Abstract
AlGaN/GaN high electron mobility transistors (HEMTs) have demonstrated their extraordinary potential in developing solid-state microsensors for detecting gases, metal ions, anions, biomolecules, and other substances due to their excellent chemical stability, high surface charge sensitivity, high temperature-tolerance performance, and low power consumption characteristics. In this paper, only three types of AlGaN/GaN HEMT-based sensors used for detecting the pH value, heavy metal ions, and harmful anions, which are suitable for water quality monitoring, will be discussed. First, we introduce the structural design, detection principle, and fabrication processes of AlGaN/GaN HEMT-based sensors. Then, surface functionalization methods for the gate region, sensing mechanisms, and the sensitivity and selectivity performances based on different gate region treatments are reviewed and analyzed. Finally, some challenging problems that hinder the practical application of the sensors are proposed.
Export citation and abstract BibTeX RIS
1. Introduction
As the source of life, water is indispensable to all animals and plants. Human beings could not survive without water. However, with population growth, water resources are becoming increasingly scarce, and water pollution is becoming more serious. Many types of toxicants and hazardous substances are running into the ground, rivers, lakes and reservoirs that seriously pollute ground water and surface water as a result of the rapid development of industry and agriculture technology. Thus, it has become increasingly important to monitor the harmful pollutants in water. There are many traditional methods, including ion chromatography, gas chromatography, liquid chromatography, fluorescence, capillary electrophoresis and electrochemical methods, that have been developed [1–6]. Although they have high detection sensitivity, most of these methods require expensive instruments, well-trained operators and long testing times, and furthermore, it is difficult to achieve real-time and fast detection. Therefore, portable, low-cost and real-time sensors with excellent sensitivity and selectivity are highly desirable.
Semiconductor-based sensors have been intensively studied owing to rapid developments in semiconductor manufacturing and wireless network technology as well as strategies for surface functionalization [7–10]. Since the first ion-sensitive sensor based on a Si field-effect transistor (FET) was reported [11], FET-based sensors have been widely used in the detection of toxic gases, heavy metal ions, harmful anions, organic compounds, viruses, bacteria, cells, etc [12–20]. Silicon-based sensors are still the predominant commercialized semiconductor-based sensors that are used due to the maturity of Si chip manufacturing technology. However, silicon-based sensors are not suited for operation in harsh environments, such as high temperature, high pressure or corrosive ambient conditions, because of their narrow band gap and high chemical activity [21, 22]. Recently, wide band-gap group-III nitride compound semiconductors that feature high temperature/high power capability, excellent chemical stability, and high surface charge sensitivity have been developed as favorable sensors for detecting gases such as hydrogen, carbon monoxide, and oxygen [23–25], organic and inorganic ions [26, 27], and biomolecules, including glucose, prostate, saliva, and DNA [28–32].
This review discusses the recent development of AlGaN/GaN high electron mobility transistor (HEMT)-based sensors. We focus on structural design, surface functionalization methods, and sensing mechanisms of sensors for applications in detecting pH values, heavy metal ions, and harmful anions in water.
2. Detection principle and structural design of AlGaN/GaN HEMT-based sensors
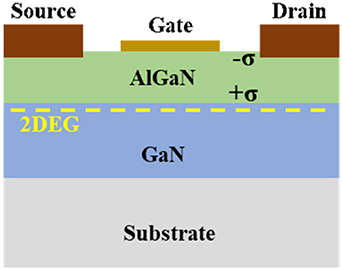
A two-dimensional electron gas (2DEG) channel can be formed at the AlGaN/GaN heterostructure interface, as shown in figure 1, due to the spontaneous and piezoelectric polarization of nitride semiconductors; this 2DEG channel is very close to the surface and extremely sensitive to the surface charge, and the change in the surface potential will modulate the 2DEG concentration and change the channel conductivity [9, 33–36]. To ensure charge neutrality in the undoped AlGaN/GaN heterostructure, the negative sheet charge of the 2DEG has to be balanced by positive adsorbed charges at the heterostructure surface [33]. A reduction in the positive surface charge due to adsorption of negative ions should cause a decrease in the 2DEG sheet carrier concentration and a corresponding increase in the sheet resistance of the device, and vice versa. Thus, AlGaN/GaN HEMT-based sensors can respond quite sensitively to the adsorption of charged analytes in the surrounding environment. This is the basic mechanism that causes a perceptible change in the surface-near 2DEG channel conductivity for sensor applications.
Figure 1. The schematic structure of AlGaN/GaN HEMTs.
