Abstract
Bladder cancer has a 60%–70% recurrence rate most likely due to any residual tumour left behind after a transurethral resection (TUR). Failure to completely resect the cancer can lead to recurrence and progression into higher grade tumours with metastatic potential. We present here a novel therapy to treat superficial tumours with the potential to decrease recurrence. The therapy is a heat-based approach in which bladder tumour specific single-walled carbon nanotubes (SWCNTs) are delivered intravesically at a very low dose (0.1 mg SWCNT per kg body weight) followed 24 h later by a short 30 s treatment with a 360° near-infrared light that heats only the bound nanotubes. The energy density of the treatment was 50 J cm−2, and the power density that this treatment corresponds to is 1.7 W cm−2, which is relatively low. Nanotubes are specifically targeted to the tumour via the interaction of annexin V (AV) and phosphatidylserine, which is normally internalised on healthy tissue but externalised on tumours and the tumour vasculature. SWCNTs are conjugated to AV, which binds specifically to bladder cancer cells as confirmed in vitro and in vivo. Due to this specific localisation, NIR light can be used to heat the tumour while conserving the healthy bladder wall. In a short-term efficacy study in mice with orthotopic MB49 murine bladder tumours treated with the SWCNT-AV conjugate and NIR light, no tumours were visible on the bladder wall 24 h after NIR light treatment, and there was no damage to the bladder. In a separate survival study in mice with the same type of orthotopic tumours, there was a 50% cure rate at 116 days when the study was ended. At 116 days, no treatment toxicity was observed, and no nanotubes were detected in the clearance organs or bladder.
Export citation and abstract BibTeX RIS
1. Introduction
Bladder cancer has one of the highest costs-per-patient ratios for diagnosis and treatment due to the elevated rate of recurrence [1–3]. Approximately 75%–85% of patients have Ta/T1 stage non-muscle invasive bladder cancer (NMIBC) that can be treated using surgical transurethral resection (TUR). TUR is usually followed by intravesical chemotherapy and immunotherapy, which has proven to be effective in increasing remission periods; however, 70% of patients still experience tumour relapse [1–5]. Recurrence is believed to occur due to four main mechanisms: incomplete resection, re-implantation, disperse microscopic tumours, or new tumour development [1, 3, 4]. A previous study tested the frequency of each mechanism and determined incomplete resection and cell re-implantation after TUR to be the primary causes of most relapses [1]. Unfortunately, there has been limited improvement in therapeutic options for bladder cancer patients over the past 20 years [5]. Due to this void in research as well as the rapidly increasing costs associated with cycles of diagnostics and treatment, we have developed a novel approach to thermally ablate tumours using specifically targeted single-walled carbon nanotubes (SWCNTs) and near-infrared (NIR) laser light.
Photothermal ablation is the use of light to heat biologic tissue above 60 °C over a short time period [6, 7]. Exposure to high temperatures leads to tissue death via necrosis and can be used as an alternative for chemoresistive as well as hyperthermic resistive tumours [6–8]. A previous study comparing the response of breast cancer to hyperthermia (39 °C–45 °C), a slow heating of tissue, versus ablation showed that ablation temperatures were able to eradicate hyperthermia resistive tumours [7, 9].
A recent study of treating mice with bladder cancer used NIR light in combination with anti-epidermal growth factor receptor (EGFR) coated gold nanoparticles obtained promising results using a procedure that required surgery to expose the bladder [10]. In the current study, we have focused on using targeted SWCNTs and have developed a clinically translatable procedure to irradiate the bladder without the need for surgery.
SWCNTs are gaining more attention as versatile nanoparticles in diagnostics and therapeutics for drug delivery, photodynamic therapy, and NIR contrast enhancement [11–14]. SWCNTs have an intrinsic excitation in the NIR range (700–1100 nm) that is released as vibrational energy. This energy is converted into heat that induces cell death via coagulative necrosis, rupturing of cell membranes, and denaturing of proteins [8, 15, 16]. Biological tissue has an 'optimal window' within the NIR range making the tissue easily light penetrable up to a few millimetres [8, 12].
