Abstract
Lead halide perovskite materials are thriving in optoelectronic applications due to their excellent properties, while their instability due to the fact that they are easily hydrolyzed is still a bottleneck for their potential application. In this work, water-resistant, monodispersed and stably luminescent cesium lead bromine perovskite nanocrystals coated with CsPb2Br5 were obtained using a modified non-stoichiometric solution-phase method. CsPb2Br5 2D layers were coated on the surface of CsPbBr3 nanocrystals and formed a core–shell-like structure in the synthetic processes. The stability of the luminescence of the CsPbBr3 nanocrystals in water and ethanol atmosphere was greatly enhanced by the photoluminescence-inactive CsPb2Br5 coating with a wide bandgap. The water-stable enhanced nanocrystals are suitable for long-term stable optoelectronic applications in the atmosphere.
Export citation and abstract BibTeX RIS
1. Introduction
Inorganic metal halide perovskite (CsPbX3, where X is a halogen element such as F, Br or I) nanocrystals have attracted considerable attention as a versatile material platform for the realization of a new generation of solution-processible optoelectric devices, including light emitting diodes (LEDs) [1], lasers [2], photodetectors [3] and solar cells [4]. Perovskite nanocrystals have excellent optoelectronic properties, such as large bandgap tunability, efficient narrow band emission, low cost and facile synthesis process [5, 6]. However, all lead halide perovskite materials are normally very sensitive to moisture [7] due to the fact that they are easily hydrolyzed [8]. The light emission wavelength also varies during their hydrolysis and crystal-growing at ambient atmosphere. Hence, the instability of the emerging perovskite materials in a humid environment is a bottleneck for their potential applications [9].
'Coating' is an effective method for enhancing the stability of perovskite nanocrystals, such as coating with silica [10], silica spheres [11], mesoporous silica matrixes [12] and polyhedral oligomeric silsesquioxane [13]. The nanocrystals can also be dispersed in phosphate glasses [14], PMMA [5] or PMA [15] to enhance their stability. All these coating processes make nanocrystals clusters instead of being monodispersed. Surface engineering has been used, such as ligand exchange processes, to improve the luminescence efficiency but not to be stably enhanced [16]. Nevertheless, these coating structure are usually insulating, resulting in clusters or introducing a lot of defects that hinder them from use in optoelectronic applications. Until now, thin layer coated or monodispersed core–shell structures are of great interest and an unsolved issue. In particular, the wide bandgap coating layer and the narrow bandgap core may form a quantum well structure and enhance the light emission of the core. Researchers are looking for the proper materials and synthesis methods for such core–shell structures.
Recently, CsPb2Br5 has been reported with indirect bandgap and being photoluminescence (PL) inactive with a bandgap of 2.979 eV, while CsPbBr3, with a bandgap of about 2.48 eV [17–20]. Tetragonal CsPb2Br5 is a 2D material that has an obviously different crystal structure from CsPbBr3. A wide bandgap coating layer and narrow bandgap core may form a quantum well structure and enhance light emission. Dual-phase CsPbBr3–CsPb2Br5 structures usually exist during the synthesis process of CsPbBr3 nanocrystals [20]. The shape and phase evolution from CsPbBr3 to CsPb2Br5 demonstrated that the reaction could take place at or over 396.5 K in the solvent and the ultrathin CsPb2Br5 nanosheets were also synthesized by adjusting the chain length of the ligand [17]. Some electrical and optical properties of CsPb2Br5 are still rarely investigated and many blanks need to be filled. The synthesis parameters are extremely strict for the formation of inorganic metal halide perovskite nanocrystals [21]. This also makes it possible to synthesize and control the crystal growth of CsPbBr3–CsPb2Br5 system materials. Nevertheless, how to control the enlargement of the CsPb2Br5 2D layer to form a coating layer is still an issue.
In view of the aforementioned facts, CsPb2Br5 coating CsPbBr3 could be an option to improve the stability of perovskite nanocrystals. The crystal growth of perovskite materials at different crystal facets could be controlled via the synthesis parameters, such as the surfactant, ratio of reactant, temperature, and reaction time, even though the experiment parameters are extremely strict [22–28]. In this work, CsPbBr3 nanocrystals coated with CsPb2Br5 were synthesized using a modified non-stoichiometric solution-phase method by controlling the density of Cs2CO3 and PbBr2 in the precursor solution under specific synthesis parameters.
