Abstract
Reactive oxygen species (ROS) play an important role in various physiological processes of living organisms. However, their increased concentration is usually considered as a threat for our health. Plants, invertebrates, and vertebrates including humans have various enzymatic and non-enzymatic defence systems against ROS. Unfortunately, both bad condition of surrounding environment and unhealthy lifestyle can interfere with an activity of enzymes responsible for a regulation of ROS levels. Therefore, it is important to look for alternative ROS scavengers, which could be administrated to chosen tissues to prevent pathological processes such as distortion of DNA or RNA structures and oxidation of proteins and lipids. One of the most recently proposed solutions is the application of nanozymes, which could mimic the activity of essential enzymes and prevent excessive activity of ROS. In this work, nanoparticles of Au, Pt, Pd, Ru and Rh were synthesized and studied in this regard. Peroxidase-, catalase (CAT)- and superoxide dismutase (SOD)-like activity of obtained nanoparticles were tested and compared using different methods. The influence of bovine and human albumins on CAT- and peroxidase-like activity was examined. Moreover, in the case of CAT-like activity, an influence of pH and temperature was examined and compared. Determination of SOD-like activity using the methods described for the examination of the activity of native enzyme was not fully successful. Moreover, cytotoxicity of chosen nanoparticles was studied on both regular and tumor cells.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
List of abbreviation
| 4-AAP | 4-aminoantipyrine, |
| BSA | bovine serum albumin |
| CAT | catalase |
| DMSO | dimethyl sulfoxide |
| EDTA | disodium ethylenediaminetetraacetate dihydrate |
| HBPG | hyperbranched polyglycidol |
| HSA | human serum albumin |
| MEME | minimum essential medium eagle |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NBT | nitro blue tetrazolium chloride |
| NPs | nanoparticles |
| OPD | o-phenyldiamine |
| PhOH | phenol |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| XOD | xanthine oxidase microbial |
1. Introduction
Biochemically active ROS take part in essential biological processes such as oxidative phosphorylation or post-translation protein modification. ROS play role of secondary messengers in signalling pathways, indirectly react with kinases and transcriptions factors, and take part in cell proliferation and differentiation [1]. In addition, ROS show antibacterial properties, therefore they are produced by cells as defence agents against invading microbes [2, 3]. In a case of situation when a concentration of ROS in living organisms is bigger than required for previously mentioned processes, an oxidative stress appears [4]. Then ROS become harmful for organisms as they can trigger DNA and RNA mutation, oxidize amino acids and cause abnormal folding and protein aggregation. In addition, ROS leads to necrotic or apoptotic cell death [5, 6].
Living organisms have two types of defence systems against the oxidative stress: enzyme-based and non-enzymatic one. The latter is based on a fast reaction of antioxidants, such as: ascorbic acid, α-tocopherol, β-carotene, uric acid, glutathione or other tripeptides with ROS [7, 8]. In a case of human organism these antioxidants are usually obtained from a diet or synthesized. Concurrently, the enzyme systems based on peroxidase, CAT and SOD can eliminate or diminish harmful processes in cells caused by ROS [9, 10]. CAT can be found, among others, in mitochondria, peroxisomes and matrix. Moreover, it can be located on the surface of tumour cells. This enzyme prevents accumulation of ROS in living organisms through a decomposition of H2O2 to oxygen and water. In addition, CAT oxidizes nitric oxide and decomposed peroxynitrite [11]. Peroxidase in presence of H2O2 can oxidize substrates and as a result hydrogen peroxide is transformed into water [12]. SOD takes part in a transformation of superoxide to water and hydrogen peroxide. Generated H2O2, can be then involved in a reaction catalysed by peroxidase or decomposed by CAT [13]. It must be pointed out, that not only natural enzymes can be used to decompose ROS, as chosen nanoparticles (NPs) can mimic functions of natural enzymes. Nanoparticles, which exhibit such properties were named nanozymes by Scrimin, Pasquato and their co-workers [14]. Nanozymes are more stable thermally and keep their activity in a wide range of pH in comparison to natural enzymes. In addition, their acquisition is easier, cheaper and does not require usage of living organisms, therefore the interest of their application has been growing constantly.
