Abstract
Highly selective and sensitive detection of trimethylamine (TMA) was achieved by the decoration of discrete p-type Cr2O3 nanoparticles on n-type ZnO nanowire (NW) networks. Semielliptical Cr2O3 nanoparticles with lateral widths of 3–8 nm were deposited on ZnO NWs by the thermal evaporation of CrCl2 at 630 °C, while a continuous Cr2O3 shell layer with a thickness of 30–40 nm was uniformly coated on ZnO NWs at 670 °C. The response (Ra/Rg: Ra, resistance in air; Rg, resistance in gas) to 5 ppm TMA of Cr2O3-decorated ZnO NWs was 17.8 at 400 °C, which was 2.4 times higher than that to 5 ppm C2H5OH and 4.3–8.4 times higher than those to 5 ppm p-xylene, NH3, benzene, C3H8, toluene, CO, and H2. In contrast, both pristine ZnO and ZnO (core)–Cr2O3 (shell) nanocables (NCs) showed comparable responses to the different gases. The highly selective and sensitive detection of TMA that was achieved by the deposition of semielliptical Cr2O3 nanoparticles on ZnO NW networks was explained by the catalytic effect of Cr2O3 and the extension of the electron depletion layer via the formation of p–n junctions.
Export citation and abstract BibTeX RIS
1. Introduction
Oxide semiconductor nanowires (NWs) with a large surface-to-volume ratio and high crystallinity have been receiving much attention in the field of gas sensing due to their excellent gas response and thermal stability [1–8]. NW network structures have a lower tendency to form agglomerates compared to nanoparticles which easily aggregate into secondary particles due to attractive van der Waals forces. Consequently, gases can diffuse rapidly and effectively through the porous NW network, allowing a larger surface area to participate in gas sensing [9].
Oxide chemoresistive gas sensors provide benefits such as high sensitivity, cost effectiveness and reliability [10–13]. Nevertheless, the simple oxidative or reductive reactions that occur between gases and the surface of the oxide semiconductor NWs have often limited the selective detection of a specific gas. Many efforts have been made during the past decade in order to achieve selectivity in gas sensing. The main approaches for selective gas detection thus far include the manipulation of acid–base properties [14], catalytic promotion of the gas sensing reaction [15–18], and the employment of a catalytic filtering layer [19].
TMA, dimethylamine, triethylamine, and ammonia (NH3) are known to be secreted from fish after death and their concentrations increase with the decay of the fish [20, 21]. Thus, the concentration of TMA can be used as a measure of fish freshness. When inhaled, TMA can cause headaches, nausea, skin burns, and irritation to the eyes and respiratory system [22]. The allowable exposure limit set by the National Institute for Occupational Safety and Health is 10 ppm for 10 h in a long term exposure and 15 ppm for 15 min in a short term exposure. In the evaluation of fish freshness, 0–10 ppm is regarded as fresh, and decay begins at over 10 ppm of TMA emittance [23]. Various methods including gas chromatography [24], high performance liquid chromatography [25], ion mobility spectrometry [26], mass spectrometry [27], and the use of a quartz microbalance [28] have been explored to analyse the amount of TMA secreted. However, the aforementioned methods suffer from limitations such as the requirements of complicated equipment, a long sample preparation time, and professional operating skills. Among many oxide semiconductor based gas sensors, various sensing materials such as SnO2–ZnO composites [20], Th-doped SnO2 [29–31], and Al-doped ZnO [32] have also been investigated. However, most of the oxide semiconductors used for the detection of TMA require further improvements in their selectivity, gas response, and stability.
Cr2O3 with a corundum structure is a p-type oxide semiconductor and can be used for gas sensor applications [33, 34]. Considering that Cr2O3 is a catalyst for methylamine decomposition [35], the addition of Cr2O3 may be a promising approach to achieving the selective detection of TMA. Although Cr2O3-doped ZnO thick film sensors [36] and Cr2O3-sensitized ZnO nanofibre sensors [37] have been studied for the chemoresistive sensing of NH3 and C2H5OH, respectively, the selective detection of TMA using Cr2O3–ZnO nanocomposites has been barely investigated. In the present study, we prepared two different configurations of p–n junctions between p-type Cr2O3 and n-type ZnO: single crystalline ZnO NWs decorated with a discrete configuration of semielliptical Cr2O3 nanoparticles and ZnO–Cr2O3 core–shell nanocables (NCs). These sensors can be used as valuable platforms for highly selective and sensitive TMA detection due to the catalytic effect of Cr2O3 as well as the extension of the electron depletion layer via the formation of a p–n junction. The main focus of this study was directed at the design of selective and sensitive TMA sensors using different configurations of one-dimensional Cr2O3–ZnO hetero-nanostructures and the elucidation of their sensing mechanism.