Download figure:
Standard image High-resolution imageSeveral device structures have been proposed to improve the sensitivity and stability of sensors [37–40]. The basic structure consists of the AlGaN/GaN heterostructure, metal source and drain electrodes, and gate region, as shown in figure 1. For different purposes of ion detection, gate metallization can be used as part of an optional device structure. In general, for pH and anion detection in aqueous solutions, HEMT devices without gate metallization can be used to directly sense charged particles and adsorbed molecules, and surface-functionalization can enhance the sensitivity of sensors to the charged analytes.
For metal ion detection, either a metal gate with optional functionalization or a surface-functionalization nonmetallized gate region is employed. Furthermore, reference electrodes are often used to improve the stability of sensors [41]. Moreover, a normally-off AlGaN/GaN HEMT using a recessed-gate structure [38] and AlGa(In)N/GaN HEMTs with multi-segment sensitive regions [40] were also developed to improve the sensitivity of HEMT-based sensors.
3. Sensor fabrication
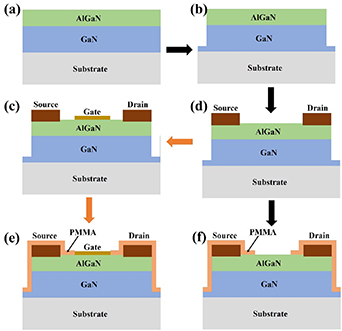
AlGaN/GaN heterostructures were epitaxially grown by metal organic chemical vapor deposition on sapphire or Si substrates that consist of an approximately 2 μm-thick undoped GaN buffer, 1 nm-thick AlN interlayer, and approximately 20 nm-thick undoped AlGaN barrier layer with an Al composition of approximately 23%, which is followed by a 2 nm-thick GaN cap layer. The 2DEG is located at the interface between the undoped GaN layer and the AlGaN layer. The device fabrication always commences with inductively coupled plasma etching to realize mesa isolation. Then, Ti/Al/Ni/Au multi-layer metals for ohmic contact are deposited, followed by a rapid thermal process at 850 °C in ambient N2 to create the draining and source electrodes. If needed, a thin metal gate electrode is subsequently deposited. After that, a thick protection layer made of a material such as polymethyl methacrylate is used to encapsulate the source/drain regions, with only the gate region open to allow the liquid solutions to cross the surface. The process flow chart of AlGaN/GaN HEMT-based sensors is shown in figure 2. The fabrication processes concerning surface functionalization and reference electrodes will be described in the later corresponding sections because of their differentiation.
Figure 2. The process flow chart of AlGaN/GaN HEMT-based sensors.
Download figure:
Standard image High-resolution image4. Applications in water quality monitoring
4.1. pH sensors
The pH value is one of the most important physical and chemical parameters of aqueous solutions, and most natural phenomena, chemical changes and production processes involving aqueous solutions are related to the pH value. Therefore, it is very necessary to monitor the pH value in the fields of industry, agriculture, medicine, and environmental protection.
The currently widely accepted pH sensing principle is based on the site-binding model proposed by Yates et al [42], which is a theoretical model specifically used to describe the 'double electric layer' behavior between the oxide layer and the liquid interface. According to this model, amphoteric hydroxyl groups are formed at oxidic surfaces that are in contact with aqueous solutions. These hydroxyl groups can obtain an H+ to form a positive charge at the oxidic surface, or combine with OH− in solution to form a negative charge at the oxidic surface or remain neutral, depending on the H+ concentration and the equilibrium constants for the relevant dissociation reactions, which can therefore lead to a pH-dependent net surface charge and an additional voltage drop at the solid/liquid interface [43].
An oxide layer can be formed easily on the III-nitride surface after being placed in air for a period of time or by thermal oxidization, and this unique property allows the use of GaN-based HEMTs as pH sensors. In 2003, Steinhoff et al [43] first demonstrated that both native and thermally oxidized GaN surfaces (GaxOy) can respond to the pH solutions. Both surfaces exhibited almost Nernstian behavior with sensitivities of 57.3 mV pH−1 and 56.6 mV pH−1 for the native and thermally oxidized GaN p–n junction, respectively, showing almost no difference in sensitivity. These devices can provide stable operation in the range from pH of 2 to 12 with a resolution better than 0.05 pH. In fact, the native GaN surface mentioned in that work is also a thin oxide layer because of the surface oxidizability of nitride semiconductors. They analyzed the as-deposited GaN surfaces via the surface sensitive Ga 2p and the O 1 s core level XPS spectra, and the result revealed that a thin surface oxide layer after exposure to the atmosphere can be formed. However, the GaxOy can be dissolved in a slightly hot acid solution, so this thin GaxOy layer may be corroded to a certain extent when detecting pH for a long period of time, which will result in the performance degradation of the native and thermally oxidized AlGaN/GaN HEMT-based sensors. Regarding this problem, we suggest some possible solutions that can improve the corrosion resistance of the devices. For example, an additional TiO2 thin film, which has higher chemical stability [44], may be deposited on the surface of the detecting region to form a composite oxide structure with the GaxOy, or an automatic cleaning apparatus can be used to wash the device surface quickly after detecting pH. In addition, periodic calibration is also a solution to this problem. Of course, an intentionally deposited oxide gate dielectric with appropriate thickness can produce the same effect and simultaneously mitigate the corrosion problem. In addition to GaxOy, Kang et al [45] used a 10 nm-thick Sc2O3 thin film as a gate dielectric of AlGaN/GaN HEMTs and achieved very high sensitivity for detecting changes in the pH of electrolyte solutions, and this was superior to the use of native oxide or UV ozone-induced oxide in the gate region. The fabricated sensors exhibited a linear change in current between the pH values of 3 and 10 for 37 μA pH−1 and showed stable operation with a resolution of <0.1 pH over the entire pH range. It was proposed that using a higher-quality oxide would be useful for improving the resolution of pH measurements.