NPs of peroxidase-like activity e.g. AuNPs can be used as labels in various analytical systems e.g. for detection of viruses [15]. Colorimetric and biochemical assays such as ELISA, based on enzyme-like activity of nanoparticles, were described for kanamycin detection in food samples [16]. Moreover, RuNPs-based biosensor was used for detection of bilirubin [17]. Ultra-low levels of mercury were determined using AuNPs- and later PtNPs-based sensors, which exhibited even better stability and sensitivity [18, 19]. PtNPs were also described as CAT, SOD, polyphenol oxidase, ferroxidase, and ascorbate oxidase mimics [20]. PdNPs were used for medical diagnostics, due to their enzyme-like activity, for example replacing HRP in a construction of various biosensors and biotests. One of examples of assays with Pd-based nanozymes is a system for glucose detection [21]. RuNPs have been less studied then NPs of Au, Ag and Pt, but their interesting enzyme-like activity was also indicated [22]. RhNPs commonly play role of catalysts in a chemical synthesis. They are used in reaction of hydrogenation and hydroformylation [23]. However, RhNPs-based colorimetric analytical systems were proposed for a determination of glucose in blood, other bioliquids and drinks or hydrogen peroxide in hygiene products [24]. SOD-like activity was not widely studied for NPs, but some works indicated, that Pt, SiO2 and MnO-based NPs are able to mimic SOD activity [25–28]. These facts give a wide space for studying ROS scavenging function of noble metal-based nanozymes and their use as active agents against oxidative stress. If such application is to be considered, more attention should be paid to their biocompatibility. A cytotoxicity is dependent on shape, size, stability, surface modifiers and concentration of NPs. Moreover, cell endocytosis and exocytosis depend on surface charge and stabilizing agent of NPs. Therefore, it is very important to provide in vitro experiments before starting to use them in vivo [29].
In this work nanoparticles of Au, Pt, Pd, Ru and Rh were synthesized and tested for their ROS scavenging activity. HBPG was used a stabilizing agent as our previous work indicated its usefulness (for AuNPs) in a wide range of pH [30]. Moreover, as analogue of poly(ethylene glycol) it can be treated as biocompatible. As NPs after administration to human organism would be in contact with solutions of complex matrix as blood or interstitial fluid, the influence of proteins capable of forming protein corona on activity of synthesized NPs was also examined. One must remember, that nanoparticles can interact with serum albumin, fibrinogen, apolipoprotein and immunoglobulins. It can limit the accessibility of substrates to surface of NPs, which is mainly responsible for their catalytic activity [31]. It should be underlined, the vast majority of methods used for the evaluation of catalytic activity of nanozymes is directly adapted from works describing the native enzymes, which leads to many problems. It was somehow signalized in our previous articles, but it will also find a reflection in this work.
2. Experimental
2.1. Materials
NBT, xanthine and ruthenium(III) chloride hydrate were purchased from Alfa Aesar. Hydrogen peroxide, acetic acid, phosphoric acid, dimethylsulfoxide and sodium hydroxide were purchased from POCH, Avantor. Gold(III) chloride trihydrate, chloroplatinic acid solution (8 wt% in H2O), sodium tetrachloropalladium(II), rhodium(III) chloride, methionine, 4-AAP, phenol (PhOH), OPD, sodium borohydride, xanthine oxidase microbial (XOD, EC 1.17.3.2), HSA, BSA, riboflavin, boric acid, DMSO, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), MEME, MEM non-essential amino acid solution, RPMI-1640, L-glutamine, penicillin–streptomycin (10 000 units penicillin, 10 mg streptomycin/ml) and EDTA were obtained from Sigma-Aldrich. Fetal bovine serum was obtained from Life Technologies. HBPG (MW ∼ 3.2 kDa) was synthesised by ring opening polymerization [32]. Universal buffer was prepared using acetic, boric, and phosphoric acids and deionized water. Concentration of each acid amounted to 0.04 M. 0.1 M phosphate buffer was prepared in the same way. pH adjusting was done using sodium hydroxide. Cell lines: A549 (human lung epithelial carcinoma), MRC-5 (normal human lung fibroblasts) were obtained from the European Collection of Cell Cultures.
2.2. Synthesis of NPs
In scope of this research, Au, Pt, Pd, Ru, and Rh nanoparticles were synthesized. For preparation of noble metal nanoparticles, solutions of precursors (HAuCl4, H2PtCl6, Na2PdCl4 and RuCl3, 10 mM) prepared in deionized water were used. In a case of RhNPs, 125 μl of 0.2 M NaOH per 1 mg of precursor (RhCl3) was additionally added (concentration of Rh in this solution amounted also to 10 mM). For preparation of NPs, 625 μl of precursors solutions were mixed with 5 ml of HBPG solution prepared in deionized water (1 mg ml−1). After the incubation (10 min) of prepared solution, 625 μl of NaBH4 solution (50 mM) was added and then NPs solutions were left for 7 d for incubation in dark under mixing conditions. Preparation of the second type of nanoparticles (marked as M*NPs) was executed in the same way except the fact, that the hyperbranched HBPG concentration amounted to 5 mg ml−1. Nanoparticles were analysed using UV–vis spectrophotometry (Lambda 25, Perkin Elmer) and dynamic light scattering (DLS) (Zetasizer Nano, Malvern).