In order to enhance nanotube accumulation within the tumour as well as minimise non-specific heat effects, SWCNTs are conjugated to annexin V (AV), which strongly binds with phosphatidylserine (PS) present on tumour cells and tumour vasculature [17–20]. Previous literature has reported low dissociation constants between 0.1 and 2 nM for PS binding to AV for various cells [21]. PS is primarily found in an asymmetric conformation on the inner leaflet of the plasma membrane of healthy cells; however in the tumour vasculature and cancer cells, it gets externalised, making it a specific and versatile targeting receptor for bladder cancer as well as other tumours [17–20, 22]. The SWCNT-AV conjugate is delivered intravesically to the bladder for tumour specific accumulation. Intravesical delivery is commonly utilised over systemic since it provides a higher payload and the urothelium limits the absorption of particles into the bloodstream [2, 12, 16, 23].
Due to the strong association of AV to bladder tumours and the intrinsic property of nanotubes to absorb NIR light and dissipate energy as heat, this study hypothesises that targeted SWCNT-AVs in combination with a global radiating NIR fibre can be utilised as an alternative to treat NMIBC and reduce relapse rates without invasive surgery (figure 1). This study has proven that SWCNT-AVs specifically target bladder cancer cells in vitro as well as in vivo with no detectable binding to normal urothelium; combining PS-targeted nanotubes with NIR light at relatively low power level resulted in a 50% cure rate with no observable healthy tissue damage for orthotopic MB49 murine bladder tumours, thereby proving the effectiveness and clinical applicability of the proposed approach.
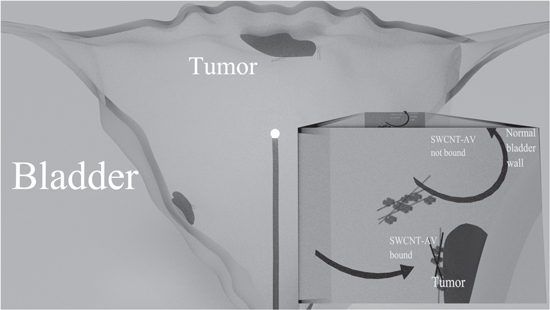
Figure 1. Representation of the structure of the bladder showing the mechanism of the SWCNT-AV and NIR light combination therapy. Cancer targeted SWCNTs are instilled into the bladder via catherization. After a clearance period for unbound nanotubes, a 360° diffusing fibre emitting a 980 nm light is threaded via a catheter into the centre of the bladder. The tumour is irradiated to heat the bound nanotubes and cause cancer cell death.
Download figure:
Standard image High-resolution image2. Methods
2.1. Materials
The plasmid encoding AV, pET-30 Ek/LIC/ANX, was previously constructed in this lab [12]. Bovine serum albumin (BSA), Alamar Blue reagent, Triton X-100, EDTA, and Tris-acetate-EDTA buffer were from Sigma-Aldrich (St Louis, MO). Sodium phosphate and sodium dodecyl sulphate (SDS) were from Mallinckrodt Chemicals (Phillipsburg, NJ). Paraformaldehyde was from Electron Microscopy Sciences (Hatfield, PA). Antifade reagent Fluoro-gel, borate buffer, fluorescein isothiocyanate (FITC), poly-L-lysine, and Slide-A-Lyzer dialysis cassettes (3.5 kDa) were from Thermo Fisher Scientific (Waltham, MA). The 2 and 100 kDa dialysis membranes were from Spectrum Laboratories (Rancho Dominguez, CA). Anti-AV (FL-319) was from Santa Cruz Biotechnology (Santa Cruz, CA). Murine bladder cancer cells (MB49) and human bladder cancer cells (J82) were from ATCC (Manassas, VA). RPMI-1640, Dulbecco's Modified Eagle, and keratinocyte-SFM cell medium were from ATCC (Manassas, VA). Fetal bovine serum (FBS) was from Atlanta Biologicals (Lawrenceville, GA). Antibiotics, penicillin and streptomycin were from Invitrogen (Grand Island, NY). (6, 5) CoMoCAT SWCNTs (average diameter 0.8 ± 0.1 nm, average length 1.5 ± 0.5 μm) were provided by Southwest Nanotechnologies, Inc. (Norman, OK). The 1, 2-distearoyl-sn-glycero-3-phosphoethanolaminepolyethylene glycol-maleimide (DSPE-PEG-maleimide; PEG molecular weight of 3.4 kDa) linker was from Creative PEGWorks (Winston Salem, NC).