2. Experimental details
2.1. Preparation
The ZnO NWs were grown directly on an alumina substrate (area, 1.5 × 1.5 mm2; thickness, 0.25 mm) with two gold electrodes (electrode length, 1 mm; separation, 0.2 mm) by thermal evaporation using mixed powders of ZnO (99.9%, Aldrich), graphite ( < 20 μm, Aldrich) and Sn (99.8%, Acros). The source (ZnO:graphite:Sn = 1:1:0.01 wt%) was loaded in an Al2O3 boat which was located in the centre of a quartz tube (diameter: 2.5 cm) and the alumina substrates were placed 5 cm downstream from the source. After evacuating the quartz tube to ∼9 × 10−2 Torr using a rotary pump, the furnace temperature was increased to 900 °C. The ZnO NWs were grown for 20 min by a reaction between the source and an Ar–O2 mixture gas (Ar, 100 sccm; O2, 1 sccm). The gold electrodes acted as catalysts for the growth of ZnO NWs by the vapour–liquid–solid mechanism, which facilitated networking between NWs with highly porous structures and increased the connectivity between the electrodes and NWs.
The semielliptical Cr2O3 nanoparticles (lateral width, 3–8 nm; height, 1–5 nm) were deposited on the ZnO NW networks by the following procedures. The as-grown ZnO NWs on the alumina substrate and the CrCl2 (99.99%, Aldrich) were placed in the right and left parts of the Al2O3 boat (length, 4 cm), respectively. After evacuating the quartz tube to ∼9 × 10−2 Torr using a rotary pump, the furnace temperature was increased to 630 or 670 °C and then was kept at the reaction temperature for 20 min. Ar gas (100 sccm) was flowed during the reaction. The discrete Cr2O3 nanoparticles were coated on ZnO NWs at 630 °C, while ZnO–Cr2O3 core–shell NCs were prepared at 670 °C.
2.2. Characterization
The phase and crystallinity of the pristine ZnO NWs, Cr2O3-decorated ZnO NWs, and ZnO–Cr2O3 core–shell NCs were analysed by x-ray diffraction (XRD, Rigaku D/MAX-2500V/PC), the morphologies of the NWs by field-emission scanning electron microscopy (FE-SEM, S-4800, Hitachi, Japan) and transmission electron microscopy (TEM, FEI Titan), and the chemical state of the NWs by x-ray photoelectron spectroscopy (XPS, Thermo, MultiLab 2000).
2.3. Gas sensing characteristics
Sensor elements with NW networks were heat treated at 550 °C for 2 h prior to the measurement of their gas sensing characteristics. The sensor was placed in a quartz tube and the temperature of the furnace was stabilized at 400 °C. A flow through technique with a constant flow rate of 500 cm3 min−1 was used. The gas concentration was controlled by changing the mixing ratio of the parent gases (5 ppm TMA, 100 ppm C2H5OH, 5 ppm p-xylene, 10 ppm NH3, 5 ppm benzene, 100 ppm C3H8, 5 ppm toluene, 100 ppm CO, and 100 ppm H2, all in air balance) and dry synthetic air. The dc-probe resistance of the sensor was measured using an electrometer interfaced with a computer.