2.3. Studies on peroxidase-like activity
Determination of peroxidase-like activity was done using spectrophotometric methods based on the use of two different substrates. In a case of o-phenylenediamine-based method a substrate mixture contained 5.8 ml of 0.1 M universal buffer, pH 4.5, 1.8 ml of H2O2 (5.00 M) stock solution and 400 μl of OPD solution (20 mM) prepared in deionized water. OPD stock solution was prepared in deionized water purged with nitrogen and then stored in a dark place. Gold nanoparticles solutions were diluted 10 times with deionized water. Pt, Pd, Ru and Rh nanoparticles were diluted 1000 times. 200 μl of a substrate mixture was distributed into wells of a polystyrene 96-well plate. Reaction was started by the addition of 50 μl of previously diluted nanoparticles or 50 μl of deionized water (blank). The evolution of absorbance was measured at 450 nm for 15 min with a step of 10 s. In a case of phenol/4-AAP-based method, to obtain substrate mixture, 6.6 ml of universal buffer, pH 7.5, was mixed with 200 μl of hydrogen peroxide stock solution (5.00 M), 400 μl of 4-AAP (50 mM), and 800 μl of phenol solution (500 mM). Prepared mixture was distributed to wells of 96-well plate (200 μl), and then reaction was started adding 50 μl of diluted nanoparticles solution. Absorbance was measured at 400 nm.
2.4. Influence of serum albumin on peroxidase-like activity of nanoparticles
Influence of bovine and HSAs on peroxidase-like activity of nanoparticles was examined using both methods. Albumins were dissolved in 5.0 ml of universal buffer (pH 4.5 or 7.5) to obtain protein stock solutions, in which concentration of albumin amounted to 100 mg ml−1. Original nanoparticles solutions, except these containing gold NPs, were diluted with deionized water 100 times. Gold nanoparticles were used directly without any dilution. Substrates mixtures were prepared in the same way as for above discussed methods. In eppendorf tubes 800 μl of deionized water, 100 μl of protein stock solution and 100 μl of NPs solutions were mixed. In a case of blank sample, additional 100 μl of deionized water was added instead of NPs. Substrate mixtures were distributed in wells of 96-well plate and the reaction was started by the addition of mixture of nanoparticles with HSA or BSA after 30 min of incubation.
2.5. CAT-like activity of NPs
2.5.1. Phenol/4-AAP-based method
Mixture X was prepared by mixing 3.5 ml of universal buffer adjusted to pH 4.5, 6.0, 7.5, 8.5, 9.5 or 10.0 with a stock solution of H2O2 (5.00 M, 500 μl). Original NPs solutions were diluted with deionized water 10 times in the case of gold and 100 times in the case of other noble metals NPs. Then 200 μl of mixture X was transferred to eppendorf tube and mixed with 50 μl of diluted solutions of NPs. Eppendorfs with opened caps, that contained solutions were mixed on a shaker for 30 min (shaking speed 400 rpm). After incubation, samples were centrifuged for another 30 min (24 088 × g). In a meantime, substrate mixture was prepared and distributed to polystyrene 96-well plate. 3.3 ml of universal buffer (pH 7.5) was mixed with 400 μl phenol (500 mM) and 200 μl of 4-AAP (50 mM). After that, three type of mixtures were prepared directly in the multi-well plate. First type contained 195 μl of substrate mixture and 50 μl of PtNPs solution, which was diluted 100 times with deionized water. For the second row, the same amount of substrate mixture was added mixed with 50 μl of deionized water, which was labeled as blank of a first type. In a separated well, 195 μl of substrate mixture was mixed with 50 μl of deionised water and 5 μl of H2O2 (5.00 M), which was labeled as a blank of a second type. Reaction was started by addition of 5 μl of previously incubated solutions after their centrifugation (NPs were separated, and supernatant taken). Absorbance was measured at 400 nm using Tecan Sunrise microplate reader. In order to calculate a degree of hydrogen peroxide decomposition a calibration curve was prepared.
2.5.2. Direct method
A series of solutions in eppendorf tubes were prepared: 1.0 ml of universal buffer adjusted to pH 7.5 or 9.5 was mixed with 25 μl of EDTA solution (7 mM), 75 μl of H2O2 solution (0.5 M), and 100 μl of each type of nanoparticles solutions diluted 10 times in deionized water or solutions of nanoparticles diluted 10 times in solutions containing albumins (HSA or BSA, their concentration after mixing with NPs solutions amounted to 10 mg ml−1). Prepared series of eppendorf tubes (caps left open) was continuously mixed for 1 h (shaking speed set to 400 rpm) at 37 °C. After incubation, 400 μl of solution from eppendorf tubes, that contained nanoparticles was mixed with 1.6 ml of universal buffer. Absorbance of prepared mixture was measured directly after the mixing at 250 nm using spectrophotometer (Lambda 25, Perkin Elmer).