Surface functionalization in the gate region using molecular groups as the sensing membranes is also an effective method for pH detection. Very recently, Ding et al proposed a molecular gated-AlGaN/GaN HEMT for pH detection [46]. The sensing surface of the sensor was modified with 3-aminopropyltriethoxysilane to provide amphoteric amine groups, which would play the role of receptors for pH detection, and the sensor exhibits good sensitivity in hydrochloric acid solution for pH detection. It was thought that the change of surface potential can explain the sensing mechanism of the molecular gated-AlGaN/GaN HEMT-based sensor. At the interface between the GaN surface and electrolyte, the processing of two reactions at the binding sites, namely, protonation and deprotonation (i.e.,–OH + H+ = –OH2+,–OH2+ + OH− = –OH + H2O and–NH2 + H+ = –NH3 +,—NH3 + + OH− = –NH2 + H2O), will positively or negatively change the surface potential, which can modulate the 2DEG concentration in the channel of AlGaN/GaN HEMT and thus change the source-drain current. In particular, they verified experimentally that the molecular gated-AlGaN/GaN HEMT modified by 3-aminopropyltriethoxysilane has much better chemical stability against HCl soaking in comparison with the oxidized AlGaN/GaN HEMT. So their result shows that using molecular gated-AlGaN/GaN HEMT modified by 3-aminopropyltriethoxysilane may be a way to solve the corrosion problem of GaxOy mentioned above.
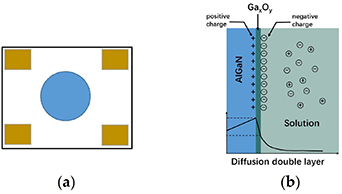
However, some studies showed that AlGaN/GaN HEMTs without surface oxide or functionalization can also be applied to detect the pH value. Podolska et al [47] developed a truly gateless four-contact AlGaN/GaN HEMT-based device without a reference electrode, as shown in figure 3(a), which is very different from the commonly used one that has a two-contact geometry with a reference electrode placed inside the solution during measurement. No AlGaN surface functionalization or treatment was applied in this device. Sheet resistance as a function of pH was extracted via the Van-der-Pauw technique based on four-point bar test structure configurations. Their results indicate that ungated AlGaN/GaN heterostructure devices are sensitive to ionic concentration in solutions, with selectivity toward negative ions over positive ions. They used the Helmholtz double-layer model to explain this negative ion sensitivity principle. The double-layer at the surface consists of a layer with a positive charge on the AlGaN surface and a layer of negatively charged ions in the liquid next to the AlGaN, as shown in figure 3(b). The negative charges most likely build up due to attraction to the positively charged (donor-like) surface states, which then creates an accumulation of negative ions close to the semiconductor surface. This means that native oxide has a minor effect and that the donor-like surface states are more likely to be playing the significant role in this case.
Figure 3. Schematic of gateless four-contact AlGaN/GaN HEMT device layout (a) [47]. Schematic of the Helmholtz double-layer model at AlGaN/GaN interface (b) [47].