3. Results and discussion
3.1. Nanostructure and chemical state analyses
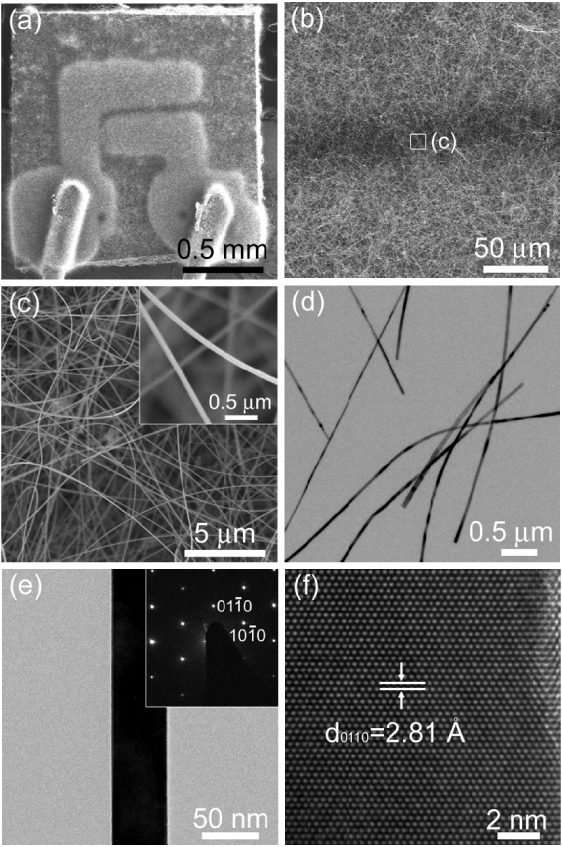
Single crystalline ZnO NWs were directly grown on an alumina substrate with two Au electrodes by thermal evaporation (figures 1(a) and (b)). The diameters of the NWs ranged from 30 to 80 nm and the NWs were several tens of micrometres long (figures 1(c) and (d)). The selected area electron diffraction (SAED) pattern confirmed that the single crystalline ZnO NWs were grown in the [ ] direction (figure 1(e)). The lattice-resolved image of a single NW showed highly crystalline (
] direction (figure 1(e)). The lattice-resolved image of a single NW showed highly crystalline ( ) fringes separated by 2.81 Å (figure (f)).
) fringes separated by 2.81 Å (figure (f)).
Figure 1. Morphologies and crystal structures of ZnO NWs: (a)–(c) SEM images of ZnO NWs grown on an alumina substrate with two Au electrodes; (d), (e) TEM image of ZnO NWs (inset, SAED pattern of ZnO NW); (f) lattice-resolved TEM image of ZnO NW.
Download figure:
Standard imageThe catalytic Cr2O3 layers were deposited on the ZnO NW networks by the thermal evaporation of CrCl2 powders at 630 °C. The overall network configuration of ZnO NWs in the sensor remained similar (figure 2(a)). The surface of ZnO NWs was made rougher by the coating of Cr2O3 via thermal evaporation of CrCl2 (figure 2(b) and inset in figure 2(b)). The small semielliptical particles on the surface of ZnO NWs (figure 2(c)) were identified as Cr2O3 in the high magnification TEM image (figure 2(d)). The highly crystalline Cr2O3 nanoparticles with semielliptical shapes were uniformly coated on the ZnO NWs, which can be confirmed by the (110) fringes of Cr2O3 separated by 2.48 Å. The lateral sizes of semielliptical Cr2O3 nanoparticles ranged from 3 to 8 nm and their heights ranged from 1 to 5 nm. The high-angle annular dark field (HAADF) scanning TEM (STEM) image and the elemental line scanning of the Cr2O3-decorated ZnO NWs by energy dispersive x-ray spectroscopy (EDS) indicated again that the semielliptical nanoparticles on the surfaces of the ZnO NWs were indeed Cr2O3 (figure 2(e)).
Figure 2. Morphologies and crystal structures of Cr2O3-decorated ZnO NWs: (a), (b) SEM images of Cr2O3-decorated ZnO NWs grown on an alumina substrate with two Au electrodes; (c), (d) TEM images of Cr2O3-decorated ZnO NWs; (e) high-angle annular dark field (HAADF) scanning TEM (STEM) image and EDS elemental line scan of Zn, Cr, and O of Cr2O3-decorated ZnO NWs.
Download figure:
Standard imageAs the thermal evaporation temperature was increased to 670 °C, the ZnO NWs were completely coated with a continuous Cr2O3 shell layer, which leads to the formation of ZnO–Cr2O3 core–shell NCs (figure 3(a)). The lattice fringes of the ZnO core are hardly visible in the high magnification TEM image due to the presence of the Cr2O3 shell layer. The formation of a crystalline Cr2O3 shell layer was confirmed by the presence of (110) fringes of Cr2O3 separated by 2.48 Å and the fast Fourier transform pattern (figure 3(b)). The shell layer was 30–40 nm thick. The formation of ZnO–Cr2O3 core–shell NCs was verified again by the high-angle annular dark field (HAADF) scanning TEM (STEM) image and the elemental line scanning profile (figure 3(c)). The presence of Cr in the central region can be attributed to the Cr2O3 shell layer located at the front and back of the ZnO core NWs. However, the significant amount of Zn in the shell region indicates that the Cr2O3 in the shell region contains a small amount of ZnO. The ionic radius of Cr3+ with the coordination number of 6 in Cr2O3 is 0.755 Å, while that of Zn2+ with the coordination number of 6 is 0.88 Å. Thus, the substitution of Zn2+ in the sites of Cr3+ can be given as a plausible reason for the presence of a Zn component in the Cr2O3 shell layer.