2.6. Influence of temperature on nanoparticles CAT-like activity
In this experiment we used a modified phenol/4-AAP method which is described above. The difference was, that we applied an increased temperature, while diluted NPs combined with a mixture X were incubated under shaking condition. Samples were incubated at 37.0 °C. The remaining part of experiment was done exactly in the same way as described above.
2.7. Influence of serum albumins on CAT-like activity of nanoparticles
Influence of serum albumins on CAT-like activity of nanoparticles was examined with modified phenol/4-AAP method described above. Modification was made during the step of preparation of NPs solutions. Before incubation with mixture X, NPs solutions were mixed with albumins solutions. In the very beginning albumins were dissolved in the universal buffer to obtain a protein stock solution (100 mg ml−1). Solutions of synthesized NPs except gold were diluted 10 times with deionized water. Subsequently in the eppendorf tube 800 μl of deionized water was mixed with 100 μl of protein stock solution and 100 μl of prepared solutions of NPs. Next, such solutions were mixed with a mixture X (pH 7.5). All subsequent steps were executed in the same way as described above.
2.8. SOD-like activity of NPs
2.8.1. Riboflavin-based method
SOD-like activity of nanoparticles was examined first with riboflavin-based method. Substrate mixture was prepared from two separate parts. First part contained 200 μl of EDTA (7.0 mM), 19.2 mg of NTB, 170 μl of riboflavin (1.0 mM) and 9.63 ml of universal buffer adjusted to pH 7.5. Second part contained 149 mg of methionine diluted in 10 ml of phosphate buffer. In a meantime, original solutions of gold NPs were diluted 10 times with deionized water. Platinum, palladium, rhodium and ruthenium NPs were diluted 100 times. After that, 50 μl of first solution was mixed with 50 μl of second solution and 70 μl of solutions of diluted nanoparticles. For further studies nanoparticles were diluted another 10 times and used in the same proportions for measurements. For the blank, 70 μl of buffer was used instead of NPs. Prepared mixture was purged with oxygen for 5 min in a dark place. Then mixture was incubated for another 10 min under the light. Absorbance was measured twice, after incubation in absence of light and after irradiation at 450 nm using spectrophotometer (Lambda 25, Perkin Elmer).
2.8.2. Xanthine-based method
To prepare a substrate mixture, 2.5 ml of phosphate buffer adjusted to pH 7.4 was mixed with 2.3 ml of deionized water, 100 μl of EDTA (7.0 mM), 1 ml of NBT (0.3 mM) and 5 ml of xanthine (0.1 mM). Gold NPs solutions were diluted 10 times with deionized water while other nanoparticles were diluted 100 times. XOD was dissolved in cold deionized water to obtain a concentration of 45 μg ml−1, directly before measurement. Then two types of solutions were prepared. First one contained 280 μl of substrate mixture and 10 μl of NPs, whereas the second type contained 280 μl of substrate mixture, 10 μl of NPs and 40 μl of XOD solution. For a blank, 10 μl of deionized water was used. Absorbance was measured at 550 nm using spectrophotometer (Lambda 25, Perkin Elmer).
2.9. NPs cytotoxicity evaluation using MTT test
MTT is a test used for in vitro evaluation of the viability of cells. MTT is based on a reduction of tetrazolium salt to colored formazan by dehydrogenases, which are active in the mitochondria of living cells. The amount of the formed formazan is proportional to the number of living cells. The general methodology used was similar to the one described in the previous works [33]. At the beginning, cell suspensions of appropriate density were prepared and 100 μl of a suspension was distributed to each well of 96-well plate. After 24 h (cell attachment to surface of the well plate was observed) medium was removed and 100 μl of NPs solutions of various concentrations (prepared in MEME culture medium without phenol red) were placed to each well. The control sample for both cell lines containing medium without NPs was also prepared. Incubation at 37 °C, in a humidified atmosphere (5% CO2) was carried out for 24 h. After the incubation, solutions were removed andsubsequently 100 μl of MTT solution in PBS (0.5 mg ml−1) was added into wells. Cells were incubated for 4 h in the incubator (protected from light). After incubation, MTT solution was removed and 100 μl of DMSO was added to each well to assure dissolving formed formazan crystals. The final step was to measure the absorbance at 570 nm using multi-mode reader Cytation 3 (BioTek). The results are presented as the percentage of cells viability compared to the control sample (cell culture incubated with medium only). Six replicates of each concentration of NPs were tested.
3. Results and discussion
All synthesized nanoparticles were examined using UV–vis spectroscopy and DLS. UV–vis analyses shown no signs of unreacted precursors, while DLS measurements allowed for estimation of hydrodynamic diameters of tested NPs (five replicates of each type of NPs). The hydrodynamic diameters were as follows: PtNPs—8.6 ± 1.4, Pt*NPs—5.4 ± 0.8, AuNPs—6.6 ± 0.9, Au*NPs—4.1 ± 0.6, PdNPs—36.8 ± 4.8, RuNPs—6.6 ± 0.9 and RhNPs—7.8 ± 1.2 nm.