2. Experimental details
2.1. Chemicals
Cesium carbonate (Cs2CO3, 99.995%), lead(II) brimine (PbBr2, 99.999%), oleic acid (OA, 90%), oleylamine (OM, 70%), 1-octadecene (ODE, 90%), and hexane (99%), were obtained from Sigma-Aldrich. All chemicals were used as-received without further purification.
2.2. Preparation of the Cs–oleate solution
A Cs–oleate solution was prepared using a method similar to, but improved on, that reported by Protesescu et al [5]. In brief, 0.4 g Cs2CO3 mixed with 1.2 ml OA and 15 ml ODE was loaded into a three-necked flask (volume 100 ml), and dried at 120 °C for 30 min. Then, they were heated to 150 °C for at least 30 min to ensure that Cs2CO3 was fully reacted with with the OA.
2.3. Synthesis of CsPbBr3/CsPb2Br5 core–shell nanocrystals and CsPbBr3 nanocrystals
2.3.1. CsPbBr3/CsPb2Br5 core–shell nanocrystals
0.94 mmol PbBr2 mixed with 5 ml ODE were heated at 120 °C for about 60 min in a three-necked flask (volume 50 ml). Subsequently, 0.5 ml OA and 0.5 ml OM were loaded into the three-necked flask. Furthermore, the solution was heated to 150 °C for at least 30 min to allow for the completely dissolution of PbBr2. Then, the Cs–oleate solution and PbBr2 solution as-prepared were heated to 190 °C. When the temperature was stable, the solution was injected into the flask promptly under magnetic stirring. Finally, the reaction solution was cooled down quickly in ice water for 30 min and put in a freeze drying oven for 30 min. The entire reaction process was carried out in an argon atmosphere.
The coated nanocrystals were kept in the original solution and washed with ethanol to remove impurities one or two times for the following characterizations since it cannot be soluble in ethanol.
2.3.2. CsPbBr3 nanocrystals
0.188 mmol PbBr2 mixed with 5 ml ODE were heated at 120 °C for about 60 min in a three-necked flask (volume 50 ml). Subsequently, 0.5 ml OA and 0.5 ml OM were loaded into the three-necked flask. Furthermore, the solution was heated to 150 °C for at least 30 min to allow for the complete dissolution of PbBr2. Then, the Cs–oleate solution as-prepared was injected into the flask promptly under magnetic stirring. Finally, the reaction solution was cooled down using ice water for 30 min and put in a freeze drying oven for 30 min. The entire reaction process was carried out in an argon atmosphere.
2.4. Characterization
X-ray powder diffraction (XRD) was performed using a D/max 2200 V x-ray powder diffractometer with Cu Kα radiation (wavelength 1.54 Å) to characterize the perovskite nanocrystals structure. The lattice parameters, volume, and crystallite sizes of the materials were extracted using the Rietveld refinement and Williamson–Hall plot methods [29, 30]. The high-resolution transmission electron microscopy (HRTEM) images were recorded using a JEM-2100F operated at 200 kV. The steady-state PL emission spectra were recorded on a Hitachi F4500 fluorescence spectrophotometer with a Xe lamp coupled to a monochromator. All spectral measurements were performed at room temperature.
3. Results and discussion
The crystal structure of CsPbBr3 nanocrystals and CsPbBr3/CsPb2Br5 core–shell nanocrystals were investigated by XRD, as shown in figures 1(a) and (b), respectively. It is worth noting that the diffraction peaks between the CsPbBr3 nanocrystals and the standard XRD spectra of the CsPbBr3-cubic are all very similar, both in location and relative strength, as shown in figure 1(a), which confirms that the CsPbBr3 nanocrystals have relatively high crystallinity with cubic structure. The lattice parameters of CsPbBr3 are a = b = c = 0.583 nm and  By contrast, for the CsPbBr3/CsPb2Br5 core–shell nanocrystals, the diffraction peaks were derived from both the CsPbBr3-cubic structure and the CsPb2Br5-tetragonal structure, as shown in figure 1(b). The lattice parameters of CsPb2Br5 are a = b = 0.848 nm, c = 1.525 nm and
By contrast, for the CsPbBr3/CsPb2Br5 core–shell nanocrystals, the diffraction peaks were derived from both the CsPbBr3-cubic structure and the CsPb2Br5-tetragonal structure, as shown in figure 1(b). The lattice parameters of CsPb2Br5 are a = b = 0.848 nm, c = 1.525 nm and  The CsPbBr3 contributes most of the percentage of materials, while the CsPb2Br5 contributes much less. This infers that the CsPbBr3/CsPb2Br5 core–shell nanocrystals contain these two crystal structures. The crystallite size (
The CsPbBr3 contributes most of the percentage of materials, while the CsPb2Br5 contributes much less. This infers that the CsPbBr3/CsPb2Br5 core–shell nanocrystals contain these two crystal structures. The crystallite size ( ) of the two materials could both be estimated at approximately 10 nm according to the Debye–Scherrer formula
) of the two materials could both be estimated at approximately 10 nm according to the Debye–Scherrer formula
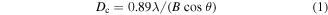
where B is the half-peak width and  in the XRD measurement is 0.154 nm under K
in the XRD measurement is 0.154 nm under K radiation of the Ni-filtered Cu target. The
radiation of the Ni-filtered Cu target. The  ranges from 10° to 80°.
