induction and plantlet regeneration of Hawaiian anthuriums. HortScience 26: 919–921; 1991.
Liu, C. M.; Xu, Z. H. An efficient procedure for micropropagation of Anthurium scherzerianum Schott (flamingo flower). Chinese J. Bot. 4: 49–55; 1992.
Matsumoto, T. K.; Kuehnle, A. R. Micropropagation of Anthurium. In: Bajaj, Y. P. S., ed. Biotechnology in agriculture and forestry 40: high-tech and micropropagation VI. New York: Springer-Verlag; 1997: 15–29.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays for tobacco tissue cultures. Physiol. Plant. 15: 473–497; 1962.
Pierik, R. L. M. Anthurium andraeanum plantlets produced from callus tissues cultivated in vitro. Physiol. Plant. 37: 80–82; 1976.
Pierik, R. L. M.; Steegmans, H. H. M.; van der Meys, J. A. J. Plantlet formation in callus tissues of Anthurium andraeanum Lind. Sci. Hort. 2: 193–198; 1974.
Rajasekaran, K.; Hein, M. B.; Davic, G. C.; Carnes, M. G.; Vasil, I. K. Endogenous growth regulators in leaf cultures of Pennisetum purpureum Schum. J. Plant Physiol. 130: 13–25; 1987.
Singh, S. K.; Syamal, M. M. A short pre-culture soak in thidiazuron or forchlorfenuron improves axillary shoot proliferation in rose micropropagation. Sci. Hort. 91: 169–177; 2001.
Skirvin, R. M.; McPheeters, K. P.; Norton, M. A. Sources and frequency of somaclonal variation. HortScience 29: 1232–1237; 1994.
Teng, W.-L. Regeneration of Anthurium adventitious shoots using liquid or raft culture. Plant Cell Tiss. Organ Cult. 49: 153–156; 1997.
Thorpe, T. A.; Harvy, I. S.; Kumar, P. P. Application of micropropagation in forestry. In: Debergh, P. C.; Zimmerman, R. H., eds. Micropropagation, technology and application. Dordrecht: Kluwer Academic Publishers; 1991: 311–336.
Welander, M. Plant regeneration from leaf and stem segments of shoots raised in vitro from mature apple trees. J. Plant Physiol. 132: 738–744; 1988.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, K.P., Joseph, D., Madasser, J. et al. Direct shoot regeneration from lamina explants of two commercial cut flower cultivars of Anthurium andraeanum Hort.. In Vitro Cell.Dev.Biol.-Plant 39, 500–504 (2003). https://doi.org/10.1079/IVP2003460
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2003460