-
PDF
- Split View
-
Views
-
Cite
Cite
Peter G. Pappas, Barbara D. Alexander, David R. Andes, Susan Hadley, Carol A. Kauffman, Alison Freifeld, Elias J. Anaissie, Lisa M. Brumble, Loreen Herwaldt, James Ito, Dimitrios P. Kontoyiannis, G. Marshall Lyon, Kieren A. Marr, Vicki A. Morrison, Benjamin J. Park, Thomas F. Patterson, Trish M. Perl, Robert A. Oster, Mindy G. Schuster, Randall Walker, Thomas J. Walsh, Kathleen A. Wannemuehler, Tom M. Chiller, Invasive Fungal Infections among Organ Transplant Recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET), Clinical Infectious Diseases, Volume 50, Issue 8, 15 April 2010, Pages 1101–1111, https://doi.org/10.1086/651262
Close - Share Icon Share
Abstract
Background. Invasive fungal infections (IFIs) are a major cause of morbidity and mortality among organ transplant recipients. Multicenter prospective surveillance data to determine disease burden and secular trends are lacking.
Methods. The Transplant-Associated Infection Surveillance Network (TRANSNET) is a consortium of 23 US transplant centers, including 15 that contributed to the organ transplant recipient dataset. We prospectively identified IFIs among organ transplant recipients from March, 2001 through March, 2006 at these sites. To explore trends, we calculated the 12-month cumulative incidence among 9 sequential cohorts.
Results. During the surveillance period, 1208 IFIs were identified among 1063 organ transplant recipients. The most common IFIs were invasive candidiasis (53%), invasive aspergillosis (19%), cryptococcosis (8%), non-Aspergillus molds (8%), endemic fungi (5%), and zygomycosis (2%). Median time to onset of candidiasis, aspergillosis, and cryptococcosis was 103, 184, and 575 days, respectively. Among a cohort of 16,808 patients who underwent transplantation between March 2001 and September 2005 and were followed through March 2006, a total of 729 IFIs were reported among 633 persons. One-year cumulative incidences of the first IFI were 11.6%, 8.6%, 4.7%, 4.0%, 3.4%, and 1.3% for small bowel, lung, liver, heart, pancreas, and kidney transplant recipients, respectively. One-year incidence was highest for invasive candidiasis (1.95%) and aspergillosis (0.65%). Trend analysis showed a slight increase in cumulative incidence from 2002 to 2005.
Conclusions. We detected a slight increase in IFIs during the surveillance period. These data provide important insights into the timing and incidence of IFIs among organ transplant recipients, which can help to focus effective prevention and treatment strategies.
Solid organ transplantation is an effective life-sparing modality for thousands of patients worldwide with organ failure syndromes. In the United States alone, >29,000 solid organ transplant procedures were performed in 2008 [1]. In spite of important advances in surgical technique and immunosuppressive regimens, there remain substantial risks for post-transplantation infection, of which invasive fungal infections (IFIs) are among the most important [2–6]. The most commonly reported IFIs among organ transplant recipients are invasive candidiasis, cryptococcosis, and invasive mold infections, such as aspergillosis and zygomycosis [2–15]. The incidence of IFIs varies in frequency and specific etiology according to the type of organ transplant procedure and transplant center [16–19]. An in-depth understanding of the overall burden of IFIs in this population is generally lacking.
The Transplant Associated Infection Surveillance Network (TRANSNET) was established in 2001 to perform prospective surveillance among all transplant recipients at selected centers for the purpose of understanding the burden of IFIs, to better define patients at risk, to understand current approaches to the diagnosis of IFIs in transplant recipients, and to describe the outcome of these infections. The group consists of 23 active transplant centers in the United States. Here, we report the results of a 5 year prospective study from 15 TRANSNET centers that provided infection surveillance data among organ transplant recipients, representing a large and geographically diverse surveillance study focusing on post-transplantation IFIs. The emphasis of this report is to define the overall and organ-specific burden of disease, to estimate the incidence of disease 1 year after transplantation, to assess trends in incidence, and to describe outcome 1 year after diagnosis.