3.1. Peroxidase-like activity of tested nanoparticles
A peroxidase-like activity of various nanoparticles is usually utilized for a construction of tests and sensors, in case of which, nanoparticles are responsible for a generation of colored products in a reaction of hydrogen peroxide with various chromogenic substrates such as ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)), TMB (tetramethylbenzidine), OPD etc. Such reaction leads also to a transformation of hydrogen peroxide to water. Together with CATs, peroxidases are responsible for a decomposition of H2O2 in vivo. The list of potential substrates for peroxidase in-vivo is wide, however among them cytochrome C and ascorbic acid are described as the most typical in the case of human organism. Besides the typical examination of catalytic activity of nanozymes, conducted studies were extended by adding proteins i.e. BSA and HSA to the samples. Such a procedure at least to minimal extent mimicked the environment of human organism. Moreover, studies were performed at two different pH using appropriately chosen substrates for peroxidase (PhOH/4-AAP for pH 7.5 and OPD for pH 4.5, whereas 7.5 is close to the typical pH of human blood and 4.5 can represent extreme endosomal conditions [34]). Besides the direct effect of pH on the activity of nanozymes, it also influences their interactions with proteins, because it affects their charge (pI for both amounts to ∼4.8) and thus structure. The results of conducted studies are depicted in figure 1.
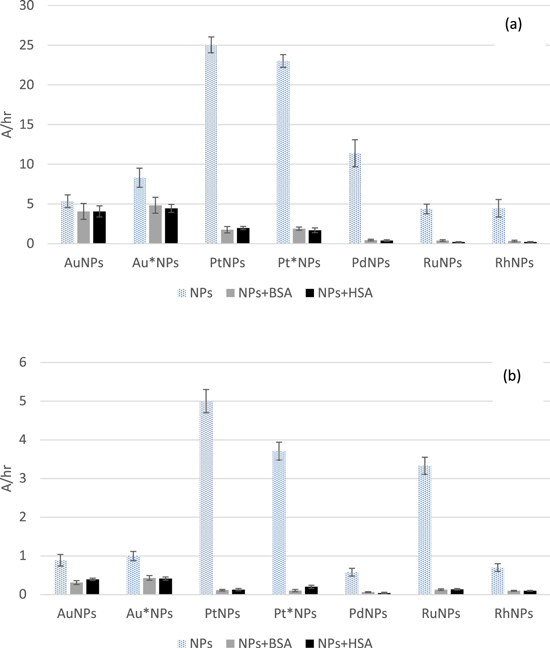
Figure 1. Peroxidase-like activity of nanoparticles in presence of bovine serum albumin (BSA) and human serum albumin (HSA) against o-phenyldiamine (a) and phenol/4-aminoantipyrine (b) expressed as an increase of absorbance (450, 500 nm, respectively) in time. Four samples of each type of NPs were tested.
Download figure:
Standard image High-resolution imageIt must be underlined, that the concentration of gold in tested samples was 100× times higher than of other metals. In the case of both substrates, the highest activity was observed for platinum nanoparticles, and among them the bigger NPs (stabilized with HBPG in the concentration of 1 mg ml−1) exhibited better behavior. Depending on the applied substrate (and resulting/required pH) the second best were PdNPs (at 4.5), which exhibited about a half of activity of platinum NPs, or RuNPs (at 7.5), whose activity was only slightly worse than Pt*NPs. The activity of nanoparticles was expressed as an increase of absorbance of the sample (caused by a product of the reaction) in time. Due to the fact, that the enzyme-like activity of nanoparticles originates from high surface available for substrates (high-energy atoms), one could expect, that the smaller are the nanoparticles, the higher should be their activity. However, in many cases ultrasmall nanoparticles exhibit worse activity, than the bigger ones. It can be due to a high curvature and inability of flat molecules of substrates to adsorb on curved surface. Obviously, it depends also on the composition of nanoparticles and as it can be seen in a figure 1 smaller Au*NPs (4.1 nm) are more active then bigger AuNPs (6.6 nm), while for platinum smaller Pt*NPs (5.4 nm) are worse than bigger PtNPs (8.6 nm).
One must notice a distinct deterioration of measured activity after introduction of proteins into the samples. The relatively smallest change was observed for gold NPs, but it has to be pointed out, that the ratio of NPs to proteins was higher in their case (100 times than for other NPs). For the other nanoparticles, the loss of activity amounted to more than 90%. It is likely related to a formation of protein crown, which blocks the accessibility of substrates to the active surface of metal nanoparticles. Moreover, proteins can undergo side reactions with hydrogen peroxide and ·OH generated on the surface of nanoparticles (other radicals can be also formed), and in this way reduce the amount of expected colored products of catalytic reactions. The examined proteins were used in a smaller concentration (10 mg ml−1), than typically observed e.g. for human blood, therefore after introducing such nanoparticles to human organism even stronger inhibition of their activity could be potentially observed. The drop of activity was slightly smaller for NPs tested at pH 4.5, however the observed effect was more than pronounced.