2.2. Cell lines and culture conditions
MB49 cells were transfected with tdTomato (Td) and luciferase (Luc), and J82 cells were transinfected with Td. Normal bladder progenitor cells were HPV immortalised as described [24]. MB49-Td-Luc cells were grown in RPMI-1640 medium, and J82-Td cells were grown in Dulbecco's Modified Eagle's Medium with glucose. Both cell line media were enriched with 10% FBS and penicillin/streptomycin antibiotics (100 U ml−1 and 100 μg ml−1, respectively). Normal immortalised bladder cells were grown in keratinocyte-SFM medium supplemented with 50 μg ml−1 bovine pituitary extract, 5 ng ml−1 epidermal growth factor, and penicillin/streptomycin antibiotics (50 U ml−1 and 50 μg ml−1, respectively). All cells lines were grown at 37 °C with 5% CO2.
2.3. AV and SWCNT-AV production
Recombinant AV and the SWCNT-AV conjugate were produced as previously described [12]. Briefly, E. coli transfected with a plasmid encoding AV, pET-30 Ek/LIC/ANX, was grown and purified using immobilised metal affinity chromatography (IMAC) with immobilised Ni2+ to isolate the AV. Freeze dried CoMoCAT SWCNTs were solubilized in 1% SDS using two cycles of probe sonication at 20 W and centrifugation at 29, 600 g for 30 min each. The suspended SWCNTs were then reacted with DSPE-PEG-maleimide for 30 min at room temperature followed by an 8 h dialysis in distilled water to remove excess linker and SDS. The dialysed conjugate was then reacted with the AV, which contains one cysteine group, for 2 h and blocked with 1.5 mg ml−1 L-cysteine. The final product, SWCNT-AV, was dialysed in 20 mM sodium phosphate buffer for 8 h to remove excess AV and L-cysteine. AV was characterised via SDS-PAGE, and SWCNT-AVs were characterised by UV–vis–NIR spectroscopy.
2.4. Binding strength
The dissociation constant for AV was determined as previously described [12] using biotin conjugated AV on 70% confluent MB49-Td-Luc and J82-Td cells. Briefly, MB49 and J82 cells were grown on 24-well plates to 70% confluence followed by fixation with 4% paraformaldehyde. Cells were incubated with 0–25 nM AV in media either supplemented with 0.5% bovine serum albumin (BSA) and 2 mM CaCl2 for or 5 mM ethylenediaminetetraacetic acid (EDTA) for 2 h at 37 °C. Excess, unbound AV-biotin was washed and 2 μg ml−1 of streptavidin–horse radish peroxidase (HRP) was added for 1 h at room temperature. Excess stretavidin-HRP was washed and cells were incubated with 0.4 mg ml−1 of O-phenylenediamine with hydrogen peroxide for 30 min. Absorbance at 450 nm was read on a BioTek Synergy HT microtiter plate reader (Winooski, VT). Specific binding was determined by subtracting total binding (medium supplemented with calcium) from non-specific binding (medium supplemented with EDTA).
2.5. Fluorescent microscopy of SWCNT-AV in vitro
MB49-Td-Luc and J82-Td cells were grown to 70% confluence on cover slips. SWCNT-AVs were tagged with FITC as previously described in [12]. SWCNT-AV-FITCs (20 mg l−1) in 2 mM CaCl2 were incubated with either MB49-Td-Luc or J82-Td cells for 2 h followed with PBS washing. The cells were fixed in 4% paraformaldehyde. Images were taken on a Nikon fluorescence microscope with a mercury arc lamp source. Images were taken with 480/30 (FITC) and 560/40 (TdTomato) filters at 40X (0.75 numerical aperture). Controls of only Td labelled cells and SWCNT-AV-FITC were imaged.