Patients and Methods
Fifteen TRANSNET sites performed organ transplantation and provided prospective surveillance data on these patients. The period of IFI surveillance was March 2001 through March 2006 and included all organ transplant recipients who developed an IFI during this period, regardless of when or where their transplant occurred (surveillance cohort). In addition, a registry of patients who underwent transplantation during the surveillance period at study sites was kept (incidence cohort). Data collected on all incidence cohort patients included demographic data (age, sex, and race/ethnicity), underlying disorder, and type of transplant. Follow-up information on patients in the incidence cohort included date of last follow-up and patient status (ie, whether the patient was alive or dead).
Definitions and case identification. Only proven and probable IFIs as defined by the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria [20] were included as cases for this study (which was conducted in its entirety prior to the publication of the revised EORTC/MSG criteria). Cases were reviewed to determine individual case validity by a data review committee. All patients who were determined to have an IFI, regardless of original transplantation date, were registered as a case with the central unit (University of Alabama at Birmingham [UAB]), and a corresponding case report form was completed.
IFIs were identified prospectively through routine review of monthly logs of transplant recipients; through review of perIFIs were identified prospectively through routine review of monthly logs of transplant recipients; through review of pertinent culture data, serological studies, and histopathological findings; and through routine direct contact with transplant physicians and coordinators. Case data included infection site, culture data, method(s) of diagnosis, date and type(s) of transplant(s), history of rejection, comorbid conditions, past or current infections, recent immunosuppressive and antifungal treatment data, and patient status 3 months after initial diagnosis of IFI. For patients who underwent multiple sequential transplant procedures, the date of first transplantation was used to calculate time to infection. Among those patients who died, the cause of death (due to IFI or another cause) was determined by the investigator and by autopsy data, if available.
Internal validation of case-finding was performed retrospectively. Because of the predicted high incidence of IFIs among lung transplant recipients, internal validation was restricted to this group (11 sites). At the conclusion of the prospective surveillance phase of the study, each investigator was provided with a list of randomly selected lung transplant recipients from their site transplant logs who were not identified as having experienced an IFI, and the investigator was asked to review these patients for previously unidentified IFI events. Cases identified in this manner were included in the database. This internal method of validating the effectiveness of case finding suggested that very few cases (<5%) were missed during initial surveillance and that this is an unlikely explanation for large observed differences in incidence among sites. Moreover, a recently published focused retrospective review of invasive aspergillosis cases at a selected TRANSNET site suggested excellent identification and capture of cases [21].
Microbiologic methods. Available cultures and pathologic specimens were processed at the participating hospitals. Species identification was performed using routine methods at the participants' affiliated laboratories. Available fungal isolates were forwarded to the UAB Fungal Reference Laboratory and then to the Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch to confirm identification and for storage. CDC species identification was used when there were discrepant results. Culture-negative cases of IFI in which histopathological findings showed acute branching hyphae consistent with an invasive mold were classified as being due to unspecified molds. Cases of aspergillosis that were diagnosed on the basis of a culture yielding an Aspergillus species that was not further identified or on the basis of a positive galactomannan assay result as the only microbiologic criteria were classified as being due to unspecified Aspergillus species.
Data analysis. Initial data entry and verification were performed by the Biostatistical Unit at UAB. Final data cleaning and data analysis were performed at the CDC. The descriptive analysis of IFIs reflects infections that occurred during the study period (March 2001 through March 2006) independent of when or where the transplantation occurred (surveillance cohort). Box-and-whisker plots were generated to summarize time to IFI from transplant.
Data from the incidence cohort were pooled to obtain cumulative incidences (CIs) of first IFI for all transplantations (overall) and within each transplant type. Pathogen-specific CIs were also calculated. CI estimates for transplant-related infections within the first 12 months after the first transplantation were estimated using the cmprisk risk package, version 2-1-7 [22], in R, version 2.6.1 [23]. Cumulative incidences were estimated accounting for the competing risks of infection-free death, retransplantation, and return to chronic dialysis (for renal transplant recipients). The CI estimates are based on time from first transplantation to the first IFI. Site-to-site variability was explored by stratifying overall and transplant-specific CI curves by site. One-year survival after diagnosis of an IFI was estimated for the IFIs in the incidence cohort using the Kaplan-Meier method.
The trend of CI during the surveillance period was also described. We divided the incidence cohort into 9 separate and sequential subcohorts, based on the 4-month time period during which an individual's transplantation occurred. The 12-month CIs of first IFI for the 9 subcohorts were calculated and then plotted against the time periods. We also described the trend of CI of Aspergillus and Candida IFIs among all sites.