Figure 3. Morphologies and crystal structures of ZnO–Cr2O3 core–shell NCs: (a), (b) TEM images and (c) high-angle annular dark field (HAADF) scanning TEM (STEM) image and EDS elemental line scan of Zn, Cr, and O of ZnO–Cr2O3 core–shell NCs.
Download figure:
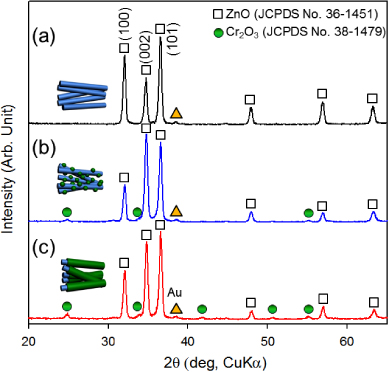
Standard imageThe pristine ZnO NWs were identified as wurtzite phase (JCPDS 36-1451), characterized by primary (101), (100), and (002) peaks (figure 4(a)). Cr2O3 with a corundum structure (JCPDS 38-1479) and wurtzite ZnO phases were found to coexist in Cr2O3-decorated ZnO NWs (figure 4(b)) and ZnO–Cr2O3 core–shell NCs (figure 4(c)). Compared to Cr2O3-decorated ZnO NWs, the XRD pattern of ZnO–Cr2O3 core–shell NWs showed a slightly stronger intensity of Cr2O3 peaks (figures 4(b) and (c)). Further characterization in order to confirm the oxidation states of the Cr in both Cr2O3-decorated ZnO NWs and ZnO–Cr2O3 core–shell NCs has been carried out using XPS (figure 5). The Cr (2p2/3) and Cr (2p1/2) peaks were centred near 577 and 586 eV, respectively (figure 5), indicating that the bonding states of chromium in both Cr2O3-decorated ZnO NWs and ZnO–Cr2O3 core–shell NCs are in the form of Cr2O3. The Cr concentrations of Cr2O3-decorated ZnO NWs and ZnO–Cr2O3 core–shell NCs were determined by XPS to be 11.8 and 18.8 at.%.
Figure 4. X-ray diffraction (XRD) patterns of (a) pristine ZnO NWs, (b) Cr2O3-decorated ZnO NWs, and (c) ZnO–Cr2O3 core–shell NCs.
Download figure:
Standard imageFigure 5. X-ray photoelectron spectroscopy survey results of (a) pristine ZnO nanowires (NWs), Cr2O3-decorated ZnO NWs and ZnO–Cr2O3 core–shell nanocables (NCs), and (b) the binding energies for Cr (2p1/2) and Cr (2p2/3) peaks of Cr2O3-decorated ZnO NWs and ZnO–Cr2O3 core–shell NCs.
Download figure:
Standard image3.2. Gas sensing characteristics
The temperature dependences of the TMA responses in ZnO NWs, Cr2O3-decorated ZnO NWs, and ZnO–Cr2O3 core–shell NCs were measured to investigate the morphological and catalytic effects of Cr2O3 on the surfaces of ZnO NWs (figure 6(a)) The pristine ZnO and Cr2O3-decorated ZnO NWs showed n-type gas sensing behaviours, that is, a resistance decrease in response to reducing gases (figure 6(b)). This indicates that the conduction in the Cr2O3-decorated ZnO NWs is dominated by the n-type ZnO NW core rather than the p-type Cr2O3 surface modification. The Ra/Rg values (S = Ra/Rg: Ra, resistance in air; Rg, resistance in gas) were used as the gas responses. In contrast, the ZnO–Cr2O3 NCs showed p-type gas sensing characteristics (figure 6(b)); that is, the resistance is increased by reducing gases. This indicates that the conduction and chemoresistive variation in ZnO–Cr2O3 core–shell NCs are dominated by the p-type Cr2O3 shell layers with thicknesses of 30–40 nm on the surfaces of n-type ZnO NWs. Thus, in ZnO–Cr2O3 core–shell NCs, the Rg/Ra values were used as the gas responses.