3.2. CAT-like activity of tested nanoparticles
3.2.1. Influence of pH on nanoparticles CAT-like activity of nanoparticles
The influence of pH on CAT-like activity of NPs using PhOH/4-AAP-based method was tested at room and elevated temperature. The optimal level of pH for native CAT lies between 7 and 11, however in a framework of presented studies nanozymes were tested in a wider range (pH 4.5–10.0 in the universal buffer). One has to remember, that noble metal-based NPs, can switch their activity in dependence on both pH and presence of potential substrates and the same NPs can exhibit CAT-like and peroxidase-like activity (which is also shown throughout this work). When analyzing the results, it is important to take into account different dilutions of NPs when comparing their activity.
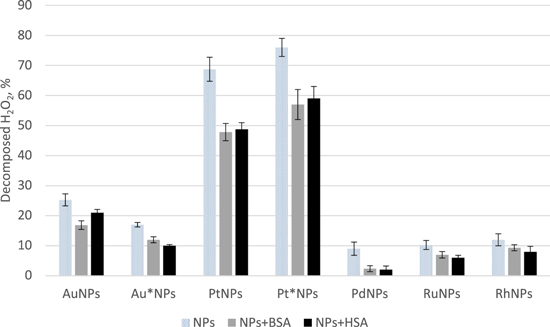
Gold NPs were diluted 10 times, while other examined solutions of NPs were diluted 100 times (with deionized water). The resulting ratio of H2O2 molecules to gold atoms contained in nanoparticles amounted to 1562, whereas for other metals it was 10 times higher. As it can be seen in figure 2 both types of gold NPs were the most active at pH 10.0. For AuNPs more than 95% of H2O2 decomposed under such conditions, while in the case of Au*NPs about 50% was decomposed after 30 min. Platinum NPs were the most active at pH 9.5 and decomposed about 98% of H2O2. Similar activity was observed at pH 10.0 in the case of both types of platinum NPs. At pH 7.5 PtNPs and Pt*NPs decomposed about 70% of H2O2, and when tested at 8.5 about 80% of H2O2 was decomposed. PdNPs increased their activity with an increase of pH and decomposed the biggest amount of hydrogen peroxide at pH 10.0 (79% of H2O2). The decomposition of hydrogen peroxide to the biggest extent by RuNPs was observed at pH 10.0 (about 45%). The activity of RhNPs was not satisfactory and the decomposition rate amounted to about 10% within a pH range from 7.5 to 10.0. It should be mentioned, that tested NPs exhibit CAT-like activity beyond pH range typical for a native CAT, however in almost all cases their activity under such conditions was lower than in the samples of a basic pH. For RhNPs the optimal pH for a decomposition of H2O2 was 6.0, but the decomposition rate amounted then to about 20% only. It suggests that the interaction between the surface of RhNPs and H2O2 is favored under such conditions, as a result of protonation/deprotonation or adsorption/desorption of OH−, which occur at the surface, and/or the decomposition reaction rate is the highest then. As the decomposition of hydrogen peroxide requires the adsorption of the molecule on the surface of a nanocatalyst, different pH-based patterns of CAT-like activity is dependent on a surface chemistry of tested metals.
Figure 2. Influence of pH on catalase-like activity of nanoparticles examined with phenol/4-aminoantipyrine-based method, expressed as the degree of decomposed hydrogen peroxide. Test carried out after incubation (30 min at room temperature) and centrifugation. Four samples of each type of NPs were tested.
Download figure:
Standard image High-resolution imageWhen considering the use of nanoparticles as ROS scavengers e.g. in form of injections, the results obtained for pH close to a physiological one should be commented more precisely. Under such conditions Pt*NPs showed the highest activity in comparison to other nanoparticles. PtNPs exhibited slightly worse activity while Pd, Ru and RhNPs demonstrated similar performance at pH 7.5. Gold NPs in higher concentration than the rest of metals caused the decomposition of 26% and 17% of H2O2 for AuNPs and Au*NPs, respectively. Moreover, the studies at pH 7.5 were performed for samples heated up to 37 °C, however only small changes in the decomposition rates were observed and they amounted to no more than 10% (usually about 5%) in favor of the higher temperature (data not shown).
Similarly to the studies on peroxidase-like activity, in the case of research on CAT-like activity based on PhOH/4-AAP method, the effect of proteins was also examined. BSA or HSA were added to samples of NPs prior to their mixing with hydrogen peroxide solution. The results of studies conducted at pH 7.5 are presented in figure 3.