ranges from 10° to 80°.
Figure 1. (a) XRD spectrum of CsPbBr3 nanocrystals compared to the standard XRD of CsPbBr3-cubic (PDF#54-0752); (b) XRD spectrum of CsPbBr3/CsPb2Br5 core–shell nanocrystals compared to the standard XRD of CsPb2Br5-tetragonal (PDF#25-0211) and CsPbBr3-cubic (PDF#54-0752). (c1) (c2) (d1) (d2): the HRTEM images of CsPbBr3 nanocrystals ((c1) and (c2)) and CsPbBr3/CsPb2Br5 core–shell nanocrystals ((d1) and (d2)).
Download figure:
Standard image High-resolution imageTo further study the shape and crystal structure of the CsPbBr3/CsPb2Br5 core–shell nanocrystals, the two samples were investigated by HRTEM as shown in figures 1(c), (d). There is no obvious difference between the shape of the two kinds of nanocrystals, all of which are nanocubes at about 10 nm. The crystal size is the same as that calculated with the Debye–Scherrer formula. The CsPb2Br5 layer appears lattice spaced at 0.3 nm (facet (213)) and that of CsPbBr3 at 0.58 nm (facet (100)), as shown in figures 1(c2) and (d2). The characterizations demonstrate that the coating processes just result in several layers of CsPb2Br5 on the surface instead of changing or destroying the original crystal structure.
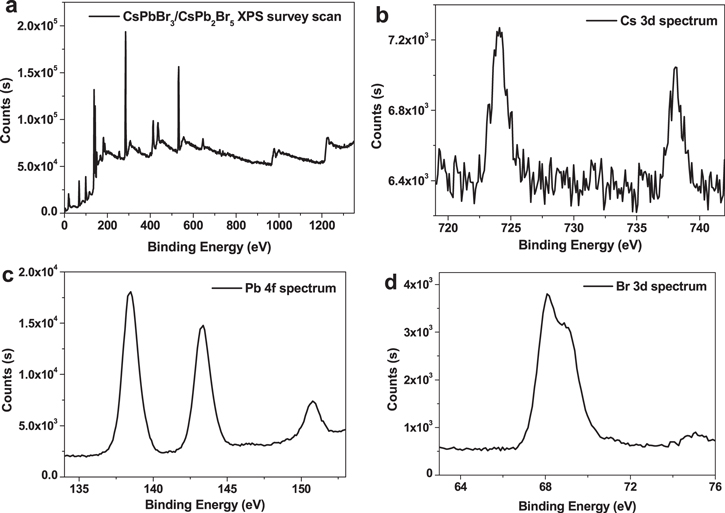
The x-ray photoelectron spectroscopy (XPS) characterizations of CsPbBr3/CsPb2Br5 core–shell nanocrystals are plotted in figure 2. There are three elements 'Cs', 'Pb' and 'Br' in the materials. The atomic ratio between 'Pb' and 'Br' is close to 2:5. The surface of the nanocrystals is made of layers of Pb–Br structures, while there is less Cs, which is all inside the nanocrystals. The structure of the CsPbBr3/CsPb2Br5 core–shell nanocrystals will be demonstrated in the following. It must be stated that there are still some precursor remains in the materials that cannot be avoided by purification during the XPS test, which may affect the results.