Figure 6. (a) Gas responses (Ra/Rg or Rg/Ra: Ra, resistance in air; Rg, resistance in gas) of ZnO NWs, Cr2O3-decorated ZnO NWs, and ZnO–Cr2O3 core–shell NCs to 5 ppm TMA over the temperature range of 300–450 °C. (b) Dynamic sensing transients to 5 ppm TMA at the sensor temperature of 400 °C.
Download figure:
Standard imageThe response of pure ZnO NWs to 5 ppm TMA was increased from 2.25 to 6.88 as the sensor temperature increased from 300 to 450 °C. The TMA responses in Cr2O3-decorated ZnO NWs showed a maximum value (17.79) at 400 °C. The responses to TMA were very small in ZnO–Cr2O3 core–shell NCs and tended to decrease with increasing sensor temperature.
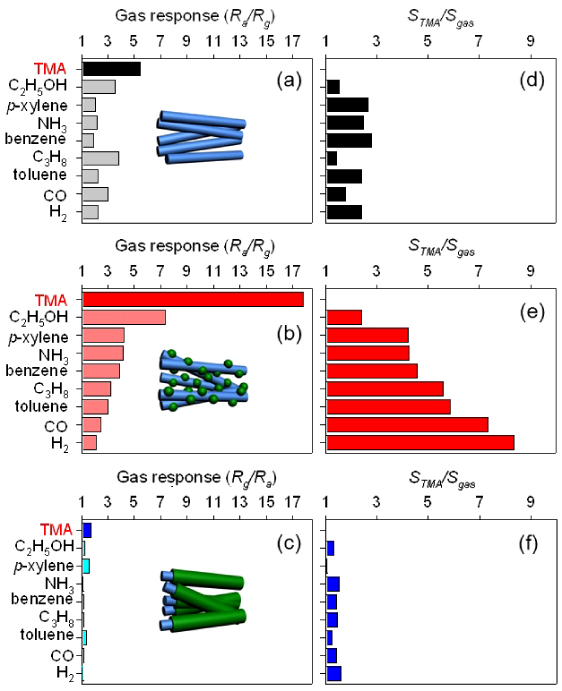
The gas responses to TMA, C2H5OH, p-xylene, NH3, benzene, C3H8, toluene, CO, and H2 of three different NWs were measured (figure 7) at 400 °C. In pristine ZnO NWs, although the response to 5 ppm TMA (Ra/Rg = 5.41) was the highest among the responses to all the gases (figure 7(a)), it was comparable to those to C3H8 (Ra/Rg = 3.80) and C2H5OH (Ra/Rg = 3.52). The decoration of Cr2O3 nanoparticles on ZnO NWs significantly enhanced the response to 5 ppm TMA up to 17.79 (figure 7(b)). This value is 2.4 times higher than the response to C2H5OH (Ra/Rg = 7.35) and significantly higher than the cross-references to other gases such as p-xylene, NH3, benzene, C3H8, toluene, CO, and H2. ZnO–Cr2O3 NCs showed similar responses to all the gases (Rg/Ra = 1.06–1.68) (figure 7(c)). In order to quantify the selective detection of TMA, the ratios of the gas responses to 5 ppm TMA and 5 ppm of another gas (STMA/Sgas: STMA, response to TMA; Sgas, response to other gas) were calculated (figures 7(d)–(f)). In pure ZnO NWs, the STMA/Sgas values ranged from 1.54 to 2.81 (figure 5(d)). In Cr2O3-decorated ZnO NWs, most of the STMA/Sgas values, with the exception of STMA/Sethanol, were in the range from 4.24 to 8.36 (figure 7(e)). Note that the STMA/Sethanol value was also enhanced significantly from 1.54 to 2.42 by the decoration of Cr2O3 on the ZnO NWs. Finally, the STMA/Sgas values of ZnO–Cr2O3 NCs ranged from 1.05 to 1.58 (figure 7(f)). These results clearly show that the coating of discrete Cr2O3 nanoparticles on ZnO NWs is a very effective way to achieve selective and sensitive detection of TMA.