2.6. In vitro cytotoxicity studies
Alamar Blue pre- and post-cell viability assays were conducted to determine the cytotoxic effects of NIR and SWCNT-AVs on both bladder cancer lines as well as normal bladder cells. Viability assays were conducted on cells at 70% confluence followed by either SWCNT-AV incubation at various concentrations for 2 h or NIR treatment for 30 s at various energy levels. Cells were washed, and a second viability assay was conducted 18 h later.
2.7. Animal handling procedures
All procedures complied with a protocol approved by Institutional Animal Care and Use Committee (IACUC) of the University of Oklahoma Health Sciences Centre. C57BL/6J female mice 10 weeks of age, weighing 20–22 g were used. Mice were on a standard chow diet. All studies on mice were conducted under anaesthesia with 2% isoflurane and 98% oxygen using a nose cone.
2.8. In vivo laser tolerance
Fibre insertion and positioning was determined via ultrasound (VEVO 2100) as shown in figure 2. The fibre was threaded through a catheter attached to an adapter with a resealable end to push the fibre through and a second end with a syringe to fill the bladder with air. An in vivo NIR tolerance test at energy densities of 50 and 100 J cm−2 and time of 30 s with a 360° radiating fibre was conducted. Mice were euthanized 24 h after treatment, and H&E staining on bladders was used to determine if damage was caused to the urothelium.
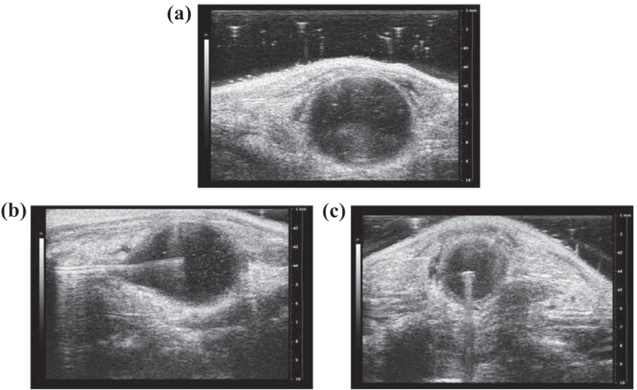
Figure 2. Ultrasound images of fibre positioning in bladder. Ultrasound images of before (a) and after diffusing fibre centreed in C57BL-6 mouse bladder in the x–z (b) and x–y (c) plane. A centred diffusing fibre ensures one session is required to treat the entire bladder and isolated residual tumour cells.
Download figure:
Standard image High-resolution image2.9. In vivo tumour model
Mice were anaesthetised and catherised with a 24 gauge catheter through the urethra. Urine was expelled and the bladder was washed with PBS. Mouse bladders were incubated with 100 μl poly-L-lysine for 20 min to disrupt the glycoaminoglycan (GAG) layer and allow for tumour implantation [25]. Bladders were inoculated with 100 μl of MB49-Td-Luc cells (5 × 105 cells ml−1) for 1 h. All animal studies were conducted once tumour presence was confirmed via bioluminescence on a Carestream X-treme Imager.
2.10. In vivo SWCNT-AV specific tumour cell binding
A mouse with an orthotopic bladder tumour was instilled with 100 μl of SWCNT-AVs at a concentration of 20 mg l−1 (0.1 mg SWCNTs kg−1) for 90 min. The mouse was euthanized 24 h later, and the bladder was collected for H&E and immunohistochemistry (IHC). IHC was conducted for SWCNT-AVs using an anti-AV to stain the AV component of the nanotube. Images were taken on a Zeiss light microscope.
2.11. Biodistribution
NIR fluorescence spectroscopy (NS MiniTracer) analysis by Applied NanoFluorescence (Houston, TX) was conducted on the bladders of saline and SWCNT-AV treated mice 24 h after instillation. A second spectroscopy was conducted on mice that were tumour free 116 days after tumour instillation. Samples for NIR fluorescence spectroscopy were prepared as previously described [26].