Results
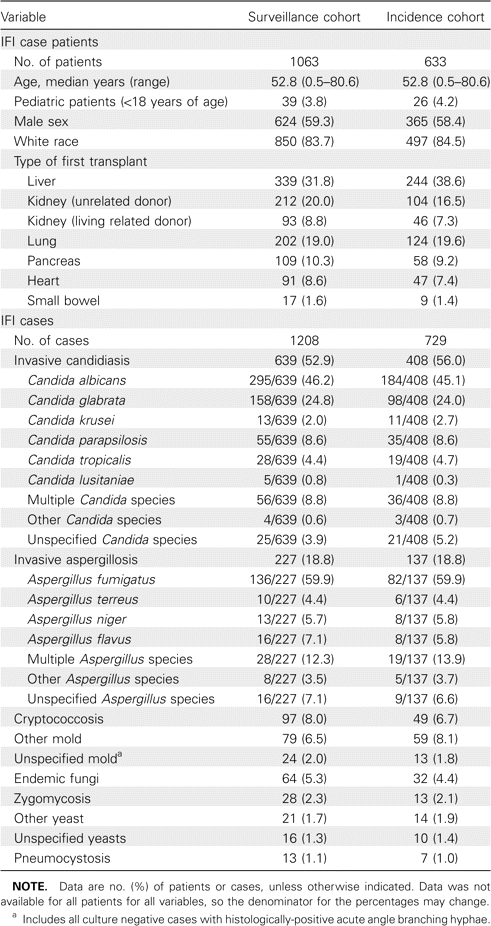
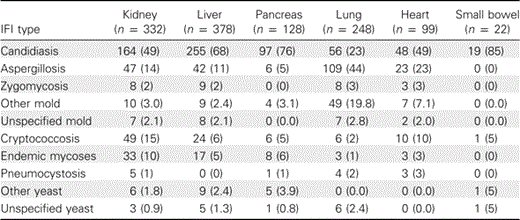
IFI among organ transplant recipients. In the surveillance cohort, there were 1208 proven (42%) and probable (58%) IFI cases among 1063 organ transplant recipients during the surveillance period (March 2001 through March 2006). During the same period, there were 729 proven and probable IFI cases among 633 organ transplant recipients in the incidence cohort. The demographic data, transplantation type, and specific IFI for both cohorts are demonstrated in Table 1. Invasive candidiasis was the most common IFI overall, followed by invasive aspergillosis, cryptococcosis, non-Aspergillus molds (excluding Zygomycetes), and the endemic fungi (including histoplasmosis, coccidioidomycosis, and blastomycosis). Proven zygomycosis and Pneumocystis jiroveci infection were uncommon in this population and accounted for <3% of the cases in each transplant type. Table 2 demonstrates the proportion of cases due to specific IFIs in each transplant type in the surveillance cohort.
Demographic Characteristics and Description of Invasive Fungal Infections (IFIs) Detected in the Transplant-Associated Infection Surveillance Network, 2001–2005
No. (%) of Invasive Fungal Infection (IFI) Cases in the Surveillance Cohort, by Transplant Type
Among all cases, invasive candidiasis was most commonly associated with candidemia (64% of cases), urinary tract involvement (11%), and peritonitis (9%). The distribution of Candida species is demonstrated in Table 1. The proportion of Candida albicans to non-albicansCandida species did not change significantly during the observation period. Among patients with invasive aspergillosis, most cases (72%) were probable. Aspergillus fumigatus was the most common species isolated, whereas infections due to Aspergillus flavus,Aspergillus niger, and Aspergillus terreus were less common. Mixed infections due to ⩾2 Aspergillus species occurred in 12% of cases and were seen almost exclusively among patients with pulmonary aspergillosis. Most cases of aspergillosis (78%) were limited to the lungs. Among cryptococcosis cases, central nervous system involvement and disease limited to the lungs were seen in 45% and 39% of cases, respectively. Cryptococcemia without other organ involvement was observed in 4% of these cases.