Figure 7. Gas responses (Ra/Rg or Rg/Ra:Ra, resistance in air; Rg, resistance in gas) to 5 ppm TMA and cross-responses to 5 ppm C2H5OH, p-xylene, NH3, benzene, C3H8, toluene, CO, and H2 at 400 °C: (a) gas response (Ra/Rg) of ZnO NWs, (b) gas response (Ra/Rg) of Cr2O3-decorated ZnO NWs, and (c) gas response (Rg/Ra) of ZnO–Cr2O3 core–shell NCs; (d) the ratios of gas responses to 5 ppm TMA and 5 ppm other gases (STMA/Sgas: STMA, response to TMA; Sgas, response to other gas) of ZnO NWs; (e) the STMA/Sgas ratios of Cr2O3-decorated ZnO NWs; (f) the STMA/Sgas ratios of ZnO–Cr2O3 core–shell NCs.
Download figure:
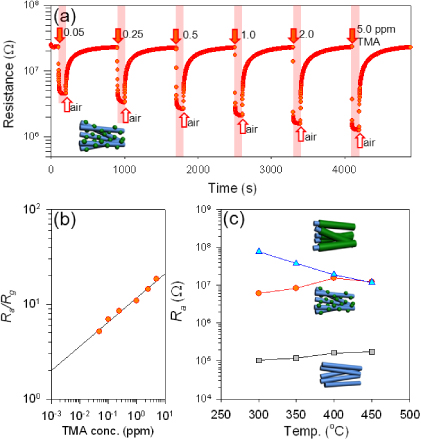
Standard imageThe sensing transients of Cr2O3-decorated ZnO NWs to 0.05–5 ppm TMA at 400 °C exhibited stable and reproducible sensing behaviours (figure 8(a)). The 90% response times, defined as the time to reach a 90% variation of resistance upon exposure to gas, ranged from ∼1 to 10 s, while the 90% recovery times were longer (358–398 s). The TMA responses were plotted as a function of concentration (figure 8(b)). To date, the responses to high concentrations of TMA have been investigated [20, 30, 32, 38–41]. Most of these works have measured the responses to TMA gas for concentrations from 50 ppm up to as high as 1000 ppm, which is significantly beyond the maximum TMA level for fish freshness. Zhu et al [35] have shown responses of 2.0, 3.3, and 49.6 to 0.5, 1.0 and 10 ppm TMA, respectively, using Cu-doped WO3. By contrast, the present Cr2O3-decorated ZnO NWs showed a remarkable response (Ra/Rg = 5.14) to 0.05 ppm TMA. From the fitting of the gas response as a function of TMA concentration, Ra/Rg values as high as 2 could be expected upon exposure to 1 ppb. Although the emittance of [TMA] > 10 ppm is often used as the criterion to evaluate the fish decay [23], the more precise evaluation of fish freshness requires the detection of [TMA] < 10 ppm. Accordingly, the present TMA sensor with ppb-level-precision can be used to evaluate extremely fresh fishes.
Figure 8. (a) TMA sensing transient of Cr2O3-decorated ZnO NWs at 400 °C, (b) gas response (Ra/Rg) of Cr2O3-decorated ZnO NWs as a function of TMA concentration at 400 °C. (c) The resistance in air (Ra) of the ZnO NW sensor, the Cr2O3-decorated ZnO NW sensor, and the ZnO–Cr2O3 core–shell NC sensor in air.
Download figure:
Standard imageThe resistance in air (Ra) of the pure ZnO NW sensor was gradually increased from 0.10 to 0.18 MΩ as the sensor temperature increased (figure 8(c)). It should be noted that the Ra values of Cr2O3-decorated ZnO NW and ZnO–Cr2O3 core–shell NC sensors are 60–300 times higher than that of the pure ZnO NW sensor. The variation of the Schottky junction between the Au contact and ZnO NWs is less probable as the reason for the change of Ra because both ZnO NWs and Cr2O3-decorated ZnO NWs make similar contacts between ZnO and Au electrodes. The Ra values of several sensors prepared under identical growth conditions were similar. Precise estimation of p–n heterojunction structures between n-type ZnO and p-type Cr2O3 is difficult because many different band gap energy (Eg) values of ZnO [42, 43] Cr2O3 [44, 45] have been reported and, in particular, little is known about the electron affinity and work function of Cr2O3. However, the electron depletion layer will be formed near the junction. This shows that not only the decoration of Cr2O3 on ZnO NWs but also the formation of ZnO–Cr2O3 core–shell NCs increases the Ra value due to the extension of the depletion layer by the formation of p–n junctions.