2.12. In vivo therapy efficacy
A therapeutic efficacy study combining SWCNT-AVs and NIR light was performed. Tumour presence was confirmed with bioluminescence imaging prior to study initiation. SWCNT-AVs (100 μl of 0.1 mg kg−1) were instilled for 90 min and followed 24 h later with NIR light treatment at an energy and power density of 50 J cm−2 and 1.67 W cm−2, respectively (time = 30 s). The mice were euthanized 24 h after NIR light treatment, and TdTomato fluorescence was measured with a Leica fluorescence dissecting microscope. Fluorescence was quantified via ImageJ analysis. A second set of mice was monitored every 3–4 days for survival post-treatment. H&E and Trichrome staining was conducted to evaluate toxicity of treatment and scar tissue formation.
2.13. Statistics
Statistical significance of in vitro studies was assessed using a one-way ANOVA and Tukey–Kramer multiple comparisons test with GraphPad Prism software. Statistical significance for tumour presence 24 h after treatment was assessed using Pearson's chi-square goodness of fit test.
3. Results
3.1. In vitro binding and tolerance
The dissociation constant of AV binding to MB49-Td-Luc and J82-Td cells was 4.14 ± 1.30 nM and 0.38 ± 0.20 nM, respectively, confirming a strong affinity of AV for bladder tumours. Once AV and PS interaction was confirmed, further evaluation of SWCNT-AVs conjugate binding affinity was visualised via fluorescence microscopy. As seen in figure 3(a), even after the conjugation of SWCNTs to AV, AV still strongly associated with mouse as well as human bladder cancer cells.
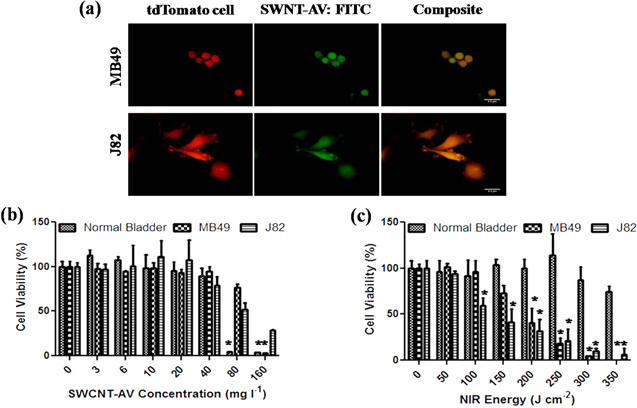
Figure 3. In vitro binding specificity and SWCNT-AV and NIR tolerance. (a) Fluorescence imaging confirming SWCNT-AV binding to murine (MB49) and human (J82) bladder cancer cells. TdTomato transinfected MB49 and J82 cells (left), FITC labelled SWCNT-AVs (middle), and composite (right). Scale = 3.2 μm. Murine and human bladder cancer lines as well as normal bladder progenitor cells were tested to determine tolerance to (b) SWCNT-AVs and (c) NIR energy. Data is presented as mean ± SE (n = 3). Statistical significance between groups is denoted by * (p < 0.01) with control, untreated samples compared against SWCNT-AV or NIR treated samples.
Download figure:
Standard image High-resolution imageTolerance of bladder cancer as well as normal bladder progenitor cells for SWCNT-AVs and NIR light was determined as seen in figures 3(b) and (c). SWCNT-AV concentrations above 40 mg l−1 were detrimental to normal bladder progenitor cells and to a lesser extent for MB49 and J82 cells. In order to ensure no damage to healthy bladder urothelium occurred due to SWCNT-AV toxicity, a conservative 20 mg l−1 concentration of nanotubes was used for all following work. Normal bladder progenitor cells had a higher tolerance to NIR energies with minimum cytotoxicity seen up to 350 J cm−2. Mouse (MB49) and human (J82) bladder cancer cell lines had noticeable decreases in viability at 150 J cm−2 and 100 J cm−2, respectively.