Download figure:
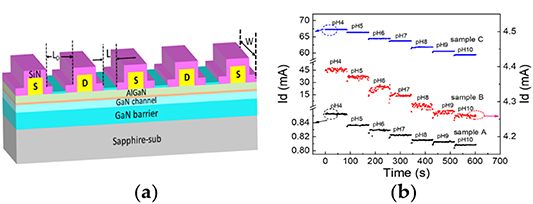
Standard image High-resolution imageEnhancing the sensitivity of the sensors has been a challenging issue. In general, the sensitivity of the sensor can be increased by increasing the sensing area in the gate region [34], which, in turn, increases the background noise and hence lowers the stability of the sensor [48]. Dong et al [40] designed and fabricated a multi-sensing-segment pH sensor by using interdigitated electrodes based on AlGaN/GaN HEMTs to deal with background noise and sensitivity issues of the sensor. The schematic structure of the multi-sensing-segment pH sensor is shown in figure 4(a), where L and W are the length and width of the open gate, respectively, and L0 is the length of the active region. Comparative study between the multi-sensing-segment pH sensor (sample C) and its conventional counterparts with a single-open-gate structure (sample A with a narrow gate width and sample B with a wide gate width) shows that the multi-sensing-segment pH sensor consisting of multiple parallel-connected sensors can guarantee good sensitivity and stability at the same time, and the sensitivity of pH sensors can be enhanced by increasing the ratio of the open gate area (L · W) to the active region area (L0 · W). The real-time measuring results of the drain current Id for the samples A, B and C are shown in figure 4(b). In addition, this structure shows an increased in the open gate width (W)/length (L), which is proportional to the drain current Id according to the equation of the drain current for standard GaN-based HEMTs:
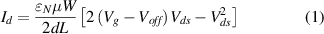
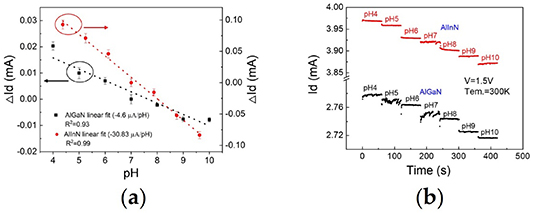
Figure 4. The schematic structure of the multi-sensing-segment AlGaN/GaN HEMT pH sensor (a) [40]. The real-time measuring results of drain current for the samples A, B and C (b) [40].
Download figure:
Standard image High-resolution imagewhere  is the dielectric constant of AlGaN, µ is the mobility of electrons, Vg is gate voltage, Voff is the threshold voltage, and Vds is the drain voltage. Thus, the multi-sensing-segment pH sensor can obtain a larger detection current in comparison with the single-sensing-area pH sensor under the same drain voltage, which can simplify the subsequent amplification circuit.
is the dielectric constant of AlGaN, µ is the mobility of electrons, Vg is gate voltage, Voff is the threshold voltage, and Vds is the drain voltage. Thus, the multi-sensing-segment pH sensor can obtain a larger detection current in comparison with the single-sensing-area pH sensor under the same drain voltage, which can simplify the subsequent amplification circuit.
To further improve the sensitivity of the emerging AlGaN/GaN HEMT-based pH sensors, some other device structures have also been developed successively. Brazzini et al [49] and Dong et al [50] proposed an AlInN/GaN HEMT structure with a thinner AlInN barrier layer that can make the 2DEG at the AlInN/GaN interface more sensitive to the change in the surface charge caused by the environment. The experimental results from Dong et al also [50] demonstrated that the AlInN/GaN device exhibited a larger current variation in terms of the pH value measurement compared with the AlGaN/GaN devices, as shown in figures 5(a)–(b). The AlInN/GaN HEMT structure only requires a very thin AlInN barrier layer to achieve the same 2DEG density as that of AlGaN/GaN HEMTs due to stronger spontaneous polarization and a larger conduction band discontinuity at the AlInN/GaN interface. In general, a 3 nm-thick barrier layer can generate the 2DEG channel for the AlInN/GaN HEMT, but for the AlGaN/GaN HEMT, at least a 10 nm barrier layer is required to form the 2DEG channel. The typical barrier thickness used in the AlGaN/GaN HEMT is approximately 25 nm, and in the AlInN/GaN HEMT, it is approximately 3–7 nm. This shorter distance between the heterostructure surface and the 2DEG channel can reduce the screening effect of intrinsic charge carriers in the barrier layer and hence makes the 2DEG channel more sensitive to the change in the surface charges. Although the AlInN/GaN HEMT seems to be a better candidate for use in ion sensors, the crystal quality and surface morphology of the AlInN/GaN heterostructure material as well as leakage current of the device need to be further improved. Moreover, Li et al [38] fabricated a normally-off AlGaN/GaN HEMT-based pH sensor with oxide film in the sensing region and found that the pH sensitivity of the sensor can be effectively improved by the enhanced transconductance of the normally-off device. Different pH sensors based on GaN HEMTs and their sensitivity parameters according to different research groups are summarized in table 1.
Figure 5. The drain current variation as a function of pH in (a) [50], and real-time measured drain current in the range of pH 4–10 in (b) [50], for AlInN/GaN and AlGaN/GaN HEMT sensors, respectively.