Figure 2. (a) XPS survey scan of CsPbBr3/CsPb2Br5 core–shell nanocrystals; (b) Cs 3d spectrum; (c) Pb 4f spectrum; (d) Br 3d spectrum. The atomic ratios of 'Cs', 'Pb' and 'Br' are 2.58%, 32.58% and 64.84%, respectively. Data were collected after 60 s of in situ Ar etching.
Download figure:
Standard image High-resolution imageThe PL decay spectra of the CsPbBr3 nanocrystals and the CsPbBr3/CsPb2Br5 core–shell nanocrystals were plotted, as shown in figures 3(a) and (b), respectively. The PL decay curve can be well fitted with a double-exponential function
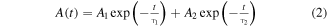
where A, A1, A2 are constants, t is time, and  and
and  represent the decay lifetimes corresponding to the intrinsic exciton relaxation and interaction between excitons and defects, respectively. The average lifetime (
represent the decay lifetimes corresponding to the intrinsic exciton relaxation and interaction between excitons and defects, respectively. The average lifetime ( ) can be calculated as
) can be calculated as
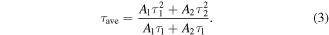
Figure 3. (a), (b) The PL decay spectra of CsPbBr3 nanocrystals and CsPbBr3/CsPb2Br5 core–shell nanocrystals, respectively.
Download figure:
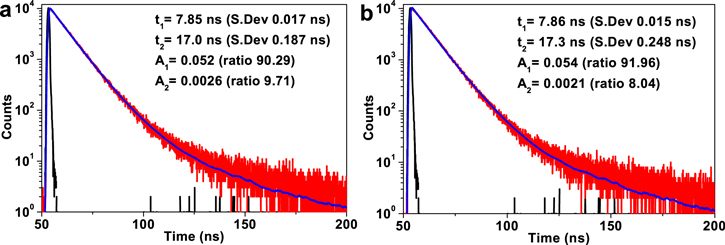
Standard image High-resolution imageThe CsPbBr3/CsPb2Br5 core–shell nanocrystals exhibit two lifetimes: τ1 of 7.85 ns accounting for 90.29% and τ2 of 17.0 ns accounting for 9.71%, respectively, which reveals the extremely high ratio of the intrinsic exciton relaxation. It infers that the two samples have fewer defect or impurity states. The CsPbBr3/CsPb2Br5 core–shell nanocrystals exhibit lifetime almost the same with the CsPbBr3 nanocrystals except only 0.3 ns difference in  This slightly longer
This slightly longer  of the CsPbBr3/CsPb2Br5 core–shell nanocrystals means that defects are reduced, but not obviously after coating, which maybe a result of the blue shift of the PL peak of the CsPbBr3/CsPb2Br5 core–shell nanocrystals compared with that of the CsPbBr3 nanocrystals. Anyway, the CsPbBr3/CsPb2Br5 core–shell nanocrystals still show the intrinsic luminescence properties of CsPbBr3 nanocrystals. It infers that the coating processes did not introduce any impurities or defects in the materials.
of the CsPbBr3/CsPb2Br5 core–shell nanocrystals means that defects are reduced, but not obviously after coating, which maybe a result of the blue shift of the PL peak of the CsPbBr3/CsPb2Br5 core–shell nanocrystals compared with that of the CsPbBr3 nanocrystals. Anyway, the CsPbBr3/CsPb2Br5 core–shell nanocrystals still show the intrinsic luminescence properties of CsPbBr3 nanocrystals. It infers that the coating processes did not introduce any impurities or defects in the materials.