3.2. In vivo laser tolerance
Tolerance studies on mice without tumours were conducted to determine the optimal energy for NIR radiation which will cause the tumour bound SWCNT AVs to heat without affecting the remainder of the urothelium. Figure 4(a) depicts H&E results from mouse bladders treated with 50 and 100 J cm−2 NIR light energy over 30 s as compared to a control bladder. Treatment with 100 J cm−2 resulted in apparent change in the urotheliumand muscle layer; however 50 J cm−2 led to no change.
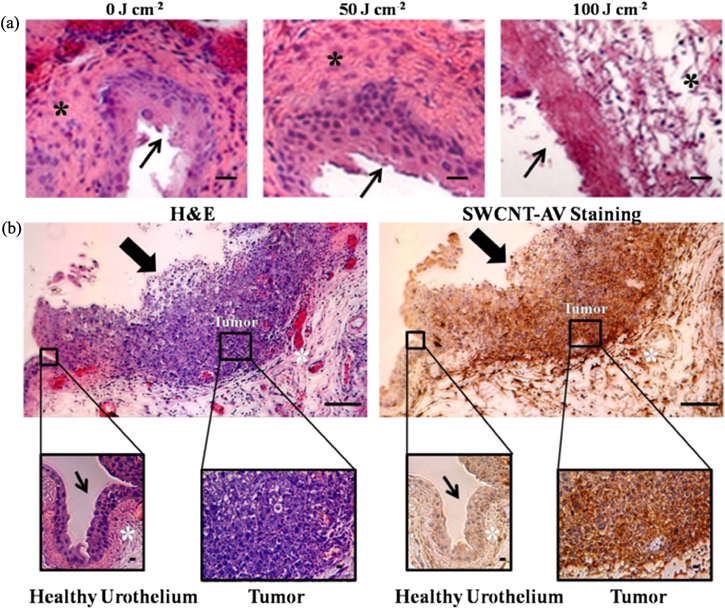
Figure 4. In vivo NIR tolerance and tumour specific SWCNT-AV binding. (a) Mice (n = 3) were treated with 0 J cm−2, 50 J cm−2, and 100 J cm−2 NIR light energy over 30 s. Representative histology images show change to the bladder wall and deeper muscle layer in mice treated with 100 J cm−2. Mice treated with 50 J cm−2 NIR energy had no visible changes 24 h after irradiation. (b) Mouse with orthotopic bladder tumour was instilled with 100 μl of 20 mg l−1 SWCNT-AVs for 90 min followed by bladder collection 24 h later when NIR laser would be applied. Within the same bladder, dark brown, positive anti-AV staining for SWCNT-AVs is seen on the tumour while minimum staining is seen on the remainder of the urothelium. Thin arrow indicates healthy bladder wall, thick arrow indicates bladder wall with tumour, and * indicates muscle layer. Scale = 10 μm.
Download figure:
Standard image High-resolution image3.3. In vivo SWCNT-AV specific tumour cell binding
In order to benefit from a targeted therapy, non-specific binding needs to be minimised to prevent heating of healthy urothelial tissue. Anti-AV IHC confirmed that SWCNT-AVs were specifically bound to the tumour, and no binding was seen along the healthy bladder wall within the same animal (figure 4(b)).
3.4. Biodistribution
NIR fluorescence spectroscopy was utilised to determine presence of 0.88 ± 0.13% of the injected dose (34.0 ±10.6% ID/g of bladder) of SWCNT-AVs in bladders of mice with tumours 24 h after SWCNT-AV instillation. A long-term biodistribution study was also conducted on the clearance organs, liver and kidney, as well as the bladder of treated mice 116 days after tumour implantation to determine clearance. No detectable nanotubes were observed in any of the clearance organs or bladder.