Download figure:
Standard image High-resolution imageTable 1. Different pH sensors based on GaN HEMTs and their sensitivity parameter.
| Device structure | Gate region treatment | Sensitivity | Refs. |
|---|---|---|---|
| AlGaN/GaN HEMT | thermally oxidized | 56 mV pH−1 | [43] |
| AlGaN/GaN HEMT | Sc2O3 | 37 μA pH−1 | [45] |
| AlGaN/GaN HEMT | PdO | 62 mV pH−1 | [51] |
| AlGaN/GaN HEMT | Al2O3 | 55.6 mV pH−1 | [28] |
| Normally-off AlGaN/GaN HEMT | Naturally oxidized | 56.3 mV pH−1 | [38] |
| AlGaN/GaN HEMT | quasi-reference electrode | 55 mV pH−1 | [41] |
| AlGaN/GaN HEMT | multi-sensing-segment | 1.35 mA pH−1 | [40] |
| AlGaN/GaN HEMT | Molecular gated | 37.17 μA pH−1 | [46] |
| AlInN/GaN HEMT | Naturally oxidized | 30.83 μA pH−1 | [50] |
| AlGaN/GaN HEMT | Naturally oxidized | 4.6 μA pH−1 | [50] |
In addition, when measuring the pH values of solutions, a separated Ag/AgCl electrode is traditionally used as the reference electrode to improve the stability of the electrode potential. However, sometimes it is limited by the large dimensions of the separated reference electrodes when measuring the pH values of microliter solutions. Recently, Xing et al [41] fabricated an AlGaN/GaN-based pH sensor by integrating a quasi-reference electrode onto the same chip, as shown in figure 6, and achieved stable pH measurements for microliter solutions. They found that pH measurement stability can be maintained with a larger junction capacitance and a smaller charge transfer resistance by optimizing the exposed area of the Au quasi-reference electrode. Based on this optimization, a sensitivity of 55 mV pH−1 was obtained at room temperature, which is very close to that of an ideal Nernst response.
Figure 6. The top view of an AlGaN/GaN based pH sensor with a quasi-reference electrode [41].
Download figure:
Standard image High-resolution image4.2. Metal ion sensors
Many heavy metals that are commonly presented in water and soil, such as mercury (Hg), lead (Pb), nickel (Ni), arsenic (As), cadmium (Cd), tin (Sn), and chromium (Cr), are not only toxic in nature but can cause cancer and neuro-degenerative diseases and affect liver and kidney functions [52–56]. Thus, it is very important to develop sensitive and selective detection methods for these heavy metals. Traditionally, there have been several methods for heavy metal detection, including spectroscopic (atomic absorption spectroscopy, Auger-electron spectroscopy, or inductively coupled plasma-mass spectrometry) or electrochemical (ion selective electrodes or polarography) approaches [57–67].
AlGaN/GaN HEMTs can be employed to realize the fast online monitoring of heavy metals. The sensitization treatment of the gate region is crucial for the detection of heavy metal ions. At present, there are three main methods used to sensitize the gate region. The first method is to use Au as a gate to bond with Hg2+ ions; the second is to functionalize the Au gate by forming an Au–S bond; and the third is to directly functionalize the surface of a nitride semiconductor in the gate region. For different treatments in the gate region, the specific sensitivity mechanism is different. Next, we will mainly take Hg2+ ion detection as an example to illustrate the sensitivity methods and mechanisms.
For an Au-gated AlGaN/GaN HEMT-based sensor without gate functionalization, the Au-mercury amalgam alloy can be formed when the Au-gate electrode is exposed to a Hg2+ ion solution [68]. The formation of the Au-mercury amalgam will change the effective surface potential of AlGaN/GaN HEMTs in the Hg2+ ion solution and hence change the concentration of the surface charge-sensitive 2DEG that can be measured by varying the source-drain current. This is the sensitivity mechanism by which the bare Au-gated AlGaN/GaN HEMTs can respond to mercury ions.
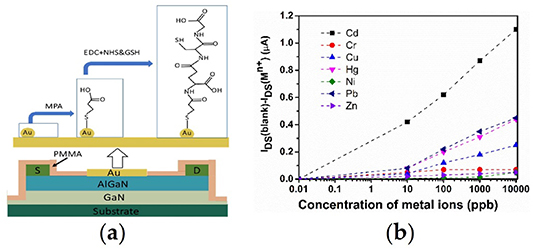
To functionalize the gate region, different research groups have proposed different methods. Wang et al [66] reported that thioglycolic acid (TGA) functionalized an Au-gated AlGaN/GaN HEMT for the detection of mercury (Hg2+) ions, as shown schematically in figure 7(a). In their sensor, a self-assembled monolayer of TGA molecules is adsorbed onto the gold gate by the formation of Au–S bonds due to a strong interaction between gold and the thiol group. The TGA molecules on the Au surface align vertically with the carboxylic acid functional group that is contained in TGA. The carboxylic acid functional group of the adjacent TGA molecules form chelates of R–COO−(Hg2+)−OOC–R with Hg2+ ions when the sensors are exposed to the Hg2+ ion solution. The charges of trapped Hg2+ ions in the R–COO−(Hg2+)−OOC–R chelates will change the polarity of the TGA molecules, which are bonded to the Au gate through–S–Au bonds, and hence change the drain current. As a result, the device exhibited a notable change in the drain current with the increase in the mercury ion concentration from 10–8 M to 10–5 M, as shown in figure 7(b).