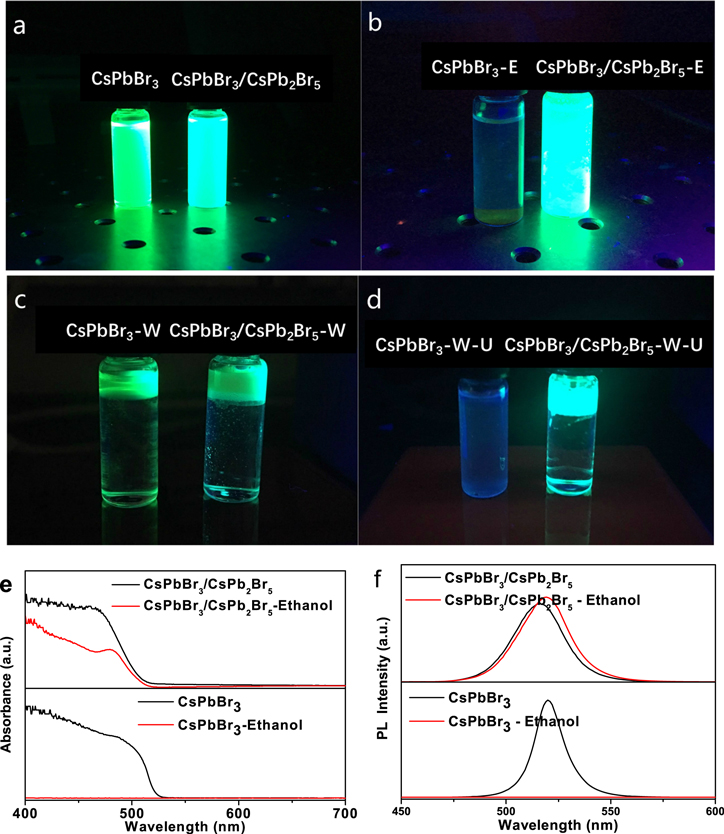
The luminescence stability in the water and ethanol atmosphere of the CsPbBr3/CsPb2Br5 core–shell nanocrystals and CsPbBr3 nanocrystals were both measured. The prepared nanocrystals appear as bright green emission as shown in figure 4(a). Firstly, we added ethanol with three times volume of nanocrystals solution. The ethanol and the solvent of nanocrystals' solution can be dissolved with each other in any ratio. After 5 s, the CsPbBr3 nanocrystals had been decomposed and its solution became nonluminous transparent solution, while the CsPbBr3/CsPb2Br5 core–shell nanocrystals could not be decomposed but could be dissolved in the mixed solution and remained luminescent, as shown in figure 4(b). Then two samples were tested with water. Water with a three times volume of nanocrystals solution was added into the solution. Water was on the bottom and nanocrystals solution was on the top, as shown in figure 4(c), because water and the solvent cannot be dissolved. Then the two samples were ultrasonicated for 2 h and left to stand for 5 min. The CsPbBr3 nanocrystal solution became nonluminous while the CsPbBr3/CsPb2Br5 core–shell nanocrystals solution remained bright green, as shown in figure 4(d). The absorption and PL spectra before and after the test were investigated, as in figures 4(e) and (f), respectively. The absorption and PL spectra of the CsPbBr3/CsPb2Br5 core–shell nanocrystals changed little after being added to ethanol, while that of the CsPbBr3 nanocrystals were not detected in the visible range from 400 nm to 700 nm. The absorption spectra of CsPbBr3/CsPb2Br5 core–shell nanocrystals shifted to blue by about 5 nm compared to that of CsPbBr3. The PL peak at 515 nm of the CsPbBr3/CsPb2Br5 core–shell nanocrystals shifted to blue by about 5 nm and was enhanced compared to that of CsPbBr3 nanocrystals at about 520 nm. In halide perovskite materials, the halide elements such as 'Cl', 'Br' and 'I' could determine the bandgap and PL spectra of the materials. In addition, the crystallite size could also affect the bandgap and PL spectra, but as a secondary factor. The blue shift is due to the interface modification of nanocrystals in CsPbBr3/CsPb2Br5 core–shell nanocrystals and the smaller core size of CsPbBr3. It must be stated that the light emission of CsPbBr3/CsPb2Br5 core–shell nanocrystals in water and ethanol also decreased, but very slowly in the cuvette in the next few days.
Figure 4. Photographs of CsPbBr3 nanocrystals and CsPbBr3/CsPb2Br5 core–shell nanocrystals excited at 365 nm in water and ethanol atmosphere. (a) CsPbBr3 nanocrystals solution and CsPbBr3/CsPb2Br5 core–shell nanocrystals solution; (b) CsPbBr3 nanocrystals solution and CsPbBr3/CsPb2Br5 core–shell nanocrystals solution added to ethanol after 5 s; (c) CsPbBr3 nanocrystals solution and CsPbBr3/CsPb2Br5 core–shell nanocrystals solution added to water; the top is the nanocrystals solution, the bottom is water; (d) CsPbBr3 nanocrystals solution and CsPbBr3/CsPb2Br5 core–shell nanocrystals solution added to water under ultrasonication for 2 h. (e), (f) Absorption spectra and PL spectra of CsPbBr3 nanocrystals and CsPbBr3/CsPb2Br5 core–shell nanocrystals, before and after ethanol being added, respectively.