Figure 3. Influence of albumins on catalase-like activity of nanoparticles examined with phenol/4-aminoantipyrine-based method, expressed as the degree of decomposed hydrogen peroxide. Test carried out after incubation (30 min at room temperature) and centrifugation. Four samples of each type of NPs were tested.
Download figure:
Standard image High-resolution imageIn the case of all tested NPs the addition of albumins caused a noticeable decrease of CAT-like activity. However, for most of the metals the effect was not so pronounced. The catalytic activity of PdNPs decreased more than 70%, but for the rest of NPs the changes were smaller and amounted to about 25%. No big differences between BSA and HSA were observed. It should be pointed out, that the ratio of proteins to metal atoms (present in nanoparticles) was 10 times smaller for gold than other metals. In contrast to decomposition of H2O2 according to peroxidase-like activity, in the case of CAT-like activity the presence of albumins does not pose such inhibiting effect, possibly due to several reasons. The mechanism of hydrogen peroxide evolution is different, and the method of CAT-like activity evaluation is not directly dependent on the second substrate (bigger than hydrogen peroxide), whose accessibility to the close proximity of nanoparticles can be limited by proteins.
To better evaluate the CAT-like activity of noble metal-based NPs the second method was also utilized. However, according to the described methodology the proportion of hydrogen peroxide to metal atoms contained in nanoparticles was totally different than in above described case and amounted to 116 in cases of all tested metals. Under applied conditions of 37 °C (no room temperature studies were conducted this time), at pH 7.5, after 60 min (in the case of previous method the total time of contact of NPs with H2O2 was 30 min, however subsequently the step of centrifugation, which lasted 30 min took place) more than 85% of hydrogen peroxide decomposed in almost all cases, except the samples containing nanoparticles stabilized with higher amount of HBPG as it can be seen in figure 4. The smaller Pt*NPs and Au*NPs caused the decomposition of about 50% of H2O2 at pH 7.5, but when tested at 9.5 the decomposition degree amounted to above 80%. The addition of BSA or HSA to the samples of pH 7.5 did not influence the degree of decomposed hydrogen peroxide to a high extent.
Figure 4. Influence of pH, bovine (BSA) and human serum albumin (HSA) on catalase-like activity of nanoparticles. Tested after incubation (60 min at 37 °C) and expressed as the degree of decomposed hydrogen peroxide. Four samples of each type of NPs were tested.
Download figure:
Standard image High-resolution image3.3. SOD-like activity of tested nanoparticles
SOD eliminates superoxide anion radical and as a result hydrogen peroxide and oxygen are formed. Methods to investigate SOD activity are usually based on an inhibition of a reaction of a chosen substrate (e.g. nitrotetrazolium blue chloride) with superoxide anion radical. Herein superoxide was generated from oxygen and riboflavin in the presence of methionine under light irradiation. The generated superoxide was supposed to react with nitrotetrazolium blue chloride and produce a formazan dye [25]. Nanoparticles can inhibit generation of formazan dye by scavenging superoxide. According to described procedures samples containing nanoparticles were incubated for 5 min in a dark place and then irradiated for 10 min with light in a presence of air. In the case of nanoparticles exhibiting ROS scavenging properties, less amount of blue colored product should be generated in comparison to the sample which contained no nanoparticles (blank). Surprisingly, in few cases (in presence of Pt, Pt*, Pd and RuNPs) a formation of higher amount of formazan in comparison to blank was observed. It means, that these nanoparticles could catalyze undesired side reaction leading to the formation of nitrotetrazolium blue chloride or be responsible for the generation of ROS under applied experimental conditions. To solve this issue, we decided to test the samples containing lower amount of NPs, but the undesired effect was still observed. Therefore, another method was proposed to asses SOD-like activity of tested NPs. It is based on oxidation of xanthine by xanthine oxidase, which leads to a generation of superoxide. The further mechanism is the same as in the previously described method and NPs should inhibit formation of formazan from nitrotetrazolium blue chloride. Unfortunately, the observations were the same as for methionine-based reaction (in most cases the absorbance was higher for samples containing NPs), which again could point to the direct activity of NPs towards reduction nitrotetrazolium blue chloride. Moreover, as noble-metal NPs exhibit the activity of various oxidoreductases, they can be also responsible for a formation of superoxide from xanthine according to xanthine oxidase-like reaction. SOD-like activity was also investigated with cytochrome C-based method but under applied conditions the colored product was unstable and rapidly decomposed after the formation.
To conclude, all three tested methods (typically used for a native enzyme) cannot be used for assessment of SOD-like activity of noble-metal nanoparticles due to a presence of side reactions. Nanoparticles of noble metals are quite versatile catalysts and therefore the more substances are present in the samples, the more reactions can take place, which causes the following of the desired process impossible using such methods.