Figure 7. A schematic of thioglycolic acid functionalized Au-gated AlGaN/GaN HEMT (a) [66]. Drain current of the sensor as a function of the Hg2+ ion concentration (b) [66].
Download figure:
Standard image High-resolution imageHowever, their experimental results seem to contradict the basic ion-sensing mechanism mentioned above. It is expected that the adsorption of Hg2+ would lead to a build-up of positive charge on the surface and cause an increase in the channel conductivity. However, a reduced source-drain current was actually measured. Although they gave a general and infallible explanation that the trapped Hg2+ ions change the polarity of the TGA molecules or change the effective surface potential of the sensor and result in a reduced source-drain current, the detailed sensitivity mechanism for the Au-gated AlGaN/GaN HEMT-based sensor needs further study.
Polyvinyl chloride (PVC) membranes with different formulations can be used as ion-selective membranes for ion FET sensors [69]. Using this method, Asadnia et al [48] developed a PVC-based membrane to functionalize the gate region of an AlGaN/GaN HEMT-based sensor and successfully detected the Hg2+ ion concentration by measuring the change of the voltage potential of the sensor with two sense terminals at a fixed channel current level even without a reference electrode. In their work, 1,10-dibenzyl-1,10-diaza-18-crown-6, a double armed crown ether composed of two flexible cation-ligating arms and a parent crown ring, was selected as a Hg2+ ionophore that can bind with the Hg2+ ions to form a molecular complex while having a low affinity for other ions. Hg2+ ionophores were added to the PVC membrane formulation similar to the optimized formulation reported by Gupta et al [70], and ionophore cavities matching the sizes of the Hg2+ ions will remain in the final PVC membrane. Thus, this PVC membrane with the specific ionophore has a high affinity for the Hg2+ ions in the solution. They explained the sensitivity mechanism as follows. The increase in captured Hg2+ ion concentration will increase the electron density of the 2DEG channel due to the adsorption of mercury ions with positive charge at the surface and therefore increase the 2DEG channel conductivity and result in a smaller voltage potential at a fixed source-drain current.
To further improve the performances of Hg2+ ion sensors, some research groups [71, 72] used 2D materials, such as MoS2 and reduced graphene oxide to functionalize or decorate the gate region of AlGaN/GaN HEMTs. Nigam et al [72] demonstrated that an MoS2-functionalized AlGaN/GaN HEMT-based sensor exhibited an excellent sensitivity of 0.64 µA ppb−1 and a detection limit of 0.01152 ppb with a rapid response time of 1.8 s, showing a linear range of detection from 0.1 ppb to 100 ppb and highly selective behavior toward Hg2+ ions. They attributed the excellent performance to the complexation of Hg2+ ions with sulfur and the electrostatic interaction between MoS2 and Hg2+ ions.
The detection limit for the metal ion concentration using an AlGaN/GaN HEMT-based sensor is generally in the range of 10−6–10−8 M, and the response time is usually a few seconds to tens of seconds. Cheng et al [73] reported an oligonucleotide-functionalized ion-sensitive AlGaN/GaN sensor, and the dynamic linear range for Hg2+ detection was determined at concentrations from 10–14 to 10–8 M, and a detection limit below the 10–14-M level was estimated.
Selectivity is another highly considered metric of sensor performance. The sensing selectivity for different metal ions depends on the surface functionalization material. Wang et al [66] found that their sensors showed excellent sensing selectivity of more than 100 for detecting mercury ions over sodium or magnesium ions, and the TGA-functionalized sensor can detect Cu2+ ions up to the same detection limit as Hg2+ ions, but the bare Au gate could not detect the Cu2+ ions. Neither sensor could detect Pb2+ ions. Kumar's group [74] also verified that the mercaptopropionic acid (MPA)- and glutathione (GSH)-functionalized AlGaN/GaN HEMT sensor exhibited good selectivity for Cd2+ ion detection. The functionalization process of MPA–GSH on the Au gate contact is shown schematically in figure 8(a), where a self-assembled monolayer of MPA is first formed on the gold electrode accompanying the activation of the carboxyl terminus, then GSH is allowed to attach to the carboxyl terminus via carbodiimide coupling, and finally the MPA–GSH-modified electrode is obtained. A cadmium ion can form a complex with GSH via the free sulfhydryl group and the carboxyl groups. The selectivity of Cd2+ ions toward other heavy metal ions, such as Cu2+, Hg2+, and Pb2+ ions, is shown in figure 8(b). The significant difference in responses toward cadmium ions and other heavy metal ions occurs because the carbonyl and sulfhydryl groups of GSH favor binding with Cd2+ ions. Meanwhile, the sensor exhibits a high sensitivity of 0.241 µA ppb−1, a fast response time of approximately 3 s, and a lower detection limit of 0.255 ppb. In addition to mercury and cadmium ions, other metal ions, such as lead, zinc, calcium, and potassium, have also been demonstrated to be detectable by the AlGaN/GaN HEMT-based sensors [75–78].