Download figure:
Standard image High-resolution imageFurthermore, the two nanocrystals solutions were also deposited on ITO glasses without any purification and passivation treatment, which were later left in water atmosphere (filled with water vapor for humidity of 100%) for a few days. The result was similar to the former test that CsPbBr3/CsPb2Br5 core–shell nanocrystals films were much more stably luminescent than nanocrystals films, as shown in figure 5. The ethanol and water test in solution and solid films verifies that the CsPbBr3/CsPb2Br5 core–shell nanocrystals are anti-water and luminescent stably in water and ethanol environments.
Figure 5. (a) (b) Photographs of the CsPbBr3 nanocrystal and CsPbBr3/CsPb2Br5 core–shell nanocrystal films on ITO glasses (size 2 cm × 2 cm) before and after being put in water atmosphere for five days, respectively. (c), (d) PL spectra of CsPbBr3 nanocrystals and CsPbBr3/CsPb2Br5 core–shell nanocrystals films on ITO glasses before and after being put in water atmosphere for a few days, respectively. The samples are put in a sealed cavity filled with water vapor kept at a humidity of 100%.
Download figure:
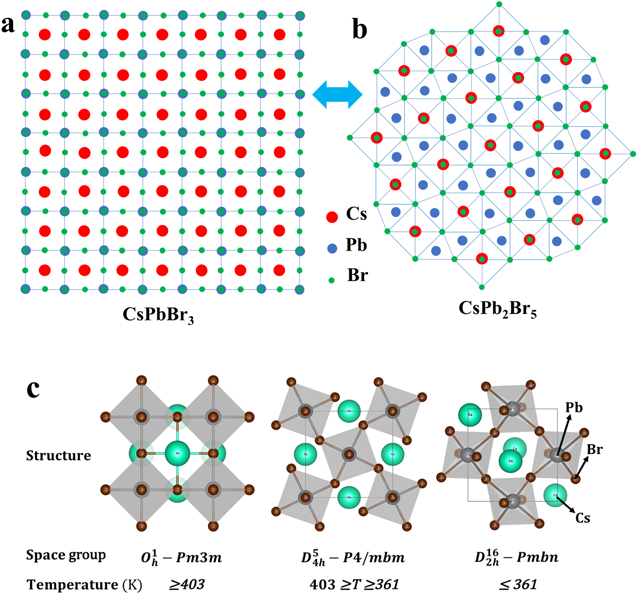
Standard image High-resolution imageThe mechanisms of the formation of the CsPbBr3/CsPb2Br5 core–shell nanocrystals will be demonstrated in the following statements. In the reaction process, CsPbBr3 nanocrystals formed with an octahedral structure of lead and bromine elements on the surface of nanocrystals during crystal growth. Under the reaction at 463.15 K, the Pb2+ and Br- ions were still superfluous, which would embed into the Pb–Br octahedral structures on the nanocrystals surface and change it to a Pb–Br capped polyhedron composed of a triangular prism and two rectangular pyramids, as shown in figure 6, which was unstable in the solution and easy to return back to CsPbBr3 structures as:
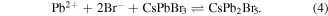
The structure of CsPbBr3 at different temperatures was simulated, as shown in figure 6(c). CsPbBr3 tends to be of  space group structure at relative high temperature, which corresponds to the XRD and HRTEM characterizations of CsPbBr3 nanocrystals. The crystal structure of CsPb2Br5 and CsPbBr3 has also been reported in previous works [17, 31]. CsPb2Br5 material appears to be a 2D structure, as shown in figure 6(b), which prefers growing horizontally at two crystal facets (100) and (110) and forms 2D layer structures with Pb and Br elements while Cs is between the two layers. As the nanocrystals grew, the evolution between CsPbBr3 and CsPb2Br5 persisted. However, CsPb2Br5 always changed back to CsPbBr3 due to the existence of sufficient Cs+ at a relatively high temperature of 463.15 K as a function as:
space group structure at relative high temperature, which corresponds to the XRD and HRTEM characterizations of CsPbBr3 nanocrystals. The crystal structure of CsPb2Br5 and CsPbBr3 has also been reported in previous works [17, 31]. CsPb2Br5 material appears to be a 2D structure, as shown in figure 6(b), which prefers growing horizontally at two crystal facets (100) and (110) and forms 2D layer structures with Pb and Br elements while Cs is between the two layers. As the nanocrystals grew, the evolution between CsPbBr3 and CsPb2Br5 persisted. However, CsPb2Br5 always changed back to CsPbBr3 due to the existence of sufficient Cs+ at a relatively high temperature of 463.15 K as a function as:
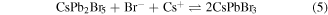