3.5. In vivo tumour ablation
Following confirmation of SWCNT-AVs binding to tumour and NIR tolerance, a short-term efficacy study was conducted for presence of tumours 24 h post treatment using an energy density of 50 J cm−2 and time of 30 s. As seen in figure 5(a), mice treated with SWCNT-AVs and NIR light combined had no detectable tumour fluorescence. A decrease in fluorescence is seen when the bladders are given SWCNT-AVs or NIR alone; however all mice still had detectable fluorescence, which can eventually lead to tumour re-growth. H&E staining (figure 5(b)) confirmed no visible damage to the bladder of control or treated mice 24 h post-treatment.
Figure 5. In vivo therapeutic efficacy study. (a) MB49 orthotopic tumours were grown to simulate NMIBC. SWCNT-AVs or PBS was delivered intravesically for 90 min. After 24 h to allow for non-specific nanotube clearance, a diffusing fibre was used to deliver 50 J cm−2 NIR light energy over 30 s. Bladders were harvested 24 h after therapy to assess tumour presence via tdTomato fluorescence (representative images above graph). Data is presented as mean ± SE (n = 6). Statistical significance between groups is denoted by * (p < 0.01). (b) H&E staining was conducted on bladders 24 h after therapy. No visible signs of damage to the bladder were detected. (c) Survival of treated and control mice (n = 6) was monitored for recurrence of bladder cancer. All the control mice died within 39 days of tumour inoculation while half the treated mice were still living at 116 days when the study was terminated. (d) Trichrome staining was conducted on the surviving mice to determine if any damage incurred which may cause scar tissue formation and thus disrupt the normal mechanical function of a bladder. No scar tissue was observed within the surviving mice. Arrow indicates bladder wall and * indicates muscle layer. Scale = 10 μm.
Download figure:
Standard image High-resolution imageRecurrence was evaluated by monitoring survival after one cycle of therapy compared to saline-treated mice as a control. The median survival was 34 days in the control group compared to 76 days in the treated group with 50% of the mice still alive at 116 days, when the study was ended (figure 5(c)). Trichrome staining (figure 5(d)) was conducted on the surviving treated mice to detect any collagen or scar tissue formation which would alter long-term normal bladder function. No scar tissue was observed.
4. Discussion
Bladder cancer has a high rate of recurrence due to incomplete resections as well as eventual chemotherapy resistance with no promising alternatives developed over the past 20 years [5]. A global heat based approach to recurrent, superficial bladder cancer has been shown here in mice to be a viable therapeutic option to specifically heat the tumour with no apparent damage to the healthy urothelium. SWCNTs are conjugated to the protein AV via a PEG linker, which provides a stealth coating to make them more biocompatible and minimise the risk of eliciting an immune response [27]. AV is a mammalian protein which binds specifically and with high affinity only to bladder cancer cells, as seen on murine and human bladder cancer cells in vitro as well as on orthotopic MB49 tumours in vivo. The strong binding ensures that the heat being generated by the nanotubes is contained within the tumour region, thus minimising non-specific damage to the healthy bladder urothelium.
Bladder progenitor cells at 70% confluence were less tolerant to the SWCNT-AV conjugate (figure 3(a)). In previous work, we found that non-confluent endothelial cells express PS on the cell surface, but do not when they are confluent [28]. Others have shown that AV is internalised on cells expressing PS on the surface by a pinocytic pathway [29]. In addition, we have shown that SWCNTs with the F3 peptide attached are internalised in non-confluent endothelial cells, which results in a loss of viability over time [30]. Therefore, it likely that the non-confluent progenitor bladder cells were damaged by internalisation of the SWCNT-AV conjugate after binding to PS on the cell surface at the concentration of the conjugate used.
In addition to successfully treating bladder tumours, this study has also shown that there was no detectable long-term nanotube presence or toxicity from the photothermal therapy at an energy density of 50 J cm−2 based on the following results: (1) the H&E analysis showing no visible damage to the bladder 24 h after treatment, and (2) the analysis of the mice surviving 116 days post-tumour instillation, which showed no nanotubes in the liver, kidney, or bladder as well as no scar tissue formation in the Trichrome stain. The energy density of 50 J cm−2 delivered over 30 s corresponds to a power density of 1.7 W cm−2, which is relatively low.