Figure 8. Functionalization process of MPA and GSH on Au gate contact of AlGaN/GaN HEMT (a). Comparison of the response of the Cd2+ ions with different heavy metal ions (b) [74].
Download figure:
Standard image High-resolution image4.3. Anion sensors
The main anions in water are Cl−, SO4 2−, HCO3 −, CO3 2−, OH−, NO3 −, PO4 3−, and As3− or As5−. If the content of these anions is too high, it will cause certain harm to the water environment and result in many problems that affect health. Therefore, it is important to effectively monitor the concentration of these anions to ensure public health [79, 80]. There are many traditional methods for detecting these anions, such as colorimetry, spectrophotometry, activation analysis, fluorometry, and ion chromatography [81–85]. Recently, AlGaN/GaN HEMTs have also been used to detect these anions by developing various surface functionalization methods.
Most surface functionalization methods are used to choose an appropriate membrane material that has excellent sensitivity and selectivity toward the target anions, and the sensitivity mechanism is similar to that of metal ion detection mentioned above. However, some novel technologies for ion detection are emerging. Recently, Jia et al [86] first employed molecular imprinting technology to functionalize the surface of AlGaN/GaN HEMT-based sensors and successfully detect trace amounts of phosphate anions.
Molecular imprinting is a technology for creating recognition sites in a macromolecular matrix using a template molecule. Within molecularly imprinted polymers (MIPs), a large number of imprinted cavities designed for the template molecule are distributed, and these cavities are consistent with the template molecules in terms of shape, size, and functional groups. Thus, MIPs have not only specific molecular recognition ability but also high affinity binding to the template molecule.
To modify the ungated region of a sensor with the phosphate ion-imprinted polymer, the AlGaN surface is oxidized, silanized and ion-imprinted successively. After oxidation by inductively coupled plasma, the sample is silanized in 10% 3-aminopropyltrimethoxy-silane (AMPS) solution. Then, the phosphate anion surface imprinting is conducted in an aqueous solution system composed of deionized water, Na3PO4 12 H2O, monomer methacryloyloxyethyl trimethyl ammonium chloride (DMC), and a cross-linker of N,N'-methylenebisacrylami-de. Finally, the graft/cross-linking polymerization reaction is performed after adding ammonium persulfate as an initiator. To remove the template phosphate anion, the device is fully washed with NaCl solution. The chemical reaction processes of ion imprinting is shown in figure 9 [86].
12 H2O, monomer methacryloyloxyethyl trimethyl ammonium chloride (DMC), and a cross-linker of N,N'-methylenebisacrylami-de. Finally, the graft/cross-linking polymerization reaction is performed after adding ammonium persulfate as an initiator. To remove the template phosphate anion, the device is fully washed with NaCl solution. The chemical reaction processes of ion imprinting is shown in figure 9 [86].
Figure 9. The chemical reaction processes of ion imprinting [86]. (a) surface-modified with coupling agent AMPS. (b) combination of monomer DMC with template PO4 3− ion via ion exchange action. (c) production of free radical on surfaces of modified silica gel particles. (d) simultaneously surface-initiated graft-polymerizing and imprinting. (e) removing template ion PO4 3.
Download figure:
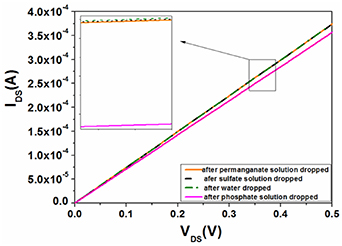
Standard image High-resolution imageThe AlGaN/GaN HEMT-based sensor functionalized by the ion imprinted polymer exhibits high sensitivity with an approximately linear dependence for phosphate concentration from 0.02 mg l−1 to 2 mg l−1, and the sensitivity and detection limit of the sensor are 3.191 μA mg−1 l−1 and 1.97 μg l−1, respectively. The outstanding advantage of this type of sensor is its superior selectivity because the imprinted caves in the template are unmatched with other interfering ions in terms of size and shape, leading them to not recognize or bind to interfering ions. Figure 10 presents the I–V characteristics of the phosphate ion-imprinted AlGaN/GaN HEMT-based sensor for the detection of different anions, showing a lack of response to other anions, such as MnO4 − and SO4 2− anions [86].