Endemic fungal infections, including histoplasmosis, blastomycosis, and coccidioidomycosis, most often presented as disseminated infection involving multiple organs. The majority (75%) of these cases were due to Histoplasmosis capsulatum. Among 48 cases of histoplasmosis, 18 (43%) occurred within 6 months after receipt of transplant; of 7 cases of coccidioidomycosis, 6 (85%) occurred within 6 months after receipt of transplant, including 2 previously reported patients who developed donor-derived coccidioidomycosis within 3 weeks after transplantation [24]. There were 9 blastomycosis cases. Cumulatively, zygomycosis, fusariosis, phaeohyphomycosis and other molds constituted ∼10% of cases. Infections due to Zygomycetes were the most common infection in this group and were categorized as pulmonary (56% of cases), sinus (13%), cutaneous (13%), or disseminated (9%). Unspecified molds, which likely included several Zygomycetes, comprised 24 cases (2%).
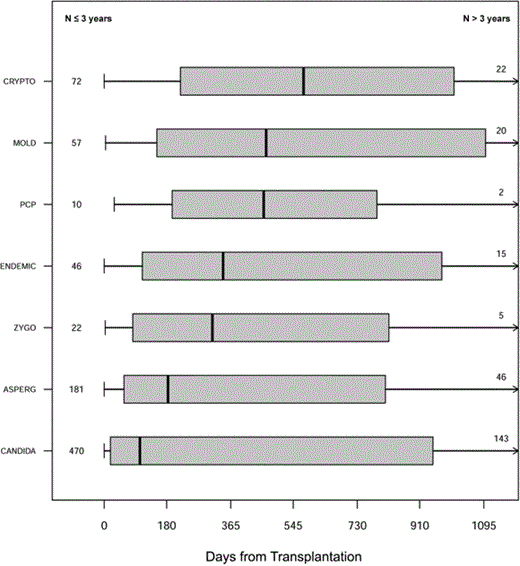
The time to diagnosis for each IFI type in the surveillance cohort is depicted in Figure 1. Median time to onset was 103 days for invasive candidiasis, 184 days for aspergillosis, 312 days for zygomycosis, 343 days for endemic fungal infections, 467 days for non-Aspergillus molds, and 575 days for cryptococcosis. As demonstrated, the majority of infections occurred >90 days after transplantation. Early infections (those occurring ⩽90 days after transplantation) were dominated by invasive candidiasis and invasive aspergillosis. Substantial numbers of IFI cases in all categories—but especially cryptococcosis, mold infections other than aspergillosis, and endemic fungal infections—occurred >3 years after transplantation.
Box and whisker graph indicating time to invasive fungal infection of a specific type. The box represents the interquartile range (25%–75%). The right whisker is truncated at 3 years. Numbers in the left hand column represent cases that occurred within 3 years after transplantation; those in the right hand column represent cases that occurred >3 years after transplantation. ASPERG, aspergillosis; CANDIDA, invasive candidiasis; CRYPTO, cryptococcosis; ENDEMIC, endemic fungi; MOLD, non-Aspergillus mold; PCP, pneumocystosis; ZYGO, zygomycosis.
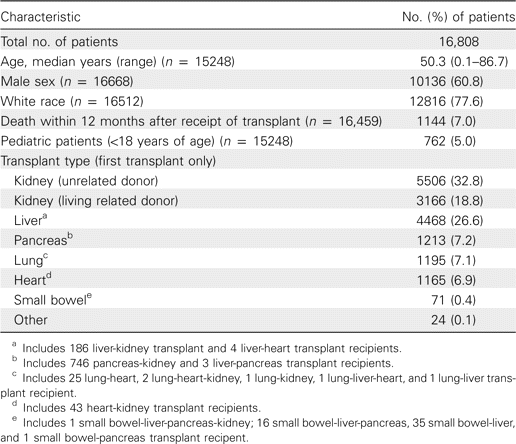
Incidence cohort. Study start and stop times differed across sites for the incidence cohort, but all 15 sites contributed from May 2002 through April 2005. Demographic data for the 16,808 persons in the incidence cohort, including age, sex, race, and type of transplant are demonstrated in Table 3. Patients who received multiple simultaneous transplants (eg, heart-lung, kidney- pancreas, and liver-small bowel transplantations) were grouped according to the organ type associated with the higher historical risk for IFI [3, 4]. Those who underwent sequential transplantation (at different dates) are classified by the first transplant received.