In an n-type gas sensor such as the Cr2O3-decorated ZnO NW sensor, the increase of Ra will lead to enhanced responses to reducing gases because the gas response is defined as Ra/Rg. However, in ZnO–Cr2O3 core–shell NCs, the gas sensing reaction is not simple and depends on the contact configuration between NCs. When p-type Cr2O3 layers completely cover the ZnO NWs, the chemoresistive change will occur along the p-type shell layers. Most p-type oxides are known to form a charge accumulation layer near the surface by the adsorption of negatively charged surface oxygen [46]. The oxidation of reducing gases by negatively charged surface oxygen releases electrons, which thins the near-surface hole accumulation layer and increases the gas resistance. This can explain the p-type gas sensing behaviours observed in the present study. However, the formation of longitudinal p–n junctions between the end parts of the ZnO core and the Cr2O3 shell cannot be excluded completely. In this case, conduction through the p-type Cr2O3 shell layer seems to dominate over conduction via the n-type ZnO core. The incorporation of the ZnO component into the Cr2O3 shell layer, as shown in figure 3c, can be also taken into account. In order to investigate the effect of ZnO incorporation on the gas sensing characteristics of crystalline Cr2O3 NWs, single crystalline or pseudo-single-crystalline Cr2O3 NWs are needed as counterparts for comparison. However, no report on the preparation of single crystalline NWs via vapour phase synthesis is available in the literature, probably due to the high melting temperature (2435 °C) and low vapour pressure of the Cr source. This should be studied further after the preparation of crystalline Cr2O3 NWs.
The enhancement of TMA selectivity by decorating Cr2O3 onto the ZnO NW sensor should be understood in relation to the catalytic effect of Cr2O3 and the extension of the p–n junction between Cr2O3 and the ZnO NW. The former is related to the chemical affinity between Cr2O3 and amine-containing gases. TMA has a very similar molecular structure to methylamine (MA), the simplest primary amine. TMA has two additional methyl groups replacing the hydrogens in MA. The nitrogen in both MA and TMA has a lone pair of electrons that can be donated and bonded. The adsorption and dissociation of MA on the surface of several transition metals including Cr has been experimentally investigated [47]. Borck et al [35] investigated the adsorption and dissociation of methylamine on the surface of Cr2O3 using density functional theory. It has been confirmed that MA adsorption on Cr2O3 involves the bonding of the lone-pair electrons on the nitrogen atom to an exposed cation (Cr in this case) atom on the oxide surface. In detail, the bonding at the surface of Cr2O3 results from the donation of the methylamine lone-pair electron density to a partially empty cation orbital. This explains the promotion of TMA adsorption by the presence of the Cr2O3 layer.
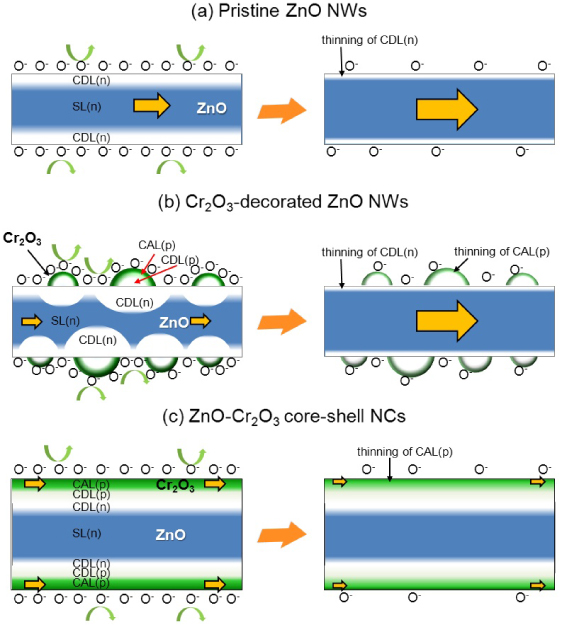
The p–n junction is another important factor in the determination of the gas response [48, 49]. In order to explain this, the schematic gas sensing mechanisms of ZnO NW sensors in relation to the presence and/or configuration of p-type Cr2O3 overlayers are illustrated in figure 9. In pristine ZnO NWs, the adsorption of negatively charged oxygen establishes a carrier (electron) depletion layer (CDL(n)) near the surface (figure 9(a), left). The oxidation of reducing gases by the reaction with negatively charged oxygen (O−) injects electrons into the semiconducting core (n-type semiconducting layer, SL(n)), which decreases the sensor resistance in proportion to the concentration of reducing gas (figure 9(a), right). When a discrete configuration of Cr2O3 nanoparticles is uniformly coated on the surface of the ZnO NWs, the CDL(n) within the ZnO NWs will extend along the radial direction from beneath the semielliptical Cr2O3 nanoparticles (figure 9(b), left), which significantly narrows the conducting core part. The electron injection