Figure 10. I–V characteristics of the phosphate ion imprinted AlGaN/GaN HEMT-based sensor for the detection of different anions [86].
Download figure:
Standard image High-resolution imageRen's group [39] demonstrated real-time chloride anion detection using InN-gated AlGaN/GaN HEMTs. The InN thin film on the gate area of the HEMT can provide fixed surface sites for reversible anion coordination. The drain current that the HEMT sensor exhibited increases as a function of the chloride ion concentration when the sensors are exposed to 100 nM NaCl at 200 s. The gate potential changes when the InN gate metal encounters chloride ions, and this causes the piezoelectrically induced charge density in the HEMT channel to increase. The sensor was tested over a range of chloride ion concentrations from 100 nM to 100 M and can also be integrated into a wireless data transmission system for remote sensing applications.
These results suggest that AlGaN/GaN HEMT-based sensors are also effective for detecting other anions through appropriate gate functionalization. For example, iodine and nitrate anions can also be detected by the AlGaN/GaN HEMT-based sensors. Different anion sensor-based AlGaN/GaN HEMTs from different research groups and their performances are summarized in table 2.
Table 2. Different anion sensors based Al(In)GaN/GaN HEMTs and their performances.
| Target anion | Functionalization | Detection limit | Selectivity | Refs. |
|---|---|---|---|---|
| I− | without | >1.0 × 10−5 M | − | [87] |
| Cl− | Anodized AgCl | 1.0 × 10−8 M | − | [88] |
| Cl− | InN gate | 1.0 × 10−7 M | − | [39] |
| NO3 − | polyvinyl chloride | 1.0 × 10−6 M | excellent | [89] |
| PO4 3− | ion imprinting | 1.97 µg l−1 | excellent | [86] |
| PO4 3− | ion imprinting | 0.09 µg l−1 | excellent | [37] |
5. Summary and outlook
This paper has given a brief overview of AlGaN/GaN HEMT-based sensors used for pH, metal ion and anion concentration detection for application in water quality monitoring. First, the detection principle and basic device structure were presented. Then, we focused on surface functionalization methods in the gate region of AlGaN/GaN HEMTs and their sensing mechanisms. AlGaN/GaN HEMT-based sensors have been greatly developed thanks to the unremitting efforts of several research teams, and high sensitivity and excellent selectivity have also been achieved and can meet the requirements of water quality monitoring for the trace target substances. In particular, improved technology for reference electrode-free or integrated reference electrodes in GaN HEMTs are urgently needed for the development of microsensors to realize in situ real-time monitoring of water quality, and unique surface functionalization technology with excellent selectivity, such as ion imprinting, is also very critical to promote the practical processing of AlGaN/GaN HEMT-based sensors.
However, current research concerning AlGaN/GaN HEMT-based sensors is still in its infancy and mainly focuses on the structural design and surface functionalization to improve the sensitivity. AlGaN/GaN HEMT-based sensors have not yet reached adequate maturity to be commercialized, and many challenging problems that need to be solved still remain. These issues include: i) the effects of ambient temperature and the pH value of an aqueous solution on the sensitivity and selectivity of sensors should be further studied to correct the accuracy and define the application scope of the sensors under different monitoring environments; ii) the stability and repeatability that determine whether the sensors can be commercialized need to be further improved by developing better device structures and processes; iii) there is a need to improve the selective recognition ability of sensors by surface functionalization or surface modification; iv) the integration of sensors into the mini-instrument chip is highly desirable to facilitate in situ real-time monitoring of water quality; and v) although the basic detection principle is clear, the more detailed sensing mechanism related to the specific surface functionalization process needs to be accurately understood, which is beneficial to the improvement of device structure design and the improved selection of surface functionalization membranes.
In summary, we reviewed the recent developments and remaining challenges of AlGaN/GaN HEMT-based sensors for application in water quality monitoring and hope this will be useful for further studies and development of GaN-based microsensors.
Author contributions:
Writing-original draft preparation, Hui Guo; writing-review and editing, Xiuling Jia, Yan Dong and Dunjun Chen; supervision, Dunjun Chen, Jiandong Ye, Rong Zhang and Youdou Zheng. All authors have read and agreed to the published version of the manuscript.
Funding:
This work is supported by the NSFC (61634002), the NSAF (U1830109), and the Jiangsu Provincial Department of Water Resources, China (2019048).
Conflicts of interest:
The authors declare no conflict of interest.