Characteristics of All Patients Included in the Incidence Cohort
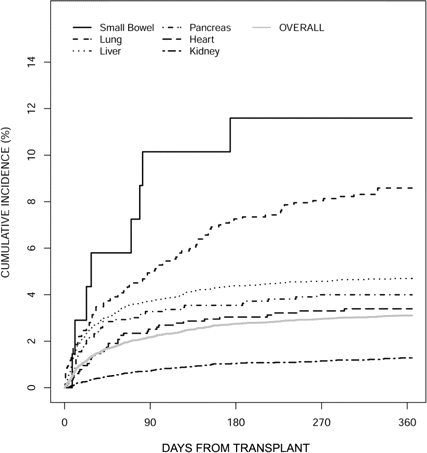
Twelve-month cumulative incidence of IFI. To estimate the 12-month CI, we limited the cohort to 16,459 organ transplant recipients with follow-up data. In the 12 months after transplantation, 921 persons died, 274 kidney transplant recipients returned to chronic dialysis, 283 persons received a subsequent transplant, and 501 persons had their first IFI prior to any of the competing risks. The overall 12-month CI for any IFI was 3.1%. Figure 2 shows the overall 12-month CI curve as well as the cumulative incidence stratified by transplant type. The greatest probability of IFI was associated with small bowel transplantation (11.6%), although this estimate represents only 1 transplant center. Twelve month CI estimates for lung and heart-lung (8.6%; 11 sites), liver (4.7%; 15 sites), pancreas and kidney-pancreas (4.0%; 15 sites), and heart transplant recipients (3.4%; 13 sites) were lower. Kidney transplant recipients had the lowest overall risk of IFI (1.3%; 15 sites) throughout the period of observation. There was considerable site-to-site variability in CI of any IFI (estimates ranging from 1.2% to 6.1%) and within specific organ transplant populations, including liver (0%–15.5%), pancreas (0%–20.0%), and lung and heart-lung transplants (0%–25.9%).
Cumulative incidence curve of first invasive fungal infection (IFI) according to transplant type. The gray line represents overall IFI incidence for all organ transplant types.
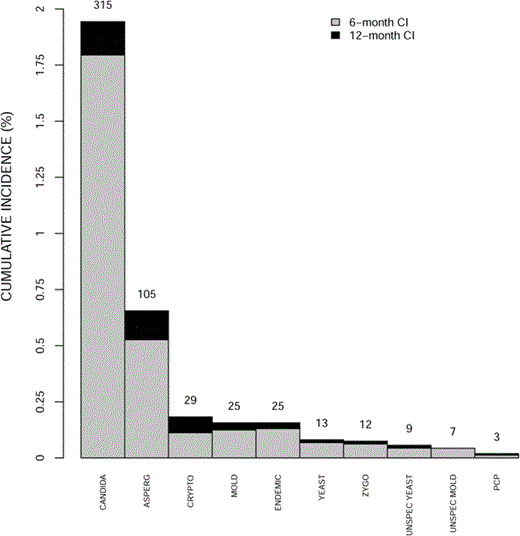
Figure 3 compares the 12-month CI estimates of first IFI of each specific IFI type, accounting for the competing risks described above. Invasive candidiasis had the highest 12-month CI estimate (1.9%) followed by invasive aspergillosis (0.7%). Cryptococcosis, mold infections other than aspergillosis or zygomycosis, and endemic fungal infections had estimates of ∼0.2%. All other IFI types had estimates <0.1%.
Bar graph demonstrating cumulative incidence (CI) of specific invasive fungal infection (IFI) at 6 months and 12 months after transplantation The number of IFIs contributing to the 12-month CI is given for each column. ASPERG, aspergillosis; CANDIDA, invasive candidiasis; CRYPTO, cryptococcosis; ENDEMIC, endemic fungi; MOLD, non-Aspergillus mold; PCP, pneumocystosis; UNSPEC, unspecified; ZYGO, zygomycosis.