A recent study using a similar photothermal based approach for bladder cancer showed promising results with anti-EGFR coated gold nanoparticles; however delivery of the NIR light required surgery to expose the bladder, which is not as clinically translatable compared to the minimally-invasive intravesical NIR light delivery proven effective in this study [10]. A global NIR light also ensures that the entire bladder is treated at the same time, thus decreasing the possibility of incomplete treatment which is a major cause of recurrence currently. Our approach also requires significantly less power (1.7 W cm−2 versus 10 W cm−2 for the gold nanoparticles) which minimises healthy tissue damage for a non-surgical approach [10]. In contrast to the previous work, the proposed therapy also provides a unique adjuvant benefit of blocking PS, which is a global immunosuppressive signal in cancer [20]. A recent study tested the effects of blocking PS via AV and saw significant regression of tumour size as well as decreases in tumour angiogenesis factors that can be a synergistic addition to the novel therapy [22].
A preliminary 48 h study was conducted to evaluate the therapeutic efficacy of the proposed therapy along with a 24 h post-treatment period to observe any signs of therapy induced distress. No visible signs of discomfort or pain were observed. At the end of the study, bladders treated with only SWCNT-AVs had some decrease in tumour presence, possibly due to the effect of the SWCT-AVs accumulating in the tumour cells in conjunction with the possible adjuvant nature of AV blockade of immunosuppressive PS [20, 22]. There is also a decrease in fluorescence for mice treated with only the NIR laser, which might be due to the increased susceptibility of malignant cells to heat damage because of their reduced ability to efficiently dissipate heat as seen in the in vitro tolerance study [6]. Although there were some decreases in tumour fluorescence for only SWCNT-AV or NIR treated mice, all mice still had tumours which will lead to recurrence. On the other hand, all mice treated with SWCNT-AVs in combination with NIR had no detectable tumour presence 24 h post-therapy.
Since tumour re-growth is the major problem with the current standard of care treatment for NMIBC and results in 70% of patients having tumour relapse [1–5], a survival study was conducted comparing saline treated control mice and combination treated mice. The photothermal therapy resulted in a 50% cure rate in the survival study, which is higher than clinical treatments used today. This is a remarkable result, given that this treatment consists of one 30 s irradiation versus the usual combination of resection, chemotherapy, and immunotherapy for the current standard of care. The cure rate should be able to be increased by transitioning to a two- or three-cycle therapy to ensure all tumours are treated. No visible change was seen in the bladder treated with this therapy, making a multi-cycle therapy a possible alternative to further increase therapy efficacy.
5. Conclusions
This study has shown success in the treatment of bladder cancer in mice with a targeted photothermal therapy using a very low dose of SWCNTs (0.1 mg SWCNTs kg−1) delivered intravesically combined with a 30 s NIR light treatment at a relatively low power density (1.7 W cm−2). This treatment approach is much simpler and less invasive than for the current standard of care that normally consists of a combination of resection, chemotherapy, and immunotherapy, and in a 116 day survival study with only one cycle of therapy a higher cure rate was found compared to what patients now experience. It is also significant that no treatment toxicity was observed and no nanotubes were detected in the clearance organs or bladder at the end of the survival study. In future work, it would be desirable to evaluate the potential of a multi-cycle therapy since no signs of treatment toxicity were detected.
Acknowledgments
This study was supported by the University of Oklahoma Institute for Biomedical Engineering, Science, and Technology and by the Peggy and Charles Stephenson Cancer Centre. We thank the following for their contributions: Cancer Tissue Pathology Core at the Stephenson Cancer Centre at the University of Oklahoma for performing tissue processing and immunohistochemistry services; Chase Allan Brown for the figure schematic; Molecular Imaging Core at the Stephenson Cancer Centre for the use of the animal imaging equipment; Dr Rajagopal Ramesh's laboratory for transfecting the MB49-TdTomato-luciferase cells; Dr Michael Ihnat's laboratory for the use of their fluorescent dissection microscope; and Noble Microscopy Facility at the University of Oklahoma, Norman, OK for use of their compound microscope.