at a relatively high temperature of 463.15 K. As the reaction was in progress, the amount of Cs+ decreased much faster than that of Pb2+ due to the superfluous Pb2+ during the reaction. This led to the fact that the core of the nanocrystals was mainly CsPbBr3 structure. Li et al realized the evolution from CsPbBr3 nanocubes to tetragonal CsPb2Br5 nanosheets above the temperature of 396.5 K (when the temperature is above 396.5 K the change in Gibbs free energy of the reaction is ΔG < 0) by varying the ligands successfully [17]. The temperature is another key factor for the synthetic processes except for the excess Pb2+ and Br−. More and more CsPB2Br5 formed and extended to layers on the crystal surface as the density of Cs+ decreased a lot at the end of the reaction. At the end of the synthetic process, the nanocrystal solution was put in ice water to cool it down rapidly when the reaction finished. The temperature decreases to zero promptly. Then, the formed CsPb2Br5 structure could not return back to CsPbBr3 structure due to ΔG > 0 as:
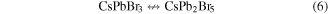
and remained as several layers on the surface of CsPbBr3 nanocrystals. The CsPb2Br5 layer could be stable on the surface of the nanocrystals and formed a coating structure after the reaction finished. Hence, 2D CsPb2Br5 grows horizontally and forms several layers on the surface of CsPbBr3 nanocrystals.
Figure 6. The crystal structure of CsPbBr3 and CsPb2Br5. (a) CsPbBr3: Pb–Br forms octahedral structures with each Cs inside eight octahedral structures (red ball: Cs, blue ball: Pb, and green ball: Br); (b) CsPb2Br5: 2D structure of Pb–Br 2D layers with Cs embedded between each two layers; (c) simulation of CsPbBr3 structures at different temperature using SPuDS software (green ball: Cs, black ball: Pb, and brown ball: Br).
Download figure:
Standard image High-resolution imageIn addition, the CsPbBr3/CsPb2Br5 core–shell nanocrystals could form a quantum well structure due to the wider bandgap of CsPb2Br5 than CsPbBr3, which infers that light emission could be enhanced. The CsPb2Br5 has an indirect bandgap, and is PL-inactive and anti-water, which makes it a good option for coating materials. CsPbBr3/CsPb2Br5 core–shell nanocrystals can be a candidate material to solve the bottleneck problem of instability for future water-stable perovskite-based applications.
4. Conclusion
In summary, water-resistant, monodispersed and stably luminescent CsPbBr3/CsPb2Br5 core–shell like structure nanocrystals were obtained and reported for the first time using a modified non-stoichiometric solution-phase method. The temperature and excess Pb2+ are two key factors for the formation of CsPbBr3/CsPb2Br5 core–shell nanocrystals. During the synthesis processes, the evolution between CsPb2Br5 and CsPbBr3 are reversible in the solution of superfluous Pb2+ and Br−. At the beginning, the formed CsPb2Br5 transformed mostly to CsPbBr3 due to the existence of Cs–oleate. However, at the end of the reaction, the solution was put in ice water to cool down. The CsPb2Br5 formed several layers on the surface of CsPbBr3 nanocrystals and did not transform back to CsPbBr3 due to ΔG > 0. The CsPb2Br5 coating with indirect bandgap and without light emission makes the CsPbBr3/CsPb2Br5 core–shell nanocrystals have a quantum well structure and be water-resistant, which greatly improves the PL properties. The water-stable enhanced nanocrystals make it possible for long-term stable optoelectronic applications in the atmosphere. Future works will focus on their electrical and optical properties, and their application to optoelectronics and biological applications.
Acknowledgments
We would like to thank the Fundamental Research Funds for the Central Universities (FRFCU) under Grant No. 2016JBM066, the National Natural Science Foundation of China (NSFC) under Grant No. 61704007 and No. 11474018 and the National Key Research and Development Program of China under Grant No. 2016YFB0401302 for financial support.