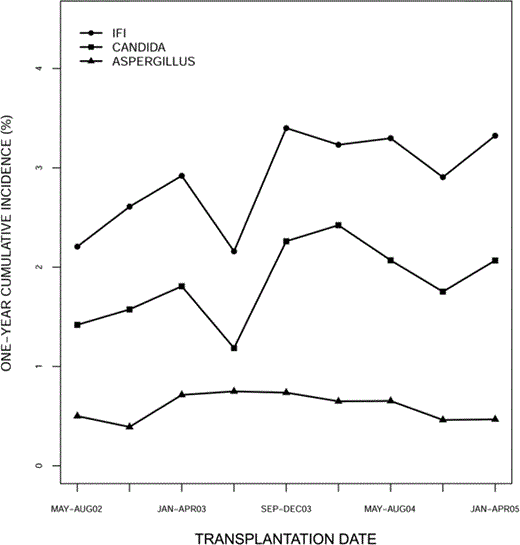
Figure 4 shows the trend of 12-month CI for first IFI, first Candida infection, and first Aspergillus infection for each of 9 sequential subcohorts over a period of 3 years. The 12-month cumulative incidence of first IFI increased from 2.2% in the subcohort that underwent transplantation during the period May–August 2002 to 3.3% in the subcohort that underwent transplantation during the period January–April 2005. As can be seen, the trend in the overall curve was driven by changes in the CI for Candida infections, which increased from 1.4% to 2.1%. No change was evident for invasive aspergillosis.
Trend graph of 1-year cumulative incidence of first invasive fungal infection (IFI), Candida infection, and Aspergillus infection for 9 subcohorts (based on 4-month periods).
Mortality. The 12 month survival after infection was 59% for patients with invasive aspergillosis, 61% for infections due to non-Aspergillus molds, 66% for invasive candidiasis, and 73% for cryptococcosis.
Discussion
It has been difficult to make an accurate assessment of the burden of IFIs or to accurately address incidence across a large and varied group of organ transplant recipients in the United States. The transplant literature is replete with single-center series, retrospective reviews, case reports, and anecdotal data [2–15]. Many of these reports emphasize especially high incidence rates of specific infections in certain transplant types [8, 11, 14, 15, 19, 20]. Most experts agree that there is a hierarchy of risk based not only on transplant type but also on underlying disease, comorbid conditions, surgical techniques, and other variables [2–6, 25]. This geographically diverse group of ∼17,000 patients with varying transplant types represents >15% of all organ transplant procedures performed in the United States during the 5-year surveillance period [1] and provides a unique opportunity for accurate comparison of infection rates between transplant types and for observing trends over a 3 year period.
These data provide robust information concerning type and timing of IFIs in the post-transplant period. The majority of these infections are relatively late, typically occurring >3 months after transplantation. Not surprisingly, invasive candidiasis was the most common IFI in each organ transplant type except among lung transplant recipients, and it was associated with a high overall mortality, similar to that seen in recent treatment trials of candidemia and other forms of invasive candidiasis [26–28]. The distribution of Candida species was similar to that which has been reported in recent national surveys among hospitalized patients [29, 30]. C. albicans and C. glabrata were the dominant species in this population, and we did not observe important trends relating to the proportion of C. albicans and non-albicansCandida species during the study period. Invasive aspergillosis remains a highly lethal, albeit less common, infection among organ transplant recipients, and it is the dominant IFI among lung transplant recipients. As noted by others [31–33], cryptococcosis and the endemic fungal infections, especially histoplasmosis, were important late complications after transplantation.
Our study is, to our knowledge, the first to evaluate secular trends of IFIs among organ transplant recipients using cumulative incidence. This method, which accounts for competing risks of IFI among separate subcohorts, is a more appropriate way of measuring transplant-associated risk. Although we were limited to a 3-year surveillance period (2002–2005) because of inadequate denominator data for the entire study period, we found that, during the 3-year surveillance period, the CI of IFIs increased among all organ transplant recipients. The increase in IFIs was reflected mainly by an increase in the incidence of Candida infections, because the CI of Aspergillus infections remained unchanged. It is important to note that this observation occurred during an era that is often characterized by prolonged and aggressive antifungal prophylaxis and empirical therapy for transplant recipients.