3.4. MTT test
Cytotoxicity of NPs on human normal foetal lung cells (MRC5) and adenocarcinomic alveolar basal epithelial cells (A549) was tested. NPs in different concentrations were added to the cell cultures to investigate their influence on viability of cells. Cytotoxicity was tested with MTT assay after 24 h of incubation (as the cell population doubling time is 22 and 23 h for A549 and MRC-5, respectively).
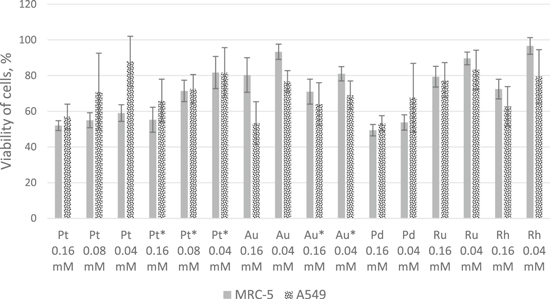
For all metals and both cell lines, an increase in cells viability correlated with decrease in NPs concentration could be observed. A material can be treated as non-cytotoxic in the case when in its presence cells keep at least 70% of the viability according to ISO 10993-1:2018 norm [35]. As it is shown on the figure 5, the most cytotoxic for both tested cell lines were PdNPs (the largest among all tested NPs), while PtNPs in all tested concentrations were cytotoxic for MRC-5 cells and only in the highest concentration for A549 cells. Smaller Pt*NPs were less cytotoxic for MRC-5 cells than bigger PtNPs. Such behavior was not observed for gold NPs. It is interesting, that all platinum NPs were more cytotoxic to MRC-5 cells, while in the case of all gold NPs the higher cytotoxicity was noted to A549 cells. RuNPs exhibited no cytotoxicity for both cell lines in both tested concentrations, while RhNPs were only cytotoxic to A549 in the higher concentration. Considering only the normal cells almost all tested NPs (except PdNPs and PtNPs) in a concentration of 0.04 mM could be used potentially safely. The cytotoxicity of tested NPs towards cancer lung cells could be beneficial, however so simple 2D model for sure do not reflect the real influence of nanoparticles on a human body. The plenitude of chemical compounds in the human organism cannot be easily mimicked and many of these substances can undergo reactions on the surface of nanoparticles forming products of unknown properties. However, the conducted studies can be used to preliminarily select the safer NPs to further and more complex research.
Figure 5. Comparison of nanoparticles' cytotoxicity on MRC-5 and A549 cells after 24 h of incubation. The number of metabolically active cells is presented as the percent referred to the control. Six replicates of each concentration of NPs were tested.
Download figure:
Standard image High-resolution imageAccording to the recent research on a localization of spherical Au nanoparticles of a diameter between 5 and 50 nm after their contact with various cells the vast majority of small (5 nm) NPs stays bound to the cell membrane (more than 95%), while the bigger ones (10–50 nm) tend to be internalized to a bigger extent (up to about 50%) [36, 37]. It may lead to different ways of the cytotoxicity as the membrane-bound NPs block the collection of nutrients and excretion of metabolic products and internalized NPs can influence the metabolic routes within the cells (assuming, that noble metal-based NPs do not undergo decomposition). In view of these information and results of conducted studies the internalization is more toxic to cells than the interaction with the cell membrane as only the biggest of synthesized NPs exhibited the some cytotoxicity (PdNPs and PtNPs).
4. Conclusions
The use of NPs of various metals becomes more and more popular and more formulations and products contain them. On the one hand their presence can be beneficial, on the other they can pose a danger as the mechanisms of their action are still barely known. The enzyme-like activity of noble metal NPs can be safely utilized for modern bioanalysis, however their application for various therapies, including ROS scavenging, should be thoroughly considered. Undoubtedly, noble metal NPs exhibit useful peroxidase- and CAT-like activity and considering the latter, it is to relatively small extent influenced by albumins, which is caused by the relative simplicity of the decomposition of hydrogen peroxide according to this mechanism. Considering the pH of various tissues of human organism including blood and the abundance of proteins, most of the tested NPs would decompose hydrogen peroxide mostly according to CAT-like activity. At the same time, as shown for SOD-like activity studies, it is hard to control the reactions, which are catalyzed by such NPs. Their limited selectivity should be treated as an advantage when applying them as labels in biotests or biosensors, but when it comes to the administration to human it should be considered as a big disadvantage. The conducted studies of NPs cytotoxicity using MTT test did not show their significant influence on both tested cell lines, but one must remember, that such models are quite simplified in comparison to the human body.
Funding
This work was financially supported by the Polish National Science Centre—(NCN) grant 2017/01/X/ST5/00034.
Conflicts of interest
The authors declare, that there is no conflict of interest.