into ZnO NWs upon exposure to reducing gases would enlarge the conduction path to a great extent by the shrinkage of the electron depletion layer (figure 9(b), right). Negatively charged oxygen is also known to be formed at the surface of p-type Cr2O3, which induces the formation of a carrier (hole) accumulation layer (CAL(p) in figure 9(b), left) near the surface of the p-type Cr2O3. The CAL(p) can be thinned by the reaction between the reducing gas and the negatively charged surface oxygen (figure 9(b), right), which can provide an additional reason for chemoresistive variation. However, considering the discrete configuration of p-type Cr2O3 nanoparticles, conduction along the continuous n-ZnO NWs is more plausible than that along the discrete Cr2O3 nanoparticles, which is supported and verified by the n-type gas sensing characteristics shown in figures 7(a) and (b). This explains the overall enhancement of gas response by the decoration of Cr2O3 nanoparticles. The highly sensitive and selective detection of TMA in Cr2O3-decorated ZnO NW sensors, accordingly, can be understood as the combined effect of the catalytic function of Cr2O3 and the radial extension of the electron depletion layer in the ZnO NWs.
Figure 9. The schematic gas sensing mechanism of (a) pristine ZnO NWs, (b) Cr2O3-decorated ZnO NWs, and (c) ZnO–Cr2O3 core–shell NCs.
Download figure:
Standard imageThe ZnO–Cr2O3 core–shell NCs do not show high responses to TMA or other gases. Although further investigation is necessary to clarify the reason for this, the following explanation can be considered plausible if one assumes a complete covering of the Cr2O3 layer on the ZnO NWs. In ZnO–Cr2O3 core–shell NCs, the adsorption of negatively charged oxygen establishes a charge (hole) accumulation layer (CAL(p) in figure 9(c), left) near the surface of the p-type Cr2O3. In addition, carrier depletion layers will be formed near the interfaces between the p-type Cr2O3 shell layer and the n-type ZnO NWs (CDL(p) and CDL(n), figure 9(c), left). The p-type gas sensing characteristics of ZnO–Cr2O3 core–shell NCs in figure 6(b) strongly indicate that conduction occurs not along the SL(n) channel in the ZnO NWs but along the thin CAL(p) channel in the Cr2O3 shell layers. The high Ra values of ZnO–Cr2O3 core–shell NCs (figure 8(c)) can be attributed to the narrow cross-sectional area for conduction along the very thin CAL(p) channel and the formation of a carrier depletion layer near the p–n junction. The high Ra value leads to low gas response (Rg/Ra) characteristics. In this configuration, the chemoresistive change, which originates from further thinning of the accumulation layer by the reducing gas (figure 9(c)), will be relatively small compared to that caused by the significant shrinkage of the electron depletion layer in the Cr2O3-decorated ZnO NW sensor (figure 9(b)).
4. Conclusions
A Cr2O3-decorated ZnO NW gas sensor was made using a two-step process. ZnO NWs were grown on alumina sensor substrates with Au electrodes by thermal evaporation and semielliptical Cr2O3 nanoparticles were deposited on the surfaces of the ZnO NWs through the thermal evaporation of CrCl2. The gas sensor showed promising results in that it had high sensitivity and selectivity towards TMA, which were attributed to the catalytic effect of Cr2O3 nanoparticles on the surfaces of the ZnO NWs and the extension of the electron depletion layer by the p–n junction. The less-agglomerated and porous network structures of NW based gas sensors provide the benefits of not only the rapid and effective diffusion of analyte gases to the entire sensing surface, but also the uniform loading of catalysts. Thus, the uniform deposition of a discrete configuration of p-type semiconductor nanostructures on the surfaces of n-type semiconductor NWs can be a powerful tool for achieving enhanced gas selectivity and sensitivity.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) National Research Laboratory (NRL) program grant funded by the Korean government (MEST) (No R0A-2008-000-20032-0).