We also observed differences in incidence after organ transplantation between participating sites, and these did not appear to be related to differences in surveillance techniques. We can only speculate as to the reasons behind these observations. Possible explanations include variability in complexity and acuity of transplant recipients, surgical technique, antifungal prophylaxis and empirical treatment strategies, immunosuppressive regimens, and approach to diagnosis. An example of the variability in immunosuppressive approach is the burgeoning use of alemtuzumab as a primary immunosuppressive agent in the immediate post-transplant period [34–36], practiced by many but not all transplant centers. Similarly, antifungal prophylaxis strategies vary widely, and these approaches are generally driven by the anecdotal experience, rather than being evidence-based [37–40]. Finally, there are institutional differences in the aggressiveness with which diagnoses of potential IFIs are pursued—for example, in the willingness to subject patients to an open-lung biopsy when other diagnostic modalities have failed.
There are several important limitations to this study. Although this study included a large denominator of organ transplant recipients, the data that were collected on all patients in the incidence cohort were inadequate to allow for accurate determination of risk; thus, these data were not included in the analysis. It must be emphasized that this study was primarily designed to determine an estimate of the national burden of IFIs among transplant recipients and not specifically as a risk factor study. The definitions that were used to identify IFIs, although widely accepted and standardized, were originally designed to define and standardize diagnostic criteria for inclusion of cancer patients with invasive mycoses into clinical trials. These definitions have proven useful for other epidemiologic surveillance studies, and we believe them to be appropriate for use in this study. Exclusion of IFI cases categorized as “possible” certainly led to an underestimate of the actual number of cases, but inclusion of these cases would have allowed many patients to be included who did not have an IFI. Finally, each site developed an aggressive strategy to screen for IFIs in their transplant population, but there was no specifically mandated strategy to which all sites were held accountable.
In summary, these data represent the first comprehensive attempt to define the incidence and influence on mortality of IFIs in organ transplant recipients in the United States. The overall incidence is lower than previous estimates. As expected, small bowel and lung transplant recipients have the highest 1- year incidence of IFIs, and invasive candidiasis is the most common IFI overall. Mold infections, especially those due to invasive aspergillosis, were particularly important among lung transplant recipients. A large number of all types of IFIs occurred >1 year after transplantation, with as many as 25% of IFIs developing >3 years after transplantation. This represents an important observation, because prevention, diagnostic, and treatment strategies need to be developed that take into account the risk of late-occurring infections. These data have significant implications for future studies that examine organ-specific risk factors for post-transplantation IFI and could also lead to new strategies towards the early diagnosis and prevention of IFIs in this growing and highly vulnerable population.
Acknowledgment
We thank Pallavi Daram, Robert Warren, and Beth Deerman (University of Alabama at Birmingham); Pamela DeTullio (University of Michigan); Deborah Berg (University of Texas Health Science Center San Antonio); Christine Kane (Fred Hutchinson Cancer Research Center); Sanjeet Dadwal, Bernard Tegtmeier, Jane Kriengkauykiat, Mary Ann Clouser, Margaret O'Donnell, and Stephen Forman (City of Hope National Medical Center); Cheryl Shoden (Mayo Clinic, Rochester); Kathleen Hinkle (University of Pennsylvania); and Mary E. Brandt, Lynette Benjamin, Karen Stamey, Shirley McClinton, S. Arunmohzi Balajee, Rui Kano, Scott Fridkin, Juliette Morgan, Rana Hajjeh, and David Warnock (Centers for Disease Control and Prevention).
Potential conflicts of interest. P.G.P. has received grant support from Merck, Pfizer, Schereing-Plough, and Astellas and served as an adhoc advisor for Novartis, Basilea, Merck, Pfizer, and Astellas. D.R.A. has received grant support and served as an ad hoc advisor for Merck, Pfizer, and Schering-Plough. T.F.P. has received research support and honoraria from Merck, Pfizer, Schering-Plough, and Nektar Therapeutics and has served as a consultant for Basilea, Merck, Nektar, and Pfizer. K.A.M. has received grant support from Merck and Enzon and served as an ad hoc advisor and/or consultant for Astellas, Basilea, Enzon, Merck, Pfizer, and Schering-Plough. J.I.I. has received honoraria from Astellas, Enzon, Pfizer, and Schering-Plough. VAM is on the speakers' bureau for Amgen, Merck, Pfizer, Schering-Plough, and Celgene. All other authors: no conflicts.
References
The findings and conclusions in this presentation/report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This information is distributed solely for the purpose of pre dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by the Centers for Disease Control and Prevention. It does not represent and should not be construed to represent any agency determination or policy.
Author notes
Author affiliations are listed at the end of the